Functions of Ionic Liquids in Preparing Membranes for Liquid Separations: A Review
Abstract
1. Introduction
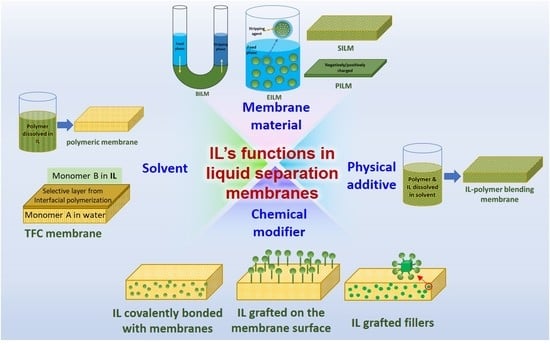
2. Functions of ILs in Developing Liquid Separation Membranes
2.1. ILs as Raw Membrane Materials
2.1.1. Bulk IL Membrane (BILM)
2.1.2. Emulsion IL Membrane (EILM)
2.1.3. Supported IL Membrane (SILM)
2.1.4. Polymerized IL Membrane (PILM)
2.2. ILs as Physical Additives
2.2.1. IL-Polymer Blending Membranes for PV
2.2.2. IL-Polymer Blending Membranes for Separating Metal/Organic from Water
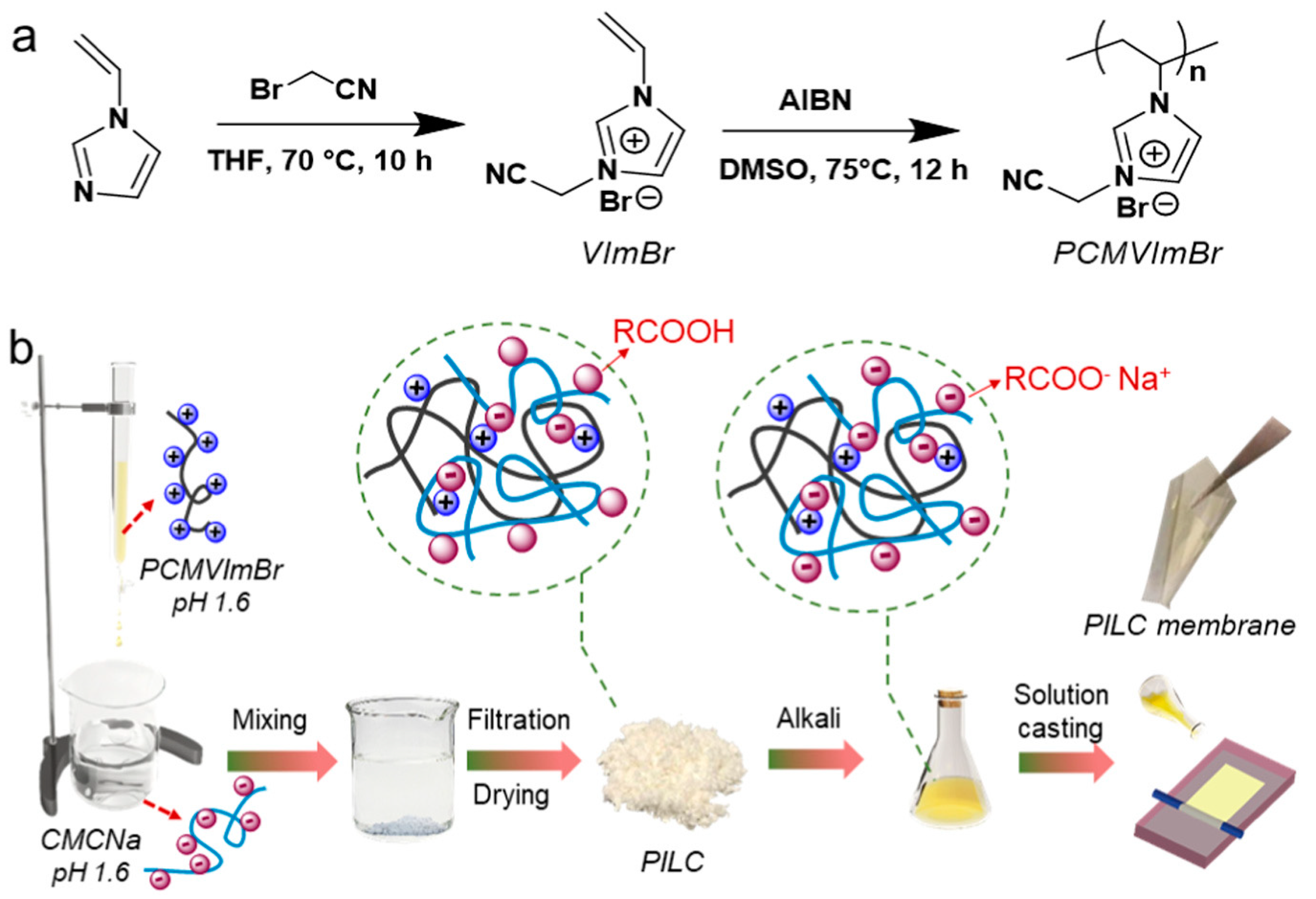
2.3. ILs as Chemical Modifiers
2.3.1. Chemically Modify Membrane
2.3.2. Chemically Modify the Membrane Additives
2.4. ILs as Solvents
2.4.1. Solvents for Polymer Dissolution
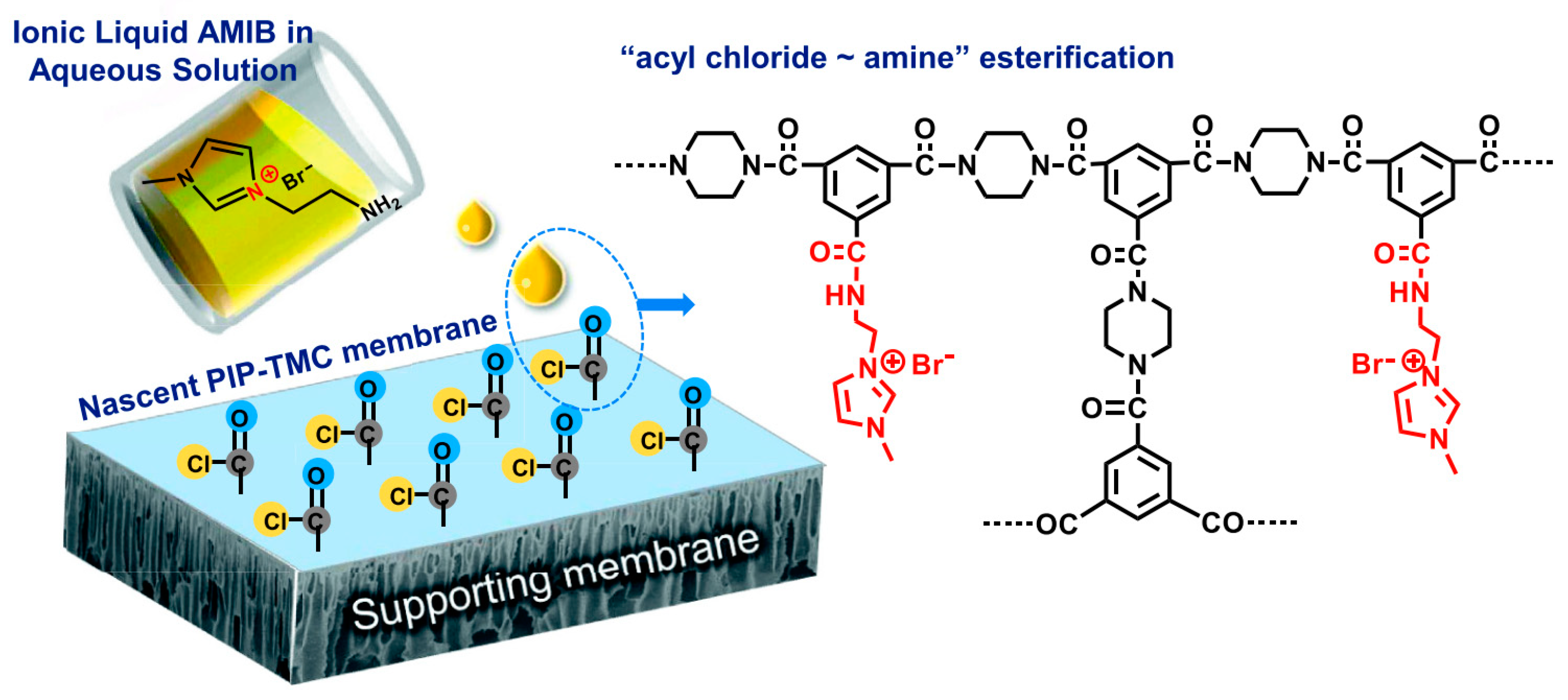
2.4.2. Solvents as Reaction Media during Membrane Fabrication
3. The Sustainability of ILs in Developing Liquid Separation Membranes
4. Conclusions and Future Directions
- (1)
- Membranes that are fabricated directly with ILs (i.e., BILM, EILM, and SILM) are mainly used for removing organics and metal ions based on extraction. Such membranes often suffer from poor stability due to either the leaching of ILs into the liquid phase or the emulsion swelling and breakage (specifically involving EILM).
