Removal of Enantiomeric Ibuprofen in a Nanofiltration Membrane Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Analytical Methods
3. Results and Discussion
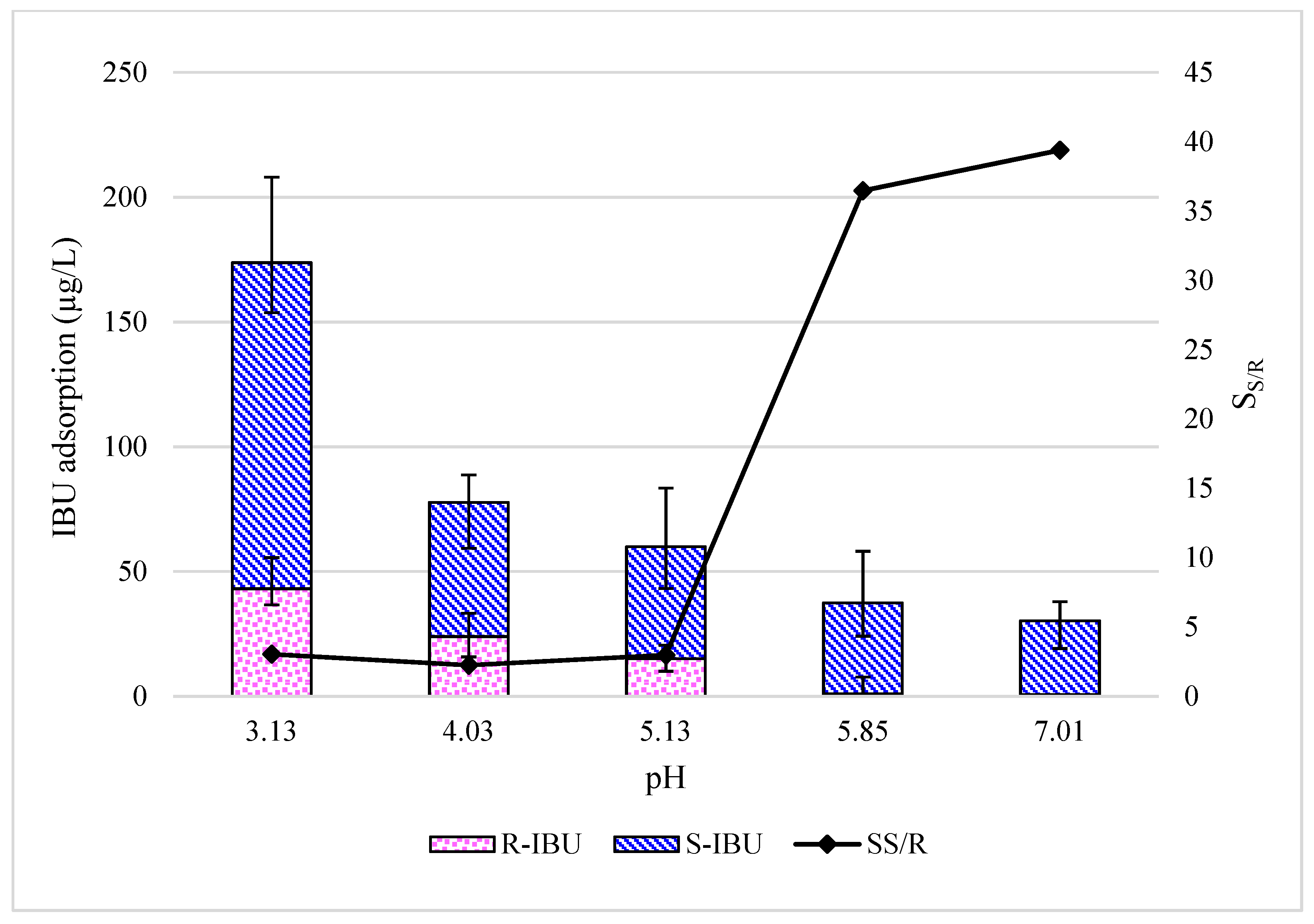
3.1. Effect of Feed pH on Adsorption
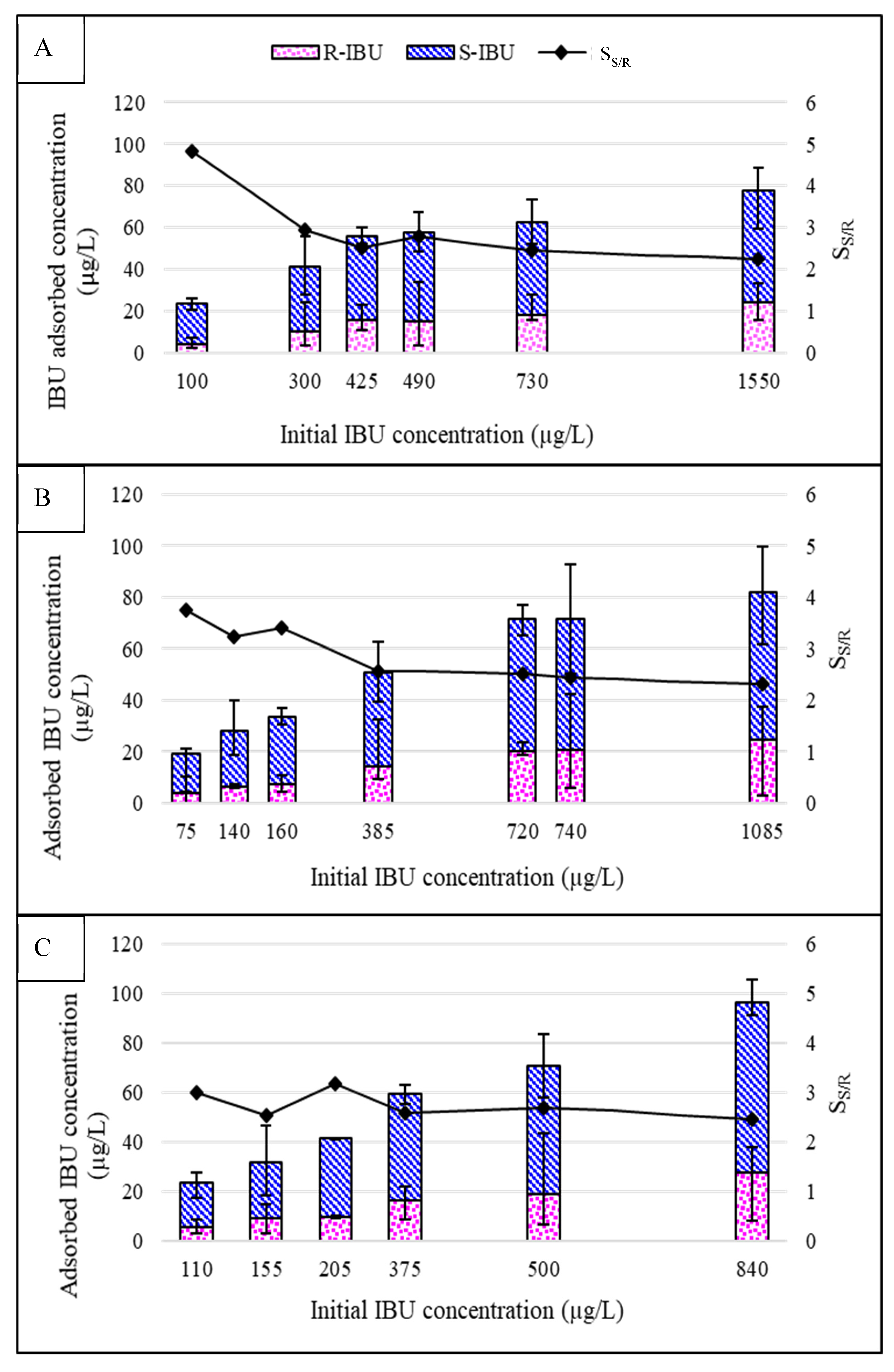
3.2. Effect of Feed Concentration on Adsorption
3.3. Adsorption Isotherm Modeling
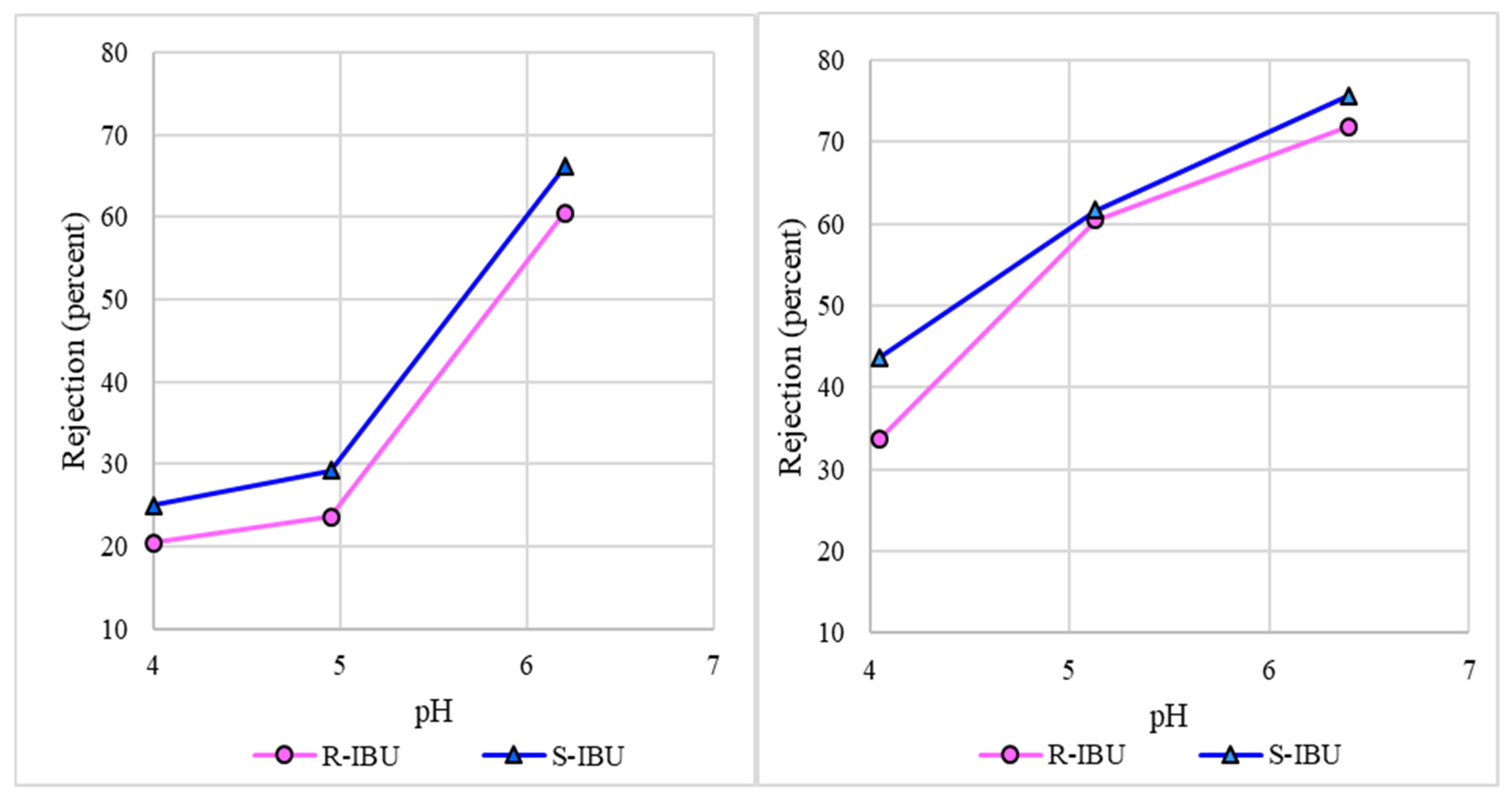
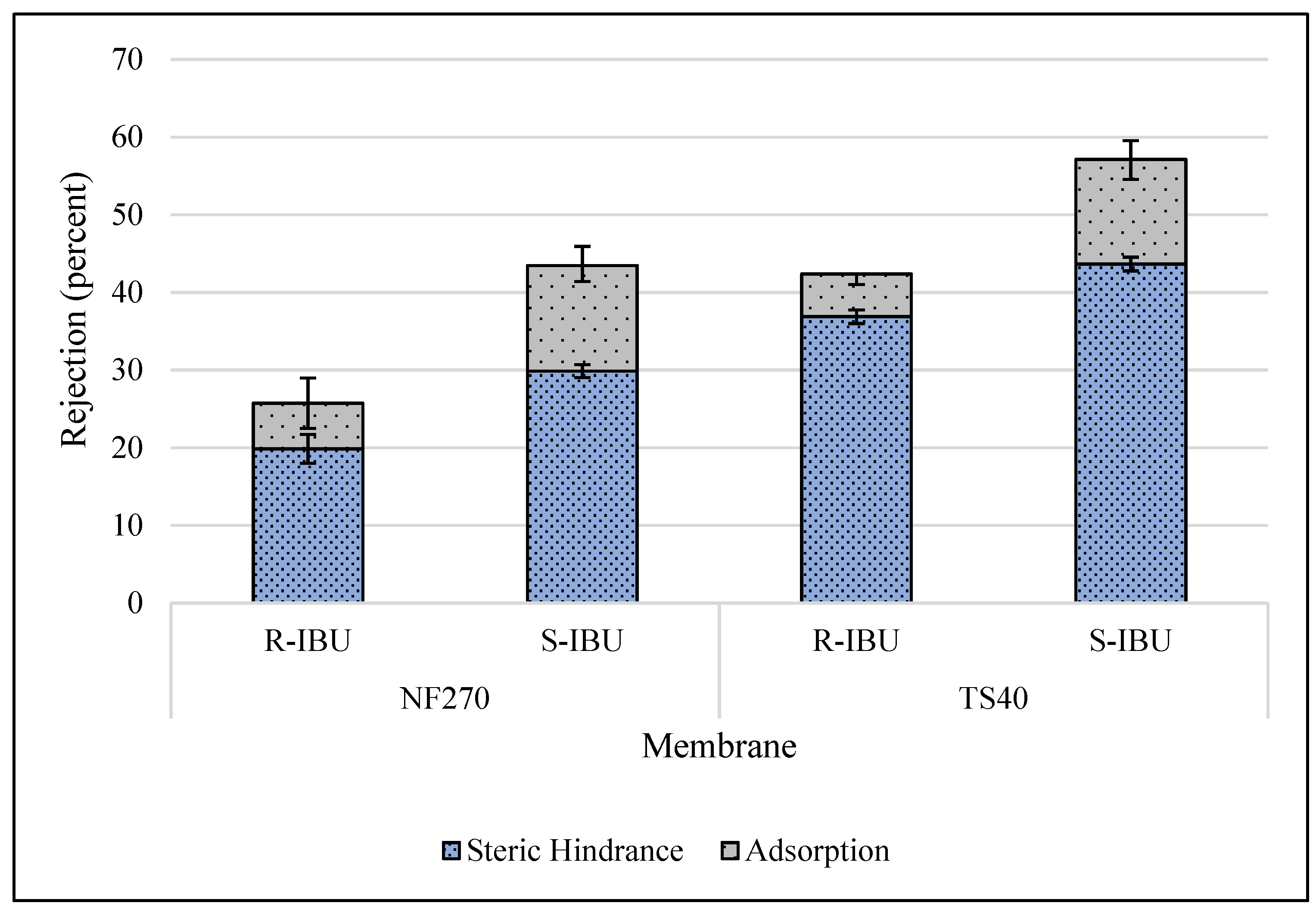
3.4. Rejection of Ibuprofen Enantiomers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bellona, C.; Drewes, J.E.; Xu, P.; Amy, G. Factors affecting the rejection of organic solutes during NF/RO treatment—A literature review. Water Res. 2004, 38, 2795–2809. [Google Scholar] [CrossRef]
- Yangali-Quintanilla, V.; Maeng, S.K.; Fujioka, T.; Kennedy, M.; Amy, G. Proposing nanofiltration as acceptable barrier for organic contaminants in water reuse. J. Membr. Sci. 2010, 362, 334–345. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C. Nanofiltration and ultrafiltration of endocrine disrupting compounds, pharmaceuticals and personal care products. J. Membr. Sci. 2006, 270, 88–100. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Ventura, F.; Barceló, D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008, 42, 3601–3610. [Google Scholar] [CrossRef]
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Jang, M.; Kim, D.; Yu, M.; Heo, J.; Yoon, Y. Removal of contaminants of emerging concern by membranes in water and wastewater: A review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Yangali-Quintanilla, V.; Sadmani, A.; McConville, M.; Kennedy, M.; Amy, G. A QSAR model for predicting rejection of emerging contaminants (pharmaceuticals, endocrine disruptors) by nanofiltration membranes. Water Res. 2010, 44, 373–384. [Google Scholar] [CrossRef]
