New Polymer Inclusion Membrane in the Separation of Nonferrous Metal Ion from Aqueous Solutions
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Equipment
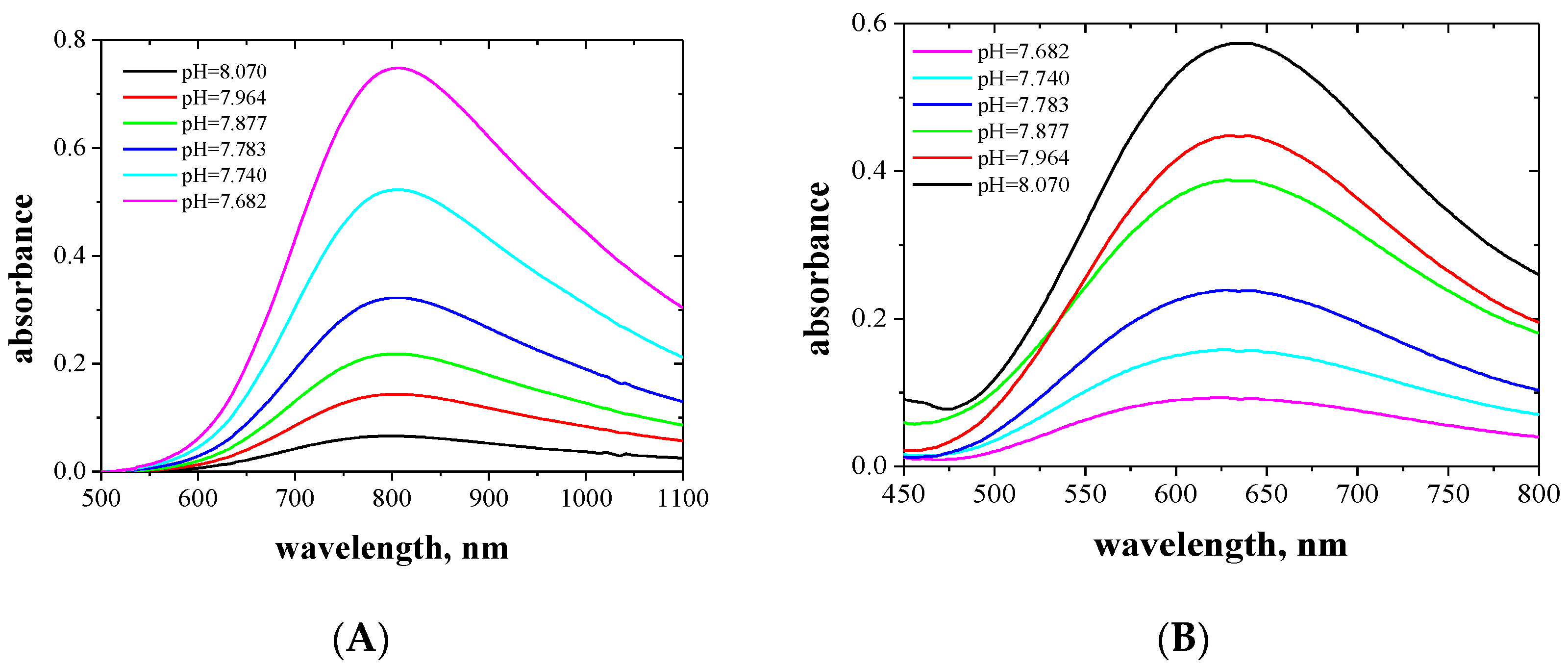
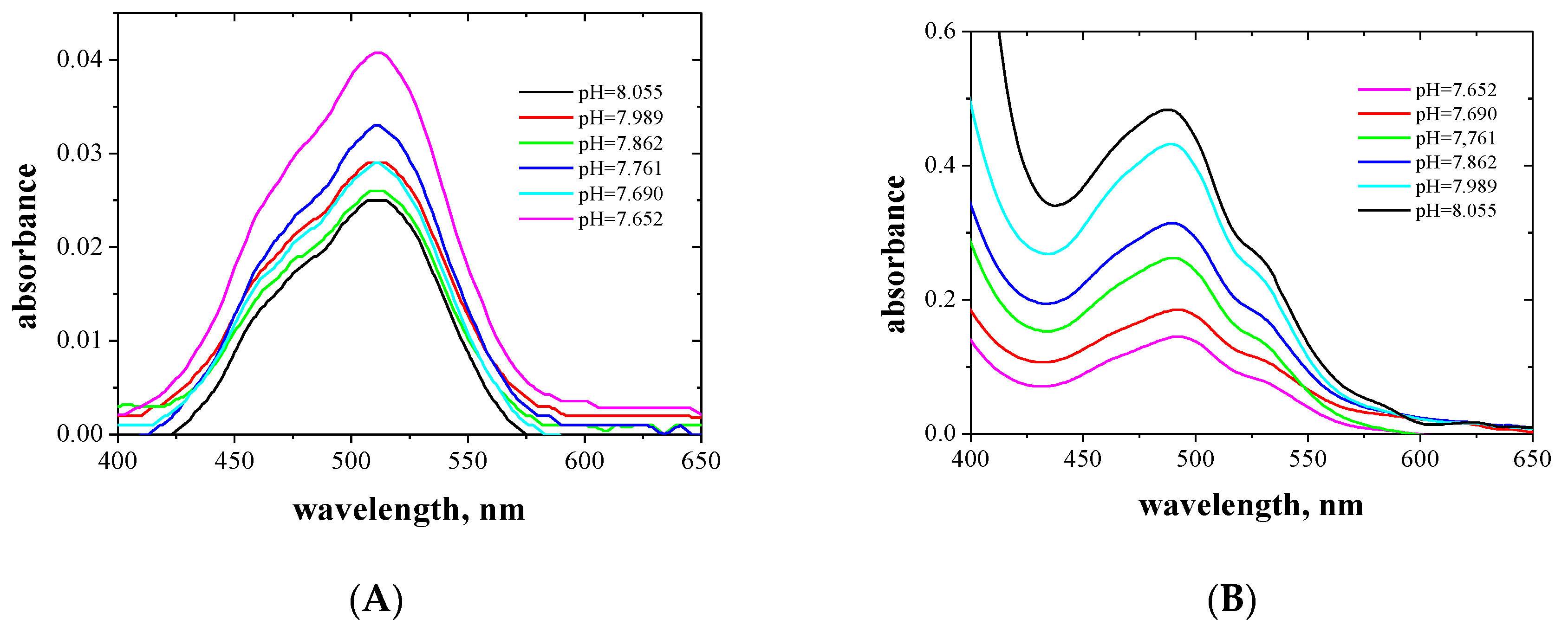
2.2. Procedure for Determination of Dissociation Constants (pKa)
2.3. Liquid-Liquid Extraction Procedure (SX)
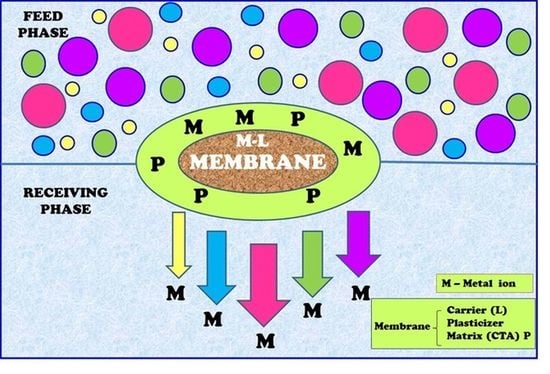
2.4. Polymer Inclusion Membrane