- (2)
- PILMs are more stable than IL membranes due to their larger molecular structures. They exhibited good performance in applications such as the removal of metal ions and organic dyes, desalination, the concentration of proteins, and oil/water separation. The PILMs have shown great potential but further studies on them are required.
- (3)
- The stability of the membranes can be improved tremendously if ILs/PILs are blended with polymers due to physical interactions such as hydrogen bonds, π-π stacking, or electrostatic interactions. These types of membranes have been widely studied for organophilic PV and separation of metal/organic from water. However, the issue of IL leaching may still exist due to weak physical interactions.
- (4)
- ILs/PILs can be used to chemically modify polymeric membranes or membrane components (like fillers) to improve the separation performance and membrane stability due to their strong covalent bonds. These membranes showed promising performance and excellent stability in PV, RO, MF, and RO, etc. Some large-scale demonstrations are needed to promote industrial applications.
- (5)
- The use of ILs as a solvent to dissolve polymers (especially those which are too rigid to be dissolved by traditional solvents) and as reaction media for the interfacial polymerization to fabricate liquid membranes are important because this option is less hazardous and more sustainable. Currently, the use of ILs to dissolve polymers and fabricate membranes have been studied a great deal. Therefore, further efforts must be directed to the study of the mechanisms for the dissolution and phase inversion of IL-polymer solutions. Since the use of ILs as reaction media for the thin-film membranes just started recently, more ILs must be investigated for applications in this area, and their reaction mechanism must be explored to fully understand how to precisely control their reaction processes.
- (6)
- To make the usage of ILs in developing liquid separation membranes more sustainable and economic, more efforts should be paid to looking for efficient methods for the regeneration, recovery, and removal of ILs in these special cases.
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.; Liu, Y.; Wang, J.; Gascon, J.; Li, J.; van der Bruggen, B. Metal-organic frameworks based membranes for liquid separation. Chem. Soc. Rev. 2017, 46, 7124–7144. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Marino, T.; Russo, F.; Criscuoli, A.; Figoli, A. TamiSolve® NxG as novel solvent for polymeric membrane preparation. J. Membr. Sci. 2017, 542, 418–429. [Google Scholar] [CrossRef]
- Cseri, L.; Szekely, G. Towards cleaner PolarClean: Efficient synthesis and extended applications of the polar aprotic solvent methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate. Green Chem. 2019, 21, 4178–4188. [Google Scholar] [CrossRef]
- Marino, T.; Galiano, F.; Molino, A.; Figoli, A. New frontiers in sustainable membrane preparation: Cyrene™ as green bioderived solvent. J. Membr. Sci. 2019, 580, 224–234. [Google Scholar] [CrossRef]
- Russo, F.; Galiano, F.; Pedace, F.; Aricò, F.; Figoli, A. Dimethyl isosorbide as a green solvent for sustainable ultrafiltration and microfiltration membrane preparation. ACS Sustain. Chem. Eng. 2019, 8, 659–668. [Google Scholar] [CrossRef]
- Le Phuong, H.A.; Ayob, N.A.I.; Blanford, C.F.; Rawi, N.F.M.; Szekely, G. Nonwoven membrane supports from renewable resources: Bamboo fiber reinforced poly(lactic acid) composites. ACS Sustain. Chem. Eng. 2019, 7, 11885–11893. [Google Scholar] [CrossRef]
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Han, D.; Row, K.H. Recent applications of ionic liquids in separation technology. Molecules 2010, 15, 2405–2426. [Google Scholar] [CrossRef]
- Noble, R.D.; Gin, D.L. Perspective on ionic liquids and ionic liquid membranes. J. Membr. Sci. 2011, 369, 1–4. [Google Scholar] [CrossRef]
- Karkhanechi, H.; Salmani, S.; Asghari, M. A review on gas separation applications of supported ionic liquid membranes. ChemBioEng Rev. 2015, 2, 290–302. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Feng, S.; Li, H.; Wan, Y.; Zhang, X. Recent development of ionic liquid membranes. Green Energy Environ. 2016, 1, 43–61. [Google Scholar] [CrossRef]