- Yangali-Quintanilla, V.; Sadmani, A.; Kennedy, M.; Amy, G. A QSAR (quantitative structure-activity relationship) approach for modelling and prediction of rejection of emerging contaminants by NF membranes. Des. Water Treat. 2010, 13, 149–155. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Verliefde, A.; Braeken, L.; Cornelissen, E.R.; Moons, K.; Verberk, J.Q.; van Dijk, H.J.; Amy, G. Assessment of a semi-quantitative method for estimation of the rejection of organic compounds in aqueous solution in nanofiltration. J. Chem. Technol. Biotechnol. 2006, 81, 1166–1176. [Google Scholar] [CrossRef]
- Ozaki, H.; Li, H. Rejection of organic compounds by ultra-low pressure reverse osmosis membrane. Water Res. 2002, 36, 123–130. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in the drinking water industry. Environ. Pollut. 2003, 122, 435–445. [Google Scholar] [CrossRef]
- Schutte, C.F. The rejection of specific organic compounds by reverse osmosis membranes. Desalination 2003, 158, 285–294. [Google Scholar] [CrossRef]
- Comerton, A.M.; Andrews, R.C.; Bagley, D.M.; Hao, C. The rejection of endocrine disrupting and pharmaceutically active compounds by NF and RO membranes as a function of compound and water matrix properties. J. Membr. Sci. 2008, 313, 323–335. [Google Scholar] [CrossRef]
- Comerton, A.M.; Andrews, R.C.; Bagley, D.M.; Yang, P. Membrane adsorption of endocrine disrupting compounds and pharmaceutically active compounds. J. Membr. Sci. 2007, 303, 267–277. [Google Scholar] [CrossRef]
- Jeffery-Black, S.; Duranceau, S.J. The influence of solute polarizability and molecular volume on the rejection of trace organics in loose nanofiltration membrane processes. Des. Water Treat. 2016, 57, 29059–29069. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Schaep, J.; Wilms, D.; Vandecasteele, C. Influence of molecular size, polarity and charge on the retention of organic molecules by nanofiltration. J. Membr. Sci. 1999, 156, 29–41. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Vanneste, J.; Tocci, E.; Jansen, J.C.; Tasselli, F.; Degrevè, J.; Drioli, E.; Van der Bruggen, B. Physicochemical Characterization of Solute Retention in Solvent Resistant Nanofiltration: The Effect of Solute Size, Polarity, Dipole Moment, and Solubility Parameter. J. Phys. Chem. B. 2011, 115, 14507–14517. [Google Scholar] [CrossRef]
- Kiso, Y.; Mizuno, A.; Jung, Y.; Kumano, A.; Ariji, A. Rejection properties of pesticides with a hollow fiber NF membrane (HNF-1). Desalination 2002, 143, 147–157. [Google Scholar] [CrossRef]
- Sadmani, A.H.M.; Andrews, R.C.; Bagley, D.M. Nanofiltration of pharmaceutically active and endocrine disrupting compounds as a function of compound interactions with DOM fractions and cations in natural water. Sep. Purif. Technol. 2014, 122, 462–471. [Google Scholar] [CrossRef]
- Breitner, L.N.; Howe, K.J.; Minakata, D. Effect of Functional Chemistry on the Rejection of Low-Molecular Weight Neutral Organics through Reverse Osmosis Membranes for Potable Reuse. Environ. Sci. Technol. 2019, 53, 11401–11409. [Google Scholar] [CrossRef]
- Davies, N.M. Clinical Pharmacokinetics of Ibuprofen: The First 30 Years. Clin. Pharm. 1998, 34, 101–154. [Google Scholar] [CrossRef]
- Evans, A.M. Comparative Pharmacology of S(+)-Ibuprofen and (RS)-Ibuprofen. Clin. Rheumatol. 2001, 20, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bonato, P.S.; Maria Perpetua, F.M.; de Carvalho, R. Enantioselective determination of ibuprofen in plasma by high-performance liquid chromatography–electrospray mass spectrometry. J. Chromatogr. B. 2003, 796, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Huang, H.; Schwab, K. Effects of Solution Chemistry on the Adsorption of Ibuprofen and Triclosan onto Carbon Nanotubes. Langmuir 2011, 27, 12960–12967. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef]
- McEachran, A.D.; Shea, D.; Bodnar, W.; Nichols, E.G. Pharmaceutical occurrence in groundwater and surface waters in forests land-applied with municipal wastewater. Environ. Toxicol. Chem. 2016, 35, 898–905. [Google Scholar] [CrossRef]
- Buser, H.; Poiger, T.; Müller, M.D. Occurrence and Environmental Behavior of the Chiral Pharmaceutical Drug Ibuprofen in Surface Waters and in Wastewater. Environ. Sci. Technol. 1999, 33, 2529–2535. [Google Scholar] [CrossRef]