- Salar-Garcia, M.J.; Ortiz-Martinez, V.M.; Hernandez-Fernandez, F.J.; Rios, A.P.d.L.; Quesada-Medina, J. Ionic liquid technology to recover volatile organic compounds (VOCs). J. Hazard. Mater. 2017, 321, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Rynkowska, E.; Fatyeyeva, K.; Kujawski, W. Application of polymer-based membranes containing ionic liquids in membrane separation processes: A critical review. Rev. Chem. Eng. 2018, 34, 341–363. [Google Scholar] [CrossRef]
- Yan, X.; Anguille, S.; Bendahan, M.; Moulin, P. Ionic liquids combined with membrane separation processes: A review. Sep. Purif. Technol. 2019, 222, 230–253. [Google Scholar] [CrossRef]
- Li, J.L.; Zhang, Y.; Zhang, S.; Liu, M.; Li, X.; Cai, T. Hyperbranched poly(ionic liquid) functionalized poly(ether sulfone) membranes as healable antifouling coatings for osmotic power generation. J. Mater. Chem. A 2019, 7, 8167–8176. [Google Scholar] [CrossRef]
- Mustafa, M.Z.u.; bin Mukhtar, H.; Md Nordin, N.A.H.; Mannan, H.A.; Nasir, R.; Fazil, N. Recent developments and applications of ionic liquids in gas separation membranes. Chem. Eng. Technol. 2019, 42, 2580–2593. [Google Scholar] [CrossRef]
- Isosaari, P.; Srivastava, V.; Sillanpää, M. Ionic liquid-based water treatment technologies for organic pollutants: Current status and future prospects of ionic liquid mediated technologies. Sci. Total. Environ. 2019, 690, 604–619. [Google Scholar] [CrossRef]
- Foong, C.Y.; Wirzal, M.D.H.; Bustam, M.A. A review on nanofibers membrane with amino-based ionic liquid for heavy metal removal. J. Mol. Liq. 2020, 297, 111793. [Google Scholar] [CrossRef]
- Aguilar, M.; Luis, C.J. Solvent Extraction and Liquid Membranes: Fundamentals and Applications Innew Materials; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Qian, W.; Texter, J.; Yan, F. Frontiers in poly(ionic liquid)s: Syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef]
- Malik, M.A.; Hashim, M.A.; Nabi, F. Ionic liquids in supported liquid membrane technology. Chem. Eng. J. 2011, 171, 242–254. [Google Scholar] [CrossRef]
- Winterton, N. Solubilization of polymers by ionic liquids. J. Mater. Chem. 2006, 16, 4281–4293. [Google Scholar] [CrossRef]
- Xing, D.Y.; Peng, N.; Chung, T.-S. Investigation of unique interactions between cellulose acetate and ionic liquid [EMIM]SCN, and their influences on hollow fiber ultrafiltration membranes. J. Membr. Sci. 2011, 380, 87–97. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Liu, Y.-R.; Nie, Y. Research review of dissolving natural polymer materials with ionic liquids and green spinning technology. J. Light Ind. 2016, 31, 1–14. [Google Scholar]
- Xing, D.Y.; Chan, S.Y.; Chung, T.S. The ionic liquid [EMIM]OAc as a solvent to fabricate stable polybenzimidazole membranes for organic solvent nanofiltration. Green Chem. 2014, 16, 1383–1392. [Google Scholar] [CrossRef]
- Hua, D.; Japip, S.; Wang, K.Y.; Chung, T.-S. Green design of poly(m-phenylene isophthalamide)-based thin-film composite membranes for organic solvent nanofiltration and concentrating lecithin in hexane. ACS Sustain. Chem. Eng. 2018, 6, 10696–10705. [Google Scholar] [CrossRef]
- Mariën, H.; Bellings, L.; Hermans, S.; Vankelecom, I.F. Sustainable process for the preparation of high-performance thin-film composite membranes using ionic liquids as the reaction medium. ChemSusChem 2016, 9, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Ortiz, A.; Ortiz, I. Progress in the use of ionic liquids as electrolyte membranes in fuel cells. J. Membr. Sci. 2014, 469, 379–396. [Google Scholar] [CrossRef]
- Shen, M.; Han, Y.; Lin, X.; Ding, B.; Zhang, L.; Zhang, X. Preparation and electrochemical performances of porous polypyrrole film by interfacial polymerization. J. Appl. Polym. Sci. 2013, 127, 2938–2944. [Google Scholar] [CrossRef]
- Yasuda, T.; Watanabe, M.J.M.B. Protic ionic liquids: Fuel cell applications. Mrs. Bull. 2013, 38, 560–566. [Google Scholar] [CrossRef]
- Leones, R.; Reis, P.M.; Sabadini, R.C.; Esperança, J.M.S.S.; Pawlicka, A.; Silva, M.M. Chitosan polymer electrolytes doped with a dysprosium ionic liquid. J. Polym. Res. 2020, 27, 45. [Google Scholar] [CrossRef]
- Bakonyi, P.; Koók, L.; Rozsenberszki, T.; Tóth, G.; Bélafi-Bakó, K.; Nemestóthy, N. Development and application of supported ionic liquid membranes in microbial fuel cell technology: A concise overview. Membranes 2020, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Colburn, A.; Wanninayake, N.; Kim, D.Y.; Bhattacharyya, D. Cellulose-graphene quantum dot composite membranes using ionic liquid. J. Membr. Sci. 2018, 556, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Zhu, L.-P.; Zhu, B.-K.; Xu, Y.-Y. High-flux and anti-fouling cellulose nanofiltration membranes prepared via phase inversion with ionic liquid as solvent. Sep. Purif. Technol. 2011, 83, 66–73. [Google Scholar] [CrossRef]
- Ślusarczyk, C.; Fryczkowska, B. Structure-property relationships of pure cellulose and GO/CEL membranes regenerated from ionic liquid solutions. Polymers 2019, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Hameed, M.S. Extraction of 4-nitrophenol from aqueous solutions using bulk ionic liquid membranes. Int. J. Curr. Eng. Technol. 2016, 6, 542–550. [Google Scholar]
- Lakshmi, A.B.; Sindhu, S.; Venkatesan, S. Performance of ionic liquid as bulk liquid membrane for chlorophenol removal. Int. J. ChemTech Res. 2013, 5, 1129–1137. [Google Scholar]
- Fortunato, R.; González-Muñoz, M.J.; Kubasiewicz, M.; Luque, S.; Alvarez, J.R.; Afonso, C.A.M.; Coelhoso, I.M.; Crespo, J.G. Liquid membranes using ionic liquids: The influence of water on solute transport. J. Membr. Sci. 2005, 249, 153–162. [Google Scholar] [CrossRef]
- Baylan, N.; Çehreli, S. Removal of acetic acid from aqueous solutions using bulk ionic liquid membranes: A transport and experimental design study. Sep. Purif. Technol. 2019, 224, 51–61. [Google Scholar] [CrossRef]
- Baylan, N.; Çehreli, S. Ionic liquids as bulk liquid membranes on levulinic acid removal: A design study. J. Mol. Liq. 2018, 266, 299–308. [Google Scholar] [CrossRef]
- Chakraborty, M.; Bart, H.-J. Highly selective and efficient transport of toluene in bulk ionic liquid membranes containing Ag+ as carrier. Fuel Process. Technol. 2007, 88, 43–49. [Google Scholar] [CrossRef]
- Branco, L.C.; Crespo, J.G.; Afonso, C.A.M. Studies on the selective transport of organic compounds by using ionic liquids as novel supported liquid membranes. Chem.-A Eur. J. 2002, 8, 3865–3871. [Google Scholar] [CrossRef]
- Branco, L.C.; Crespo, J.G.; Afonso, C.A.M. Ionic liquids as an efficient bulk membrane for the selective transport of organic compounds. J. Phys. Org. Chem. 2008, 21, 718–723. [Google Scholar] [CrossRef]
- Kogelnig, D.; Stojanovic, A.; Jirsa, F.; Körner, W.; Krachler, R.; Keppler, B.K. Transport and separation of iron(III) from nickel(II) with the ionic liquid trihexyl(tetradecyl)phosphonium chloride. Sep. Purif. Technol. 2010, 72, 56–60. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Venkatesan, S. Removal of phenolic compounds from aqueous solutions by emulsion liquid membrane containing Ionic Liquid [BMIM]+[PF6]− in Tributyl phosphate. Desalination 2012, 289, 27–34. [Google Scholar] [CrossRef]
- Lende, A.B.; Dinker, M.K.; Bhosale, V.K.; Kamble, S.P.; Meshram, P.D.; Kulkarni, P.S. Emulsion ionic liquid membranes (EILMs) for removal of Pb(ii) from aqueous solutions. RSC Adv. 2014, 4, 52316–52323. [Google Scholar] [CrossRef]
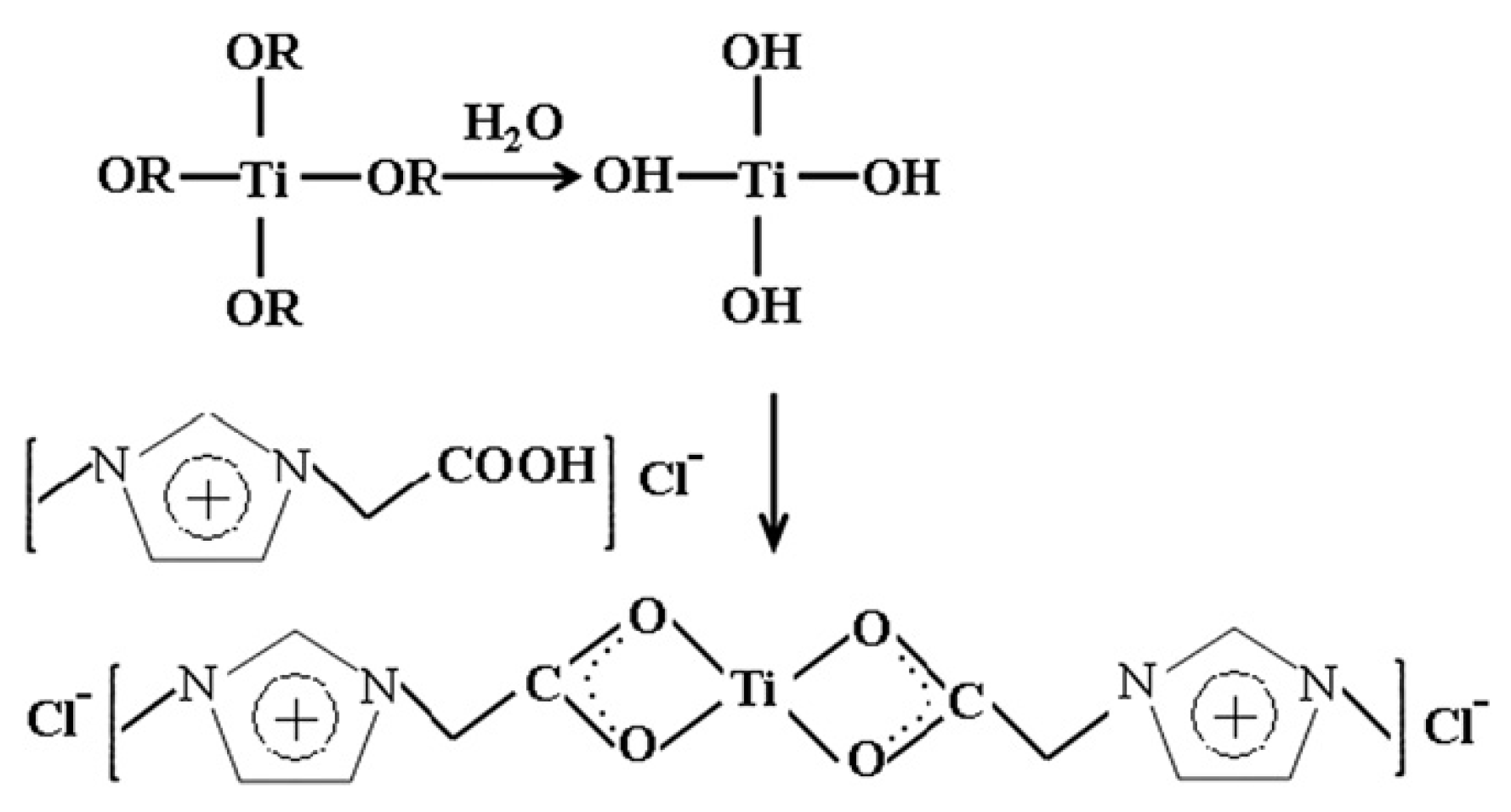
- Alguacil, F.J.; López, F.A.; García-Díaz, I.; Rodriguez, O. Cadmium(II) transfer using (TiOAC) ionic liquid as carrier in a smart liquid membrane technology. Chem. Eng. Process. Process Intensif. 2016, 99, 192–196. [Google Scholar] [CrossRef]
- Fadeev, A.G.; Meagher, M.M. Opportunities for ionic liquids in recovery of biofuels. Chem. Commun. 2001, 295–296. [Google Scholar] [CrossRef]
- Lozano, L.J.; Godínez, C.; Ríos, A.P.d.L.; Hernández-Fernández, F.J.; Sánchez-Segado, S.; Alguacil, F.J. Recent advances in supported ionic liquid membrane technology. J. Membr. Sci. 2011, 376, 1–14. [Google Scholar] [CrossRef]
- Kárászová, M.; Kacirková, M.; Friess, K.; Izák, P. Progress in separation of gases by permeation and liquids by pervaporation using ionic liquids: A review. Sep. Purif. Technol. 2014, 132, 93–101. [Google Scholar] [CrossRef]
- Riisagera, A.; Fehrmanna, R.; Haumannb, M.; Wasserscheidb, P. Supported ionic liquids: Versatile reaction and separation media. Top. Catal. 2006, 40, 91–102. [Google Scholar] [CrossRef]
- Mai, N.L.; Kim, S.H.; Ha, S.H.; Shin, H.S.; Koo, Y.-M. Selective recovery of acetone-butanol-ethanol from aqueous mixture by pervaporation using immobilized ionic liquid polydimethylsiloxane membrane. Korean J. Chem. Eng. 2013, 30, 1804–1809. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, W.; Liu, J.; Zhang, W.; Ren, Z. Extraction separation of toluene/cyclohexane with hollow fiber supported ionic liquid membrane. Korean J. Chem. Eng. 2014, 31, 1049–1056. [Google Scholar] [CrossRef]
- Li, W.; Molina-Fernández, C.; Estager, J.; Monbaliu, J.-C.M.; Debecker, D.P.; Luis, P. Supported ionic liquid membranes for the separation of methanol/dimethyl carbonate mixtures by pervaporation. J. Membr. Sci. 2020, 598, 117790. [Google Scholar] [CrossRef]