- Hashim, N.H.; Khan, S.J. Enantioselective analysis of ibuprofen, ketoprofen, and naproxen in wastewater and environmental water samples. J. Chromatogr. A. 2011, 1218, 4746–4754. [Google Scholar] [CrossRef]
- Fajobi, M.; Fayomi, O.S.I.; Akande, G.; Odunlami, O. Inhibitive Performance of Ibuprofen Drug on Mild Steel in 0.5 M of H2SO4 Acid. J. Bio. Tribo. Corros. 2019, 5, 5. [Google Scholar] [CrossRef]
- Tasić, Z.Z.; Mihajlović, M.B.P.; Simonović, A.T.; Radovanović, M.B.; Antonijević, M.M. Ibuprofen as a corrosion inhibitor for copper in synthetic acid rain solution. Sci. Rep. 2019, 9, 14710. [Google Scholar] [CrossRef]
- Bueno-Perez, R.; Martin-Calvo, A.; Gómez-Álvarez, P.; Gutiérrez-Sevillano, J.J.; Merkling, P.J.; Vlugt, T.J.H.; van Erp, T.S.; Dubbeldam, D.; Calero, S. Enantioselective adsorption of ibuprofen and lysine in metal-organic frameworks. Chem. Comm. 2014, 50, 10849–10852. [Google Scholar] [CrossRef]
- Davalos Monteiro, R.; van de Wetering, J.; Krawczyk, B.; Engelberg, D.L. Corrosion Behaviour of Type 316L Stainless Steel in Hot Caustic Aqueous Environments. Met. Mater. Int. 2019, 26, 630–640. [Google Scholar] [CrossRef]
- Kimura, K.; Amy, G.; Drewes, J.; Watanabe, Y. Adsorption of hydrophobic compounds onto NF/RO membranes: An artifact leading to overestimation of rejection. J. Membrane. Sci. 2003, 221, 89–101. [Google Scholar] [CrossRef]
- Tanaka, Y.; Saito, H.; Tsutsumi, Y.; Doi, H.; Imai, H.; Hanawa, T. Active Hydroxyl Groups on Surface Oxide Film of Titanium, 316L Stainless Steel, and Cobalt-Chromium-Molybdenum Alloy and Its Effect on the Immobilization of Poly(Ethylene Glycol). Mater. Trans. 2008, 49, 805–811. [Google Scholar] [CrossRef]