- Matsumoto, M.; Panigrahi, A.; Murakami, Y.; Kondo, K. Effect of ammonium- and phosphonium-based ionic liquids on the separation of lactic acid by supported ionic liquid membranes (SILMs). Membranes 2011, 1, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Pilli, S.R.; Banerjee, T.; Mohanty, K. Performance of different ionic liquids to remove phenol from aqueous solutions using supported liquid membrane. Desalin. Water Treat. 2014, 54, 3062–3072. [Google Scholar] [CrossRef]
- Panigrahi, A.; Pilli, S.R.; Mohanty, K. Selective separation of Bisphenol A from aqueous solution using supported ionic liquid membrane. Sep. Purif. Technol. 2013, 107, 70–78. [Google Scholar] [CrossRef]
- Abejón, R.; Rabadán, J.; Lanza, S.; Abejón, A.; Garea, A.; Irabien, A. Supported ionic liquid membranes for separation of lignin aqueous solutions. Processes 2018, 6, 143. [Google Scholar] [CrossRef]
- Jean, E.; Villemin, D.; Hlaibi, M.; Lebrun, L. Heavy metal ions extraction using new supported liquid membranes containing ionic liquid as carrier. Sep. Purif. Technol. 2018, 201, 1–9. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Lithium extraction from complex aqueous solutions using supported ionic liquid membranes. J. Membr. Sci. 2019, 580, 62–76. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Kausar, A. Research progress in frontiers of poly(ionic liquid)s: A review. Polym. Technol. 2017, 56, 1823–1838. [Google Scholar] [CrossRef]
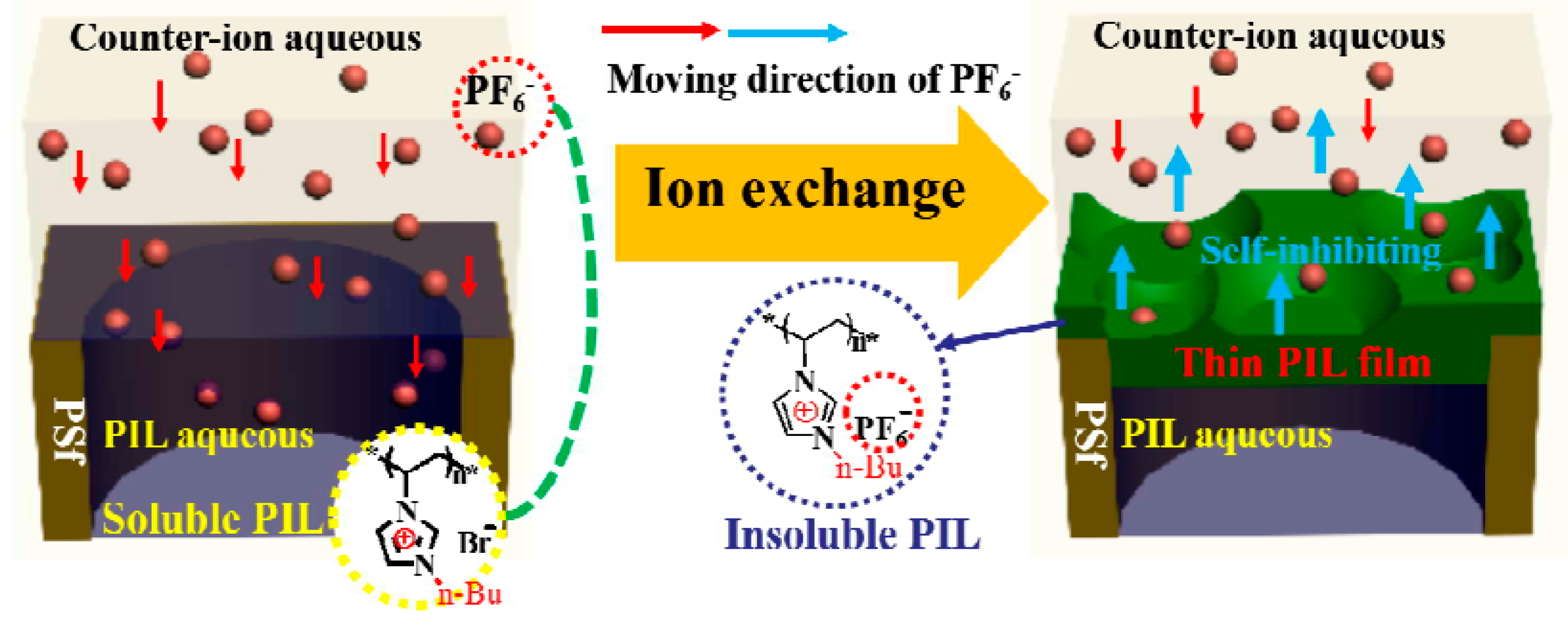
- Tang, Y.; Tang, B.; Wu, P. Preparation of a positively charged nanofiltration membrane based on hydrophilic–hydrophobic transformation of a poly(ionic liquid). J. Mater. Chem. A 2015, 3, 12367–12376. [Google Scholar] [CrossRef]
- Täuber, K.; Zimathies, A.; Yuan, J. Porous membranes built up from hydrophilic poly(ionic liquid)s. Macromol. Rapid Commun. 2015, 36, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Kohno, Y.; Gin, D.L.; Noble, R.D.; Ohno, H. A thermoresponsive poly(ionic liquid) membrane enables concentration of proteins from aqueous media. Chem. Commun. 2016, 52, 7497–7500. [Google Scholar] [CrossRef] [PubMed]
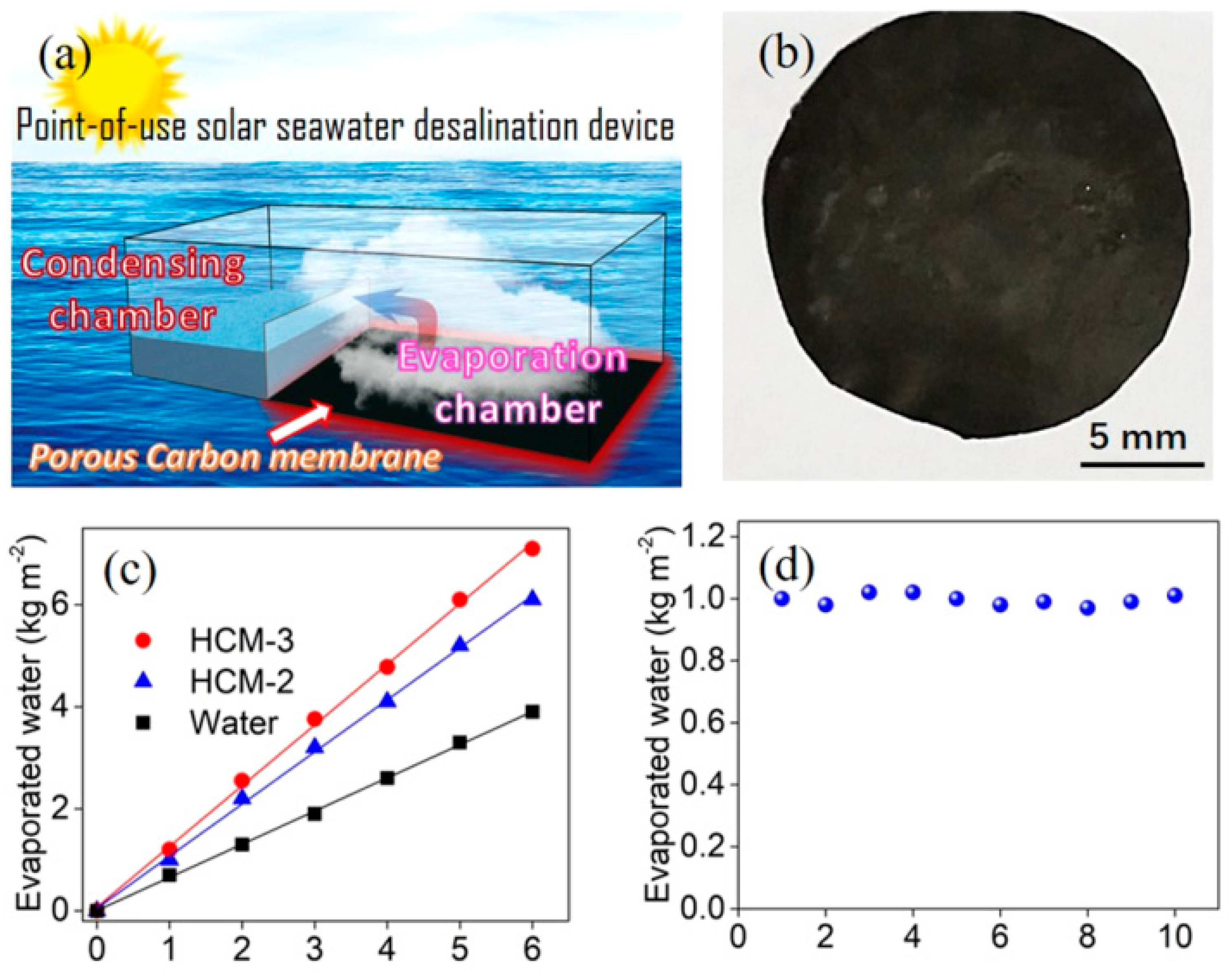
- Shao, Y.; Jiang, Z.; Zhang, Y.; Wang, T.; Zhao, P.; Zhang, Z.; Yuan, J.; Wang, H. All-poly(ionic liquid) membrane-derived porous carbon membranes: Scalable synthesis and application for photothermal conversion in seawater desalination. ACS Nano 2018, 12, 11704–11710. [Google Scholar] [CrossRef] [PubMed]
- Izák, P.; Ruth, W.; Fei, Z.; Dyson, P.J.; Kragl, U. Selective removal of acetone and butan-1-ol from water with supported ionic liquid–polydimethylsiloxane membrane by pervaporation. Chem. Eng. J. 2008, 139, 318–321. [Google Scholar] [CrossRef]
- Izák, P.; Friess, K.; Hynek, V.; Ruth, W.; Fei, Z.; Dyson, J.P.; Kragl, U. Separation properties of supported ionic liquid–polydimethylsiloxane membrane in pervaporation process. Desalination 2009, 241, 182–187. [Google Scholar] [CrossRef]
- Rdzanek, P.; Heitmann, S.; Górak, A.; Kamiński, W. Application of supported ionic liquid membranes (SILMs) for biobutanol pervaporation. Sep. Purif. Technol. 2015, 155, 83–88. [Google Scholar] [CrossRef]
- Ong, Y.T.; Tan, S.H. Pervaporation separation of a ternary azeotrope containing ethyl acetate, ethanol and water using a buckypaper supported ionic liquid membrane. Chem. Eng. Res. Des. 2016, 109, 116–126. [Google Scholar] [CrossRef]
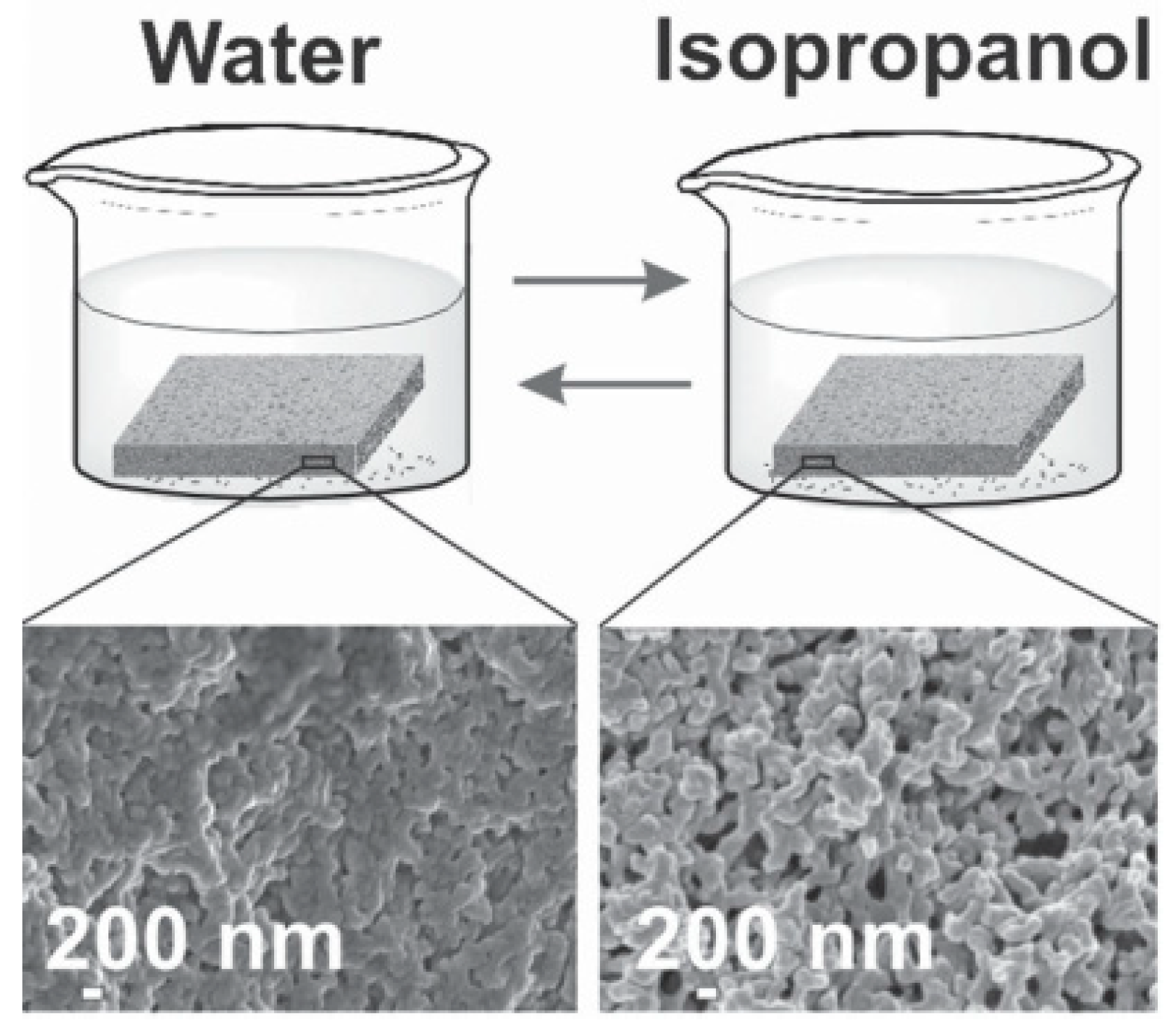
- Tang, S.; Dong, Z.; Zhu, X.; Zhao, Q. A poly(ionic liquid) complex membrane for pervaporation dehydration of acidic water-isopropanol mixtures. J. Membr. Sci. 2019, 576, 59–65. [Google Scholar] [CrossRef]
- He, Z.; Meng, M.; Yan, L.; Zhu, W.; Sun, F.; Yan, Y.; Liu, Y.; Liu, S. Fabrication of new cellulose acetate blend imprinted membrane assisted with ionic liquid ([BMIM]Cl) for selective adsorption of salicylic acid from industrial wastewater. Sep. Purif. Technol. 2015, 145, 63–74. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J. Asymmetric membrane containing ionic liquid [A336][P507] for the preconcentration and separation of heavy rare earth lutetium. ACS Sustain. Chem. Eng. 2016, 4, 2644–2650. [Google Scholar] [CrossRef]
- Elias, G.; Díez, S.; Fontàs, C. System for mercury preconcentration in natural waters based on a polymer inclusion membrane incorporating an ionic liquid. J. Hazard. Mater. 2019, 371, 316–322. [Google Scholar] [CrossRef] [PubMed]
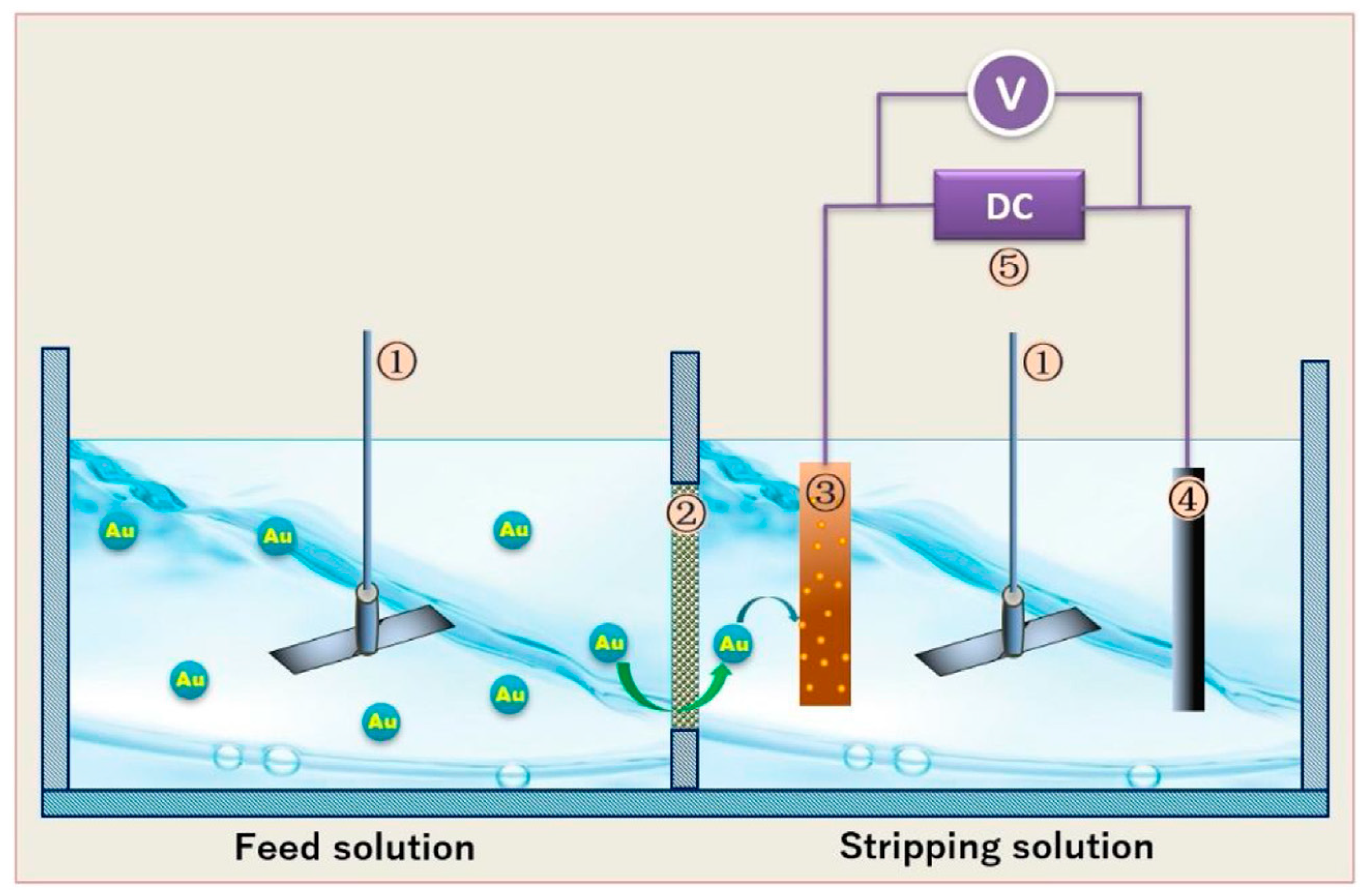
- Wang, Z.; Sun, Y.; Tang, N.; Miao, C.; Wang, Y.; Tang, L.; Wang, S.; Yang, X. Simultaneous extraction and recovery of gold(I) from alkaline solutions using an environmentally benign polymer inclusion membrane with ionic liquid as the carrier. Sep. Purif. Technol. 2019, 222, 136–144. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Wang, Y.; Liu, M.; Li, S.; Tang, L.; Wang, S.; Yang, X.; Ji, S. Improved transport of gold(I) from aurocyanide solution using a green ionic liquid-based polymer inclusion membrane with in-situ electrodeposition. Chem. Eng. Res. Des. 2020, 153, 136–145. [Google Scholar] [CrossRef]
- Xi, T.; Lu, Y.; Ai, X.; Tang, L.; Yao, L.; Hao, W.; Cui, P. Ionic liquid copolymerized polyurethane membranes for pervaporation separation of benzene/cyclohexane mixtures. Polymer 2019, 185, 121948. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, Z.; Yang, L.; Guo, H.; Han, S. Activation promoted ionic liquid modification of reverse osmosis membrane towards enhanced permeability for desalination. J. Taiwan Inst. Chem. Eng. 2017, 80, 25–33. [Google Scholar] [CrossRef]
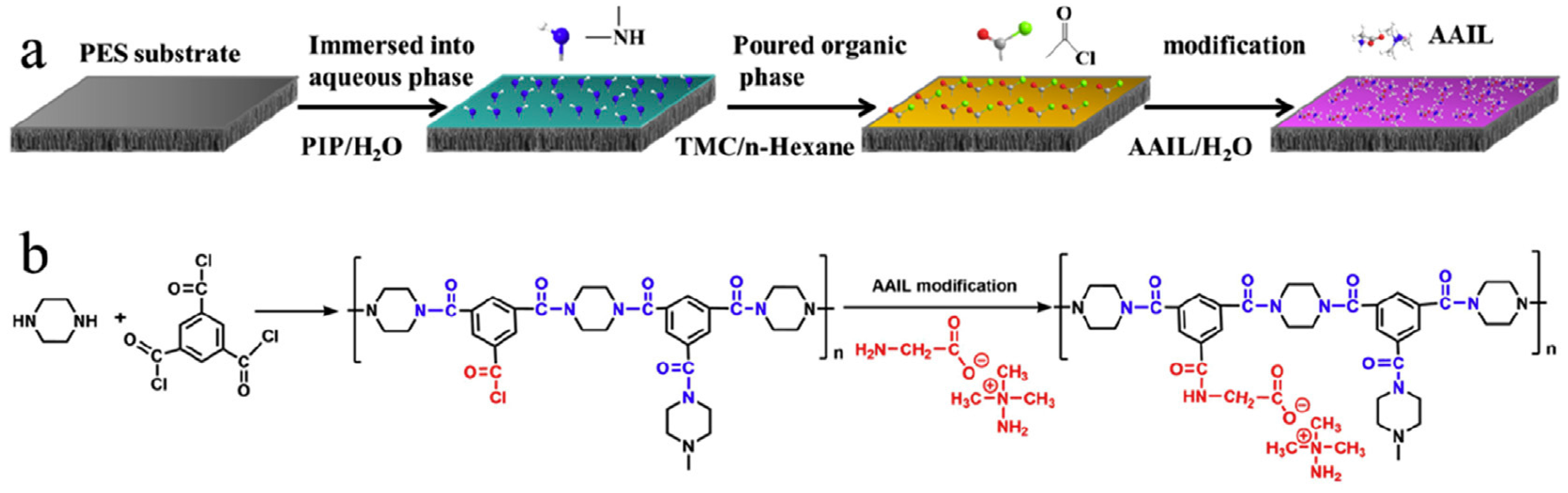
- Xiao, H.-F.; Chu, C.-H.; Xu, W.-T.; Chen, B.-Z.; Ju, X.-H.; Xing, W.; Sun, S.-P. Amphibian-inspired amino acid ionic liquid functionalized nanofiltration membranes with high water permeability and ion selectivity for pigment wastewater treatment. J. Membr. Sci. 2019, 586, 44–52. [Google Scholar] [CrossRef]
- He, B.; Peng, H.; Chen, Y.; Zhao, Q. High performance polyamide nanofiltration membranes enabled by surface modification of imidazolium ionic liquid. J. Membr. Sci. 2020, 608, 118202. [Google Scholar] [CrossRef]
- Liu, L.; Xie, X.; Zambare, R.S.; Selvaraj, A.P.J.; Sowrirajalu, B.N.; Song, X.; Tang, C.Y.; Gao, C. Functionalized graphene oxide modified polyethersulfone membranes for low-pressure anionic dye/salt Fractionation. Polymer 2018, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Ma, Y.; Ma, J.; Wang, P.; Sun, W. Preparation and characterization of PVDF/TiO2 hybrid membranes with ionic liquid modified nano-TiO2 particles. J. Membr. Sci. 2013, 427, 259–269. [Google Scholar] [CrossRef]
- Abraham, J.; Jose, T.; Moni, G.; George, S.C.; Kalarikkal, N.; Thomas, S. Ionic liquid modified multiwalled carbon nanotube embedded styrene butadiene rubber membranes for the selective removal of toluene from toluene/methanol mixture via pervaporation. J. Taiwan Inst. Chem. Eng. 2019, 95, 594–601. [Google Scholar] [CrossRef]
- Tang, W.; Lou, H.; Li, Y.; Kong, X.; Wu, Y.; Gu, X. Ionic liquid modified graphene oxide-PEBA mixed matrix membrane for pervaporation of butanol aqueous solutions. J. Membr. Sci. 2019, 581, 93–104. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Wang, Y.; Zhang, H.; Liu, J. High flux, positively charged loose nanofiltration membrane by blending with poly (ionic liquid) brushes grafted silica spheres. J. Hazard. Mater. 2015, 287, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Deng, J.; Wang, H.; Liu, J.; Zhang, Y. Improved salts transportation of a positively charged loose nanofiltration membrane by introduction of poly(ionic liquid) functionalized hydrotalcite nanosheets. ACS Sustain. Chem. Eng. 2016, 4, 3292–3304. [Google Scholar] [CrossRef]
- Barroso, T.; Temtem, M.; Hussain, A.; Aguiar-Ricardo, A.; Roque, A.C.A. Preparation and characterization of a cellulose affinity membrane for human immunoglobulin G (IgG) purification. J. Membr. Sci. 2010, 348, 224–230. [Google Scholar] [CrossRef]
- Livazovic, S.; Li, Z.; Behzad, A.R.; Peinemann, K.V.; Nunes, S.P. Cellulose multilayer membranes manufacture with ionic liquid. J. Membr. Sci. 2015, 490, 282–293. [Google Scholar] [CrossRef]
- Nevstrueva, D.; Pihlajamäki, A.; Mänttäri, M. Effect of a TiO2 additive on the morphology and permeability of cellulose ultrafiltration membranes prepared via immersion precipitation with ionic liquid as a solvent. Cellulose 2015, 22, 3865–3876. [Google Scholar] [CrossRef]
- Kim, D.; Livazovic, S.; Falca, G.; Nunes, S.P. Oil–water separation using membranes manufactured from cellulose/Ionic liquid solutions. ACS Sustain. Chem. Eng. 2018, 7, 5649–5659. [Google Scholar] [CrossRef]
- Abdellah, M.H.; Pérez-Manríquez, L.; Puspasari, T.; Scholes, C.A.; Kentish, S.E.; Peinemann, K.-V. A catechin/cellulose composite membrane for organic solvent nanofiltration. J. Membr. Sci. 2018, 567, 139–145. [Google Scholar] [CrossRef]