- Seo, P.W.; Bhadra, B.N.; Ahmed, I.; Khan, N.A.; Jhung, S.H. Adsorptive Removal of Pharmaceuticals and Personal Care Products from Water with Functionalized Metal-organic Frameworks: Remarkable Adsorbents with Hydrogen-bonding Abilities. Sci. Rep. 2016, 6, 34462. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lee, C. Elucidating the Rejection Mechanisms of PPCPs by Nanofiltration and Reverse Osmosis Membranes. Ind. Eng. Chem. Res. 2014, 53, 6798–6806. [Google Scholar] [CrossRef]
- Zhao, Y.; Kong, F.; Wang, Z.; Yang, H.; Wang, X.; Xie, Y.F.; Waite, T.D. Role of membrane and compound properties in affecting the rejection of pharmaceuticals by different RO/NF membranes. Front. Environ. Sci. Eng. 2017, 11, 20. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I.; Elimelech, M. Pharmaceutical Retention Mechanisms by Nanofiltration Membranes. Environ. Sci. Technol. 2005, 39, 7698–7705. [Google Scholar] [CrossRef]
- Nghiem, L.; Hawkes, S. Effects of membrane fouling on the nanofiltration of pharmaceutically active compounds (PhACs): Mechanisms and role of membrane pore size. Sep. Purif. Technol. 2007, 57, 176–184. [Google Scholar] [CrossRef]
- Simon, A.; Nghiem, L.D.; Le-Clech, P.; Khan, S.J.; Drewes, J.E. Effects of membrane degradation on the removal of pharmaceutically active compounds (PhACs) by NF/RO filtration processes. J. Membr. Sci. 2009, 340, 16–25. [Google Scholar] [CrossRef]
- Ge, S.; Feng, L.; Zhang, L.; Xu, Q.; Yang, Y.; Wang, Z.; Kim, K. Rejection rate and mechanisms of drugs in drinking water by nanofiltration technology. Environ. Eng. Res. 2017, 22, 329–338. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kishi, Y.; Ishigami, T.; Suga, K.; Umakoshi, H. Chiral Selective Adsorption of Ibuprofen on a Liposome Membrane. J. Phys. Chem. B. 2016, 120, 2790–2795. [Google Scholar] [CrossRef]
- Wu, X.; Kang, F.; Duan, W.; Li, L. Density functional theory calculations: A powerful tool to simulate and design high-performance energy storage and conversion materials. Prog. Nat. Sci. Mater. 2019, 29, 247–255. [Google Scholar] [CrossRef]
- Tsuneda, T. Density Functional Theory in Quantum Chemistry; Springer: Tokyo, Japan, 2014. [Google Scholar]
- Seshadri, H.; Venkatachalam, S.; Sangaranarayanan, M.; Malar, E.J.P. Adsorption of Enantiomers on Metal Surfaces: Application to D- and L-Alanine on Cu, Ni and Zn Electrodes. J. Electrochem. Soc. 2013, 160, G102–G110. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Perri, M.J.; Weber, S.H. Web-Based Job Submission Interface for the GAMESS Computational Chemistry Program. J. Chem. Educ. 2014, 91, 2206–2208. [Google Scholar] [CrossRef]
- Raschi, A.; Romano, E.; Castillo, M.; Leyton, P.; Brandán, S. Structural and Vibrational Properties of the Two Enantiomers of Etodolac. A Complete Assignment of the Vibrational Spectra. In Descriptors, Structural and Spectroscopic Properties of Heterocyclic Derivatives of Importance for Health and the Environment; Brandán, S.A., Ed.; Nova Science Publishing, Inc.: Hauppauge, NY, USA, 2015; pp. 108–131. [Google Scholar]
- McConalthy, J.; Owens, M.J. Sterochemistry in drug action. J. Clin. Psychiatry Prim. Care Companion 2003, 5, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar] [PubMed]
- Lee, S.T.; Molyneux, R.J.; Panter, K.E.; Chang, C.T.; Gardner, D.R.; Pfister, J.A.; Garrossian, M.J. Ammodendrine and N-Methylammodendrine Enantiomers: Isolation, Optical Rotation, and Toxicity. Nat. Prod. 2005, 68, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.J.; Hand, D.W.; Crittenden, J.C.; Trussell, R.R.; Tchobanoglous, G. Principles of Water Treatment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Temkin, M.J.; Pyzhev, V. Kinetics of the synthesis of ammonia on promoted iron catalysts. Acta Physicochim. 1940, 12, 217–222. [Google Scholar]
- Alsehli, B.R.M. A simple approach for determining the maximum sorption capacity of chlorpropham from aqueous solution onto granular activated charcoal. Processes 2020, 8, 398. [Google Scholar] [CrossRef]
- Hadi, M.; Samarghandi, R.; McKay, G. Equilibrium two-parameter isotherms of acid dyes sorption by activated carbons: Study of residual errors. Chem. Eng. J. 2010, 160, 408–416. [Google Scholar] [CrossRef]