- Colburn, A.; Vogler, R.J.; Patel, A.; Bezold, M.; Craven, J.; Liu, C.; Bhattacharyya, D. Composite membranes derived from cellulose and lignin sulfonate for selective separations and antifouling aspects. Nanomaterials 2019, 9, 867. [Google Scholar] [CrossRef] [PubMed]
- Falca, G.; Musteata, V.-E.; Behzad, A.R.; Chisca, S.; Nunes, S.P. Cellulose hollow fibers for organic resistant nanofiltration. J. Membr. Sci. 2019, 586, 151–161. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Taylor, A.; Serwinowski, N.; Parkerson, Z.J.; Confer, M.P.; Kammakakam, I.; Bara, J.E.; Esfahani, A.R.; Mahmoodi, S.N.; Koutahzadeh, N.; et al. Sustainable novel bamboo-based membranes for water treatment fabricated by regeneration of bamboo waste fibers. ACS Sustain. Chem. Eng. 2020, 8, 4225–4235. [Google Scholar] [CrossRef]
- Xing, D.Y.; Peng, N.; Chung, T.-S. Formation of cellulose acetate membranes via phase inversion using ionic liquid, [BMIM]SCN, as the solvent. Ind. Eng. Chem. Res. 2010, 49, 8761–8769. [Google Scholar] [CrossRef]
- Kim, D.; Le, N.L.; Nunes, S.P. The effects of a co-solvent on fabrication of cellulose acetate membranes from solutions in 1-ethyl-3-methylimidazolium acetate. J. Membr. Sci. 2016, 520, 540–549. [Google Scholar] [CrossRef]
- Xing, D.Y.; Chan, S.Y.; Chung, T.-S. Fabrication of porous and interconnected PBI/P84 ultrafiltration membranes using [EMIM]OAc as the green solvent. Chem. Eng. Sci. 2013, 87, 194–203. [Google Scholar] [CrossRef]
- Kim, D.; Nunes, S.P. Poly(ether imide sulfone) membranes from solutions in ionic liquids. Ind. Eng. Chem. Res. 2017, 56, 14914–14922. [Google Scholar] [CrossRef]
- Gsaiz, P.; Lopes, A.C.; Barker, S.E.; de Luis, R.F.; Arriortua, M.I. Ionic liquids for the control of the morphology in poly(vinylidene fluoride-co-hexafluoropropylene) membranes. Mater. Des. 2018, 155, 325–333. [Google Scholar] [CrossRef]
- Chisca, S.; Marchesi, T.; Falca, G.; Musteata, V.-E.; Huang, T.; Abou-Hamad, E.; Nunes, S.P. Organic solvent and thermal resistant polytriazole membranes with enhanced mechanical properties cast from solutions in non-toxic solvents. J. Membr. Sci. 2020, 597, 117634. [Google Scholar] [CrossRef]
- Sukma, F.M.; Çulfaz-Emecen, P.Z. Cellulose membranes for organic solvent nanofiltration. J. Membr. Sci. 2018, 545, 329–336. [Google Scholar] [CrossRef]
- Li, C.; Zhu, Y.; Lv, R.; Na, B.; Chen, B. Poly(vinylidene fluoride) membrane with piezoelectric β-form prepared by immersion precipitation from mixed solvents containing an ionic liquid. J. Appl. Polym. Sci. 2014, 131, 40505. [Google Scholar] [CrossRef]
- Kim, D.; Moreno, N.; Nunes, S.P. Fabrication of polyacrylonitrile hollow fiber membranes from ionic liquid solutions. Polym. Chem. 2016, 7, 113–124. [Google Scholar] [CrossRef]
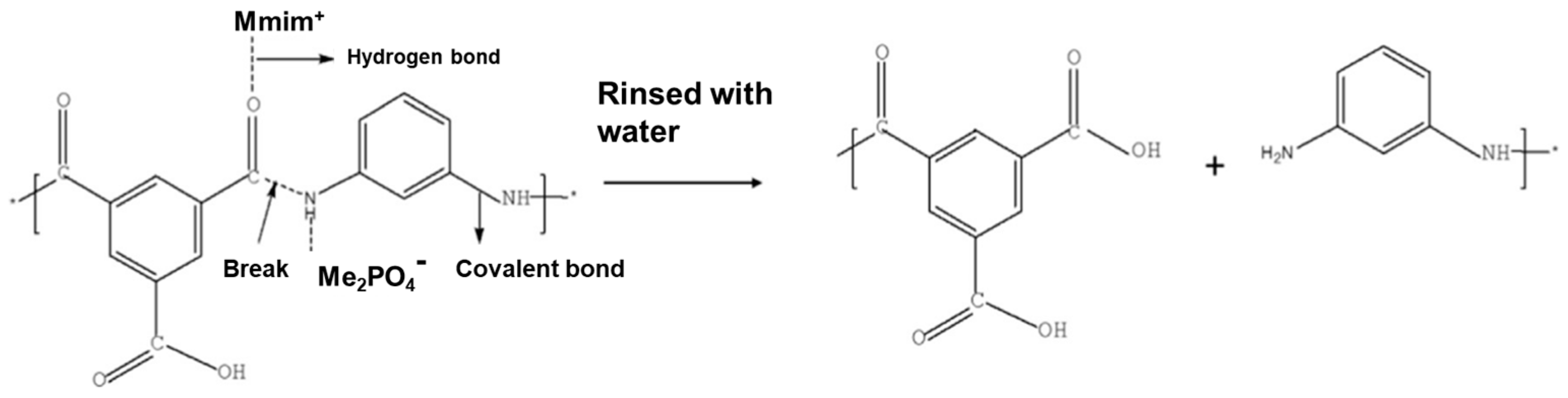
- Mariën, H.; Vankelecom, I.F.J. Optimization of the ionic liquid-based interfacial polymerization system for the preparation of high-performance, low-fouling RO membranes. J. Membr. Sci. 2018, 556, 342–351. [Google Scholar] [CrossRef]
- Hartanto, Y.; Corvilain, M.; Mariën, H.; Janssen, J.; Vankelecom, I.F.J. Interfacial polymerization of thin-film composite forward osmosis membranes using ionic liquids as organic reagent phase. J. Membr. Sci. 2020, 601, 117869. [Google Scholar] [CrossRef]
- Feng, R.; Zhao, D.; Guo, Y. Revisiting characteristics of ionic liquids: A review for further application development. J. Environ. Prot. 2010, 01, 95–104. [Google Scholar] [CrossRef]
- Fernández, J.F.; Neumann, J.; Thöming, J. Regeneration, recovery and removal of ionic Liquids. Curr. Org. Chem. 2011, 15, 1992–2014. [Google Scholar] [CrossRef]
- Kudlak, B.; Owczarek, K.; Namiesnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents -a review. Environ. Sci. Pollut. Res. 2015, 22, 11975–11992. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W. The application of nanofiltration technology in recovery of ionic liquids from spinning wastewater. Appl. Mech. Mater. 2012, 178–181, 499–502. [Google Scholar] [CrossRef]
- Mai, N.L.; Ahn, K.; Koo, Y.-M. Methods for recovery of ionic liquids-a review. Process. Biochem. 2014, 49, 872–881. [Google Scholar] [CrossRef]
- Avram, A.M.; Ahmadiannamini, P.; Qian, X.; Wickramasinghe, S.R. Nanofiltration membranes for ionic liquid recovery. Sep. Sci. Technol. 2017, 52, 2098–2107. [Google Scholar] [CrossRef]






| Polymer | IL | Applications | Year | Reference |
|---|---|---|---|---|