- Duduna, W.; Akeme, O.N.; Zinipere, T.M. Comparison of Various Adsorption Isotherm Models for Allium Cepa as Corrosion Inhibitor on Ausenitic Stainless Steel in Sea Water. Int. J. Sci. Res. 2019, 8, 961–964. [Google Scholar]
- Omanovic, S.; Roscoe, S.G. Electrochemical Studies of the Adsorption Behavior of Bovine Serum Albumin on Stainless Steel. Langmuir 1999, 15, 8315–8321. [Google Scholar] [CrossRef]
- Imamura, K.; Okamoto, T.M.; Sakiyama, T.; Nakanishi, K. Adsorption Behavior of Amino Acids on a Stainless Steel Surface. J. Colloid Interf. Sci. 2000, 229, 237–246. [Google Scholar] [CrossRef]
- Liu, T.; Chang, E.; Chiang, P. Adsorption of CECs in the nanofiltration process. Desal. Water Treat. 2013, 54, 2658–2668. [Google Scholar] [CrossRef]
- Shirley, J.; Mandale, S.; Kochkodan, V. Influence of solute concentration and dipole moment on the retention of uncharged molecules with nanofiltration. Desalination 2014, 344, 116–122. [Google Scholar] [CrossRef]
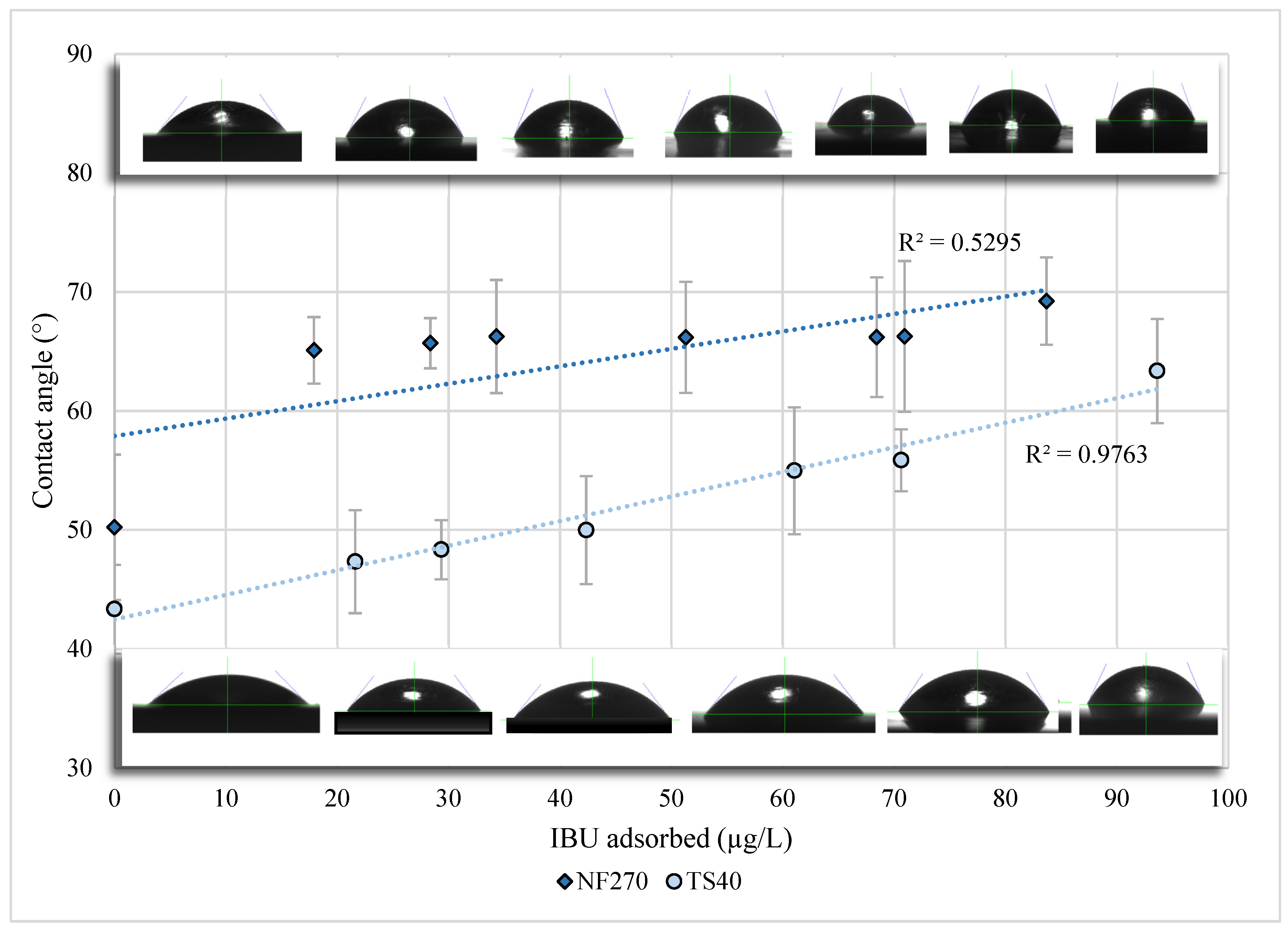
| Membrane | MWCO (Da) | Water Flux Coefficient (Lp) | MgSO4 Rejection (%) | Contact Angle (Virgin, °) | Contact Angle (Compacted, °) |
|---|---|---|---|---|---|
| NF270 | 200–400 | 0.460 | >97 | 30.6 | 50.2 |
| TS40 | 200–300 | 0.231 | >98.5 | 28.7 | 43.3 |
| Compound | Method | Energy (Hartrees) | Surface Area (Å2) | Molecular Volume (Å3) | Dipole Moment (Debeye) |
|---|---|---|---|---|---|
| R-IBU | B3LYP/6-31G* | −656.3 | 179.5 | 199.3 | 2.018 |
| S-IBU | B3LYP/6-31G* | −656.3 | 173.1 | 194.3 | 5.404 |
| Isotherm | |||||||
|---|---|---|---|---|---|---|---|
| Langmuir | |||||||
| KL (L/µg) | qa (µg/cm2) | r2 | RPD | ERRSQ | RMSE | ||
| R-IBU | Equipment | 3.93 × 10−3 | 0.031 | 0.993 | 3.73 | 3.42 × 10−3 | 2.40 × 10−2 |
| NF270 | 4.08 × 10−3 | 0.033 | 0.995 | 4.04 | 4.98 × 10−6 | 8.43 × 10−4 | |
| TS40 | 3.88 × 10−3 | 0.040 | 0.996 | 3.76 | 6.67 × 10−6 | 1.05 × 10−3 | |
| S-IBU | Equipment | 1.74 × 10−2 | 0.052 | 0.979 | 3.57 | 2.46 × 10−5 | 2.02 × 10−3 |
| NF270 | 1.44 × 10−2 | 0.057 | 0.982 | 6.45 | 6.62 × 10−5 | 3.08 × 10−3 | |