| Cellulose (surface modified) | [BMIM][Cl] | Human immunoglobulin G (IgG) purification by absorption | 2010 | [88] |
| Cellulose | [AMIM][Cl] | NF: dye rejection (<700 Da) | 2011 | [35] |
| Cellulose (TFC) | [EMIM][OAc] | UF: PEO rejection (3000 Da) NF: PEG rejection (<200 Da) | 2015 | [89] |
| Cellulose -TiO2 | [EMIM][OAc] | UF: humic acid (100 kDa) rejection | 2015 | [90] |
| Cellulose | [EMIM][OAc] | UF: oil/water separation | 2018 | [91] |
| Cellulose TFC | [EMIM][OAc] | OSN: dye rejection (500 Da) | 2018 | [92] |
| Cellulose-graphene quantum dot | [EMIM][OAc] | NF: dyes (300 < MWCO < 5000 Da) rejection | 2018 | [34] |
| Cellulose-iron/polyacrylic acid/lignin sulfonate | [EMIM][OAc] | NF: dye rejection (<300 Da) | 2019 | [93] |
| Cellulose HF | [EMIM][OAc] [EMIM][DEP] [DMIM][DMP] | UF: PEG rejection (~18 kDa) PS rejection (25 kDa) NF/OSN: Dye rejection (700–1500 Da) | 2019 | [94] |
| Cellulose-GO | [EMIM][OAc] | NF: heavy metal removal | 2019 | [36] |
| Cellulose from bamboo | [BMIM][Cl] | NF: dye rejections | 2020 | [95] |
| Cellulose acetate | [BMIM][SCN] | UF: PEG/PEO rejection | 2010 | [96] |
| Cellulose acetate HF | [BMIM][SCN] | UF: PEG/PEO rejection | 2011 | [24] |
| Cellulose acetate | [EMIM][OAc] | UF: BSA (66 kDa), γ-globulin (~140 kDa) rejection | 2016 | [97] |
| PBI | [EMIM][OAc] | OSN: dye rejection (600 Da) | 2014 | [26] |
| PBI/P84 | [EMIM][OAc] | UF: PEG/PEO rejection (~100 kDa) | 2013 | [98] |
| Extem | [EMIM][SCN] | UF: BSA (66 kDa), γ-globulin (~140 kDa) rejection DNA (6.4 kDa) | 2017 | [99] |
| PVDF-HFP | [dema][TfO] [MIM] [Tf2N] [MIM][Cl] | MF | 2018 | [100] |
| PMIA-TFC | [EMIM][OAc] | OSN: Dye rejection (470–730 Da) | 2018 | [27] |
| Polytriazole | [EMIM][DEP] | OSN: PEG rejection rom DMF (1~3 kDa) | 2020 | [101] |
| Chemical Name | Abbreviation | Formula | CAS registry Number | Molecular Weight | Density a (kg/m3) | Viscosity a (Pa×s) | Price (RMB, 100 g Weight Basis) b |
|---|---|---|---|---|---|---|---|
| 1-butyl-3-methylimidazolium tetrafluoroborate | [BMIM][BF4] | C8H15BF4N2 | 174501-65-6 | 226.03 | 1201 | 0.108 | 400 |
| 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | [BMIM][Tf2N] | C10H15F6N3O4S2 | 174899-83-3 | 419.36 | 1436 | 0.0508 | 1500 |
| 1-octyl-3-methylimidazolium chloride | [OMIM][Cl] | C12H23ClN2 | 64697-40-1 | 230.78 | 1013 | 13.3 | 300 |
| 1-butyl-3-methylimidazolium hexafluorophosphate | [BMIM][PF6] | C8H15F6N2P | 174501-64-5 | 284.19 | 1367 | 0.274 | 400 |
| 1-octyl-3-methylimidazolium hexafluorophosphate | [OMIM][PF6] | C12H23F6N2P | 304680-36-2 | 340.29 | 1236 | 0.691 | 500 |
| 1-octyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | [OMIM][Tf2N] | C14H23F6N3O4S2 | 862731-66-6 | 475.47 | 1320 | 0.0931 | 1500 |
| N-butylpyridinium tetrafluoroborate | [BPy][BF4] | C9H14BF4N | 203389-28-0 | 223.02 | 1214 | 0.1603 | 500 |
| 1-butyl-3-methylimidazolium chloride | [BMIM][Cl] | C8H15ClN2 | 79917-90-1 | 174.67 | 1082 | 0.00604 | 200 |
| N-octylpyridiniunm bis (trifluoromethyl) sulfonyl imide | [OPY][Tf2N] | C15H22F6N2O4S2 | 384347-06-2 | 472.47 | 1327 | 0.1143 | 1600 |
| 1-allyl-3-methylimidazolium chloride | [AMIM][Cl] | C7H11ClN2 | 65039-10-3 | 158.63 | 1166 | 0.82 | 300 |
| 1-ethyl-3-methylimidazolium acetate | [EMIM][OAc] | C8H14N2O2 | 143314-17-4 | 170.21 | 1100 | 0.1436 | 900 |
| 1-ethyl-3-methyimidazolium diethyl phosphate | [EMIM][DEP] | C10H21N2O4P | 848641-69-0 | 264.26 | 1144 | 0.41 | 400 |
| 1-butyl-3-methylimidazolium thiocyanate | [BMIM][SCN] | C9H15N3S | 344790-87-0 | 197.30 | 1070 | 0.05652 | 1600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, D.; Hua, D.; Hong, Y.; Ibrahim, A.-R.; Yao, A.; Pan, J.; Zhan, G. Functions of Ionic Liquids in Preparing Membranes for Liquid Separations: A Review. Membranes 2020, 10, 395. https://doi.org/10.3390/membranes10120395
Zheng D, Hua D, Hong Y, Ibrahim A-R, Yao A, Pan J, Zhan G. Functions of Ionic Liquids in Preparing Membranes for Liquid Separations: A Review. Membranes. 2020; 10(12):395. https://doi.org/10.3390/membranes10120395
Chicago/Turabian StyleZheng, Dayuan, Dan Hua, Yiping Hong, Abdul-Rauf Ibrahim, Ayan Yao, Junyang Pan, and Guowu Zhan. 2020. "Functions of Ionic Liquids in Preparing Membranes for Liquid Separations: A Review" Membranes 10, no. 12: 395. https://doi.org/10.3390/membranes10120395
APA StyleZheng, D., Hua, D., Hong, Y., Ibrahim, A.-R., Yao, A., Pan, J., & Zhan, G. (2020). Functions of Ionic Liquids in Preparing Membranes for Liquid Separations: A Review. Membranes, 10(12), 395. https://doi.org/10.3390/membranes10120395