| TS40 | 8.95 × 10−2 | 0.076 | 0.974 | 6.00 | 8.71 × 10−5 | 3.81 × 10−3 | |
| Freundlich | |||||||
| KF (L/cm2) | 1/n (-) | r2 | RPD | ERRSQ | RMSE | ||
| R-IBU | Equipment | 6.35 × 10−4 | 0.566 | 0.945 | 9.36 | 3.38 × 10−3 | 2.40 × 10−2 |
| NF270 | 4.05 × 10−4 | 0.665 | 0.995 | 3.87 | 4.53 × 10−6 | 8.05 × 10−4 | |
| TS40 | 4.74 × 10−4 | 0.677 | 0.996 | 2.78 | 7.99 × 10−7 | 3.65 × 10−4 | |
| S-IBU | Equipment | 5.89 × 10−3 | 0.350 | 0.946 | 7.97 | 7.67 × 10−5 | 3.58 × 10−3 |
| NF270 | 4.00 × 10−3 | 0.433 | 0.988 | 3.92 | 1.80 × 10−5 | 1.60 × 10−3 | |
| TS40 | 2.43 × 10−3 | 0.570 | 0.994 | 3.02 | 1.08 × 10−5 | 1.34 × 10−3 | |
| Temkin | |||||||
| KT (L/µg) | b (J/mol) | r2 | RPD | ERRSQ | RMSE | ||
| R-IBU | Equipment | 0.040 | 3.58 × 105 | 0.981 | 5.42 | 3.39 × 10−3 | 2.30 × 10−2 |
| NF270 | 0.041 | 3.25 × 105 | 0.980 | 14.0 | 7.47 × 10−6 | 1.03 × 10−3 | |
| TS40 | 0.038 | 2.65 × 105 | 0.963 | 10.4 | 1.11 × 10−5 | 1.36 × 10−3 | |
| S-IBU | Equipment | 0.185 | 2.23 × 105 | 0.974 | 3.56 | 1.11 × 10−5 | 1.36 × 10−3 |
| NF270 | 0.110 | 1.78 × 105 | 0.988 | 5.19 | 1.86 × 10−5 | 1.63 × 10−3 | |
| TS40 | 0.061 | 1.19 × 105 | 0.975 | 9.11 | 4.23 × 10−6 | 2.66 × 10−3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higgins, C.J.; Duranceau, S.J. Removal of Enantiomeric Ibuprofen in a Nanofiltration Membrane Process. Membranes 2020, 10, 383. https://doi.org/10.3390/membranes10120383
Higgins CJ, Duranceau SJ. Removal of Enantiomeric Ibuprofen in a Nanofiltration Membrane Process. Membranes. 2020; 10(12):383. https://doi.org/10.3390/membranes10120383
Chicago/Turabian StyleHiggins, Carlyn J., and Steven J. Duranceau. 2020. "Removal of Enantiomeric Ibuprofen in a Nanofiltration Membrane Process" Membranes 10, no. 12: 383. https://doi.org/10.3390/membranes10120383
APA StyleHiggins, C. J., & Duranceau, S. J. (2020). Removal of Enantiomeric Ibuprofen in a Nanofiltration Membrane Process. Membranes, 10(12), 383. https://doi.org/10.3390/membranes10120383