2.5. Transport Studies
3. Results and Discussion
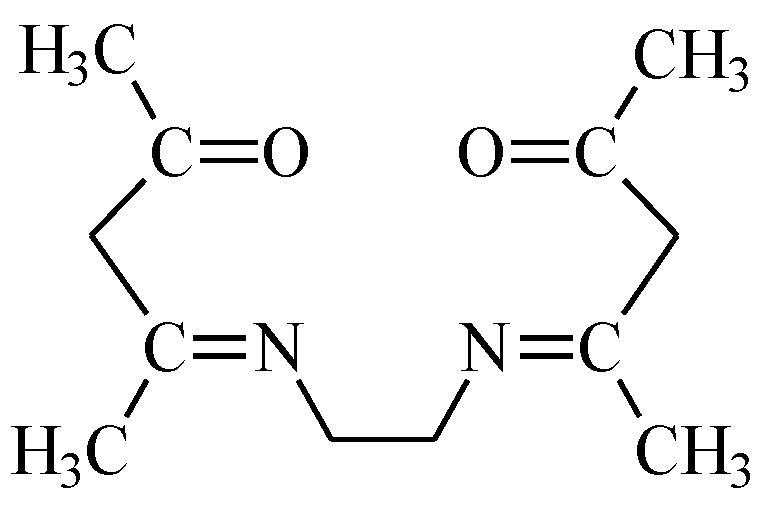
3.1. Determination of Dissociation Constants (pKa)
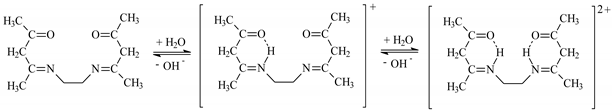
EDAB-acac + H2O ↔ HEDAB-acac+ + OH−
HEDAB-acac+ + H2O ↔ H2EDAB-acac2+ + OH−
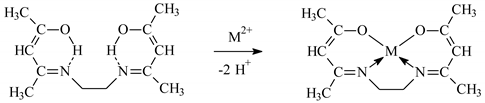
3.2. Solvent Extraction of Metal Ions by EDAB-acac
3.3. Determination of the Stability Constants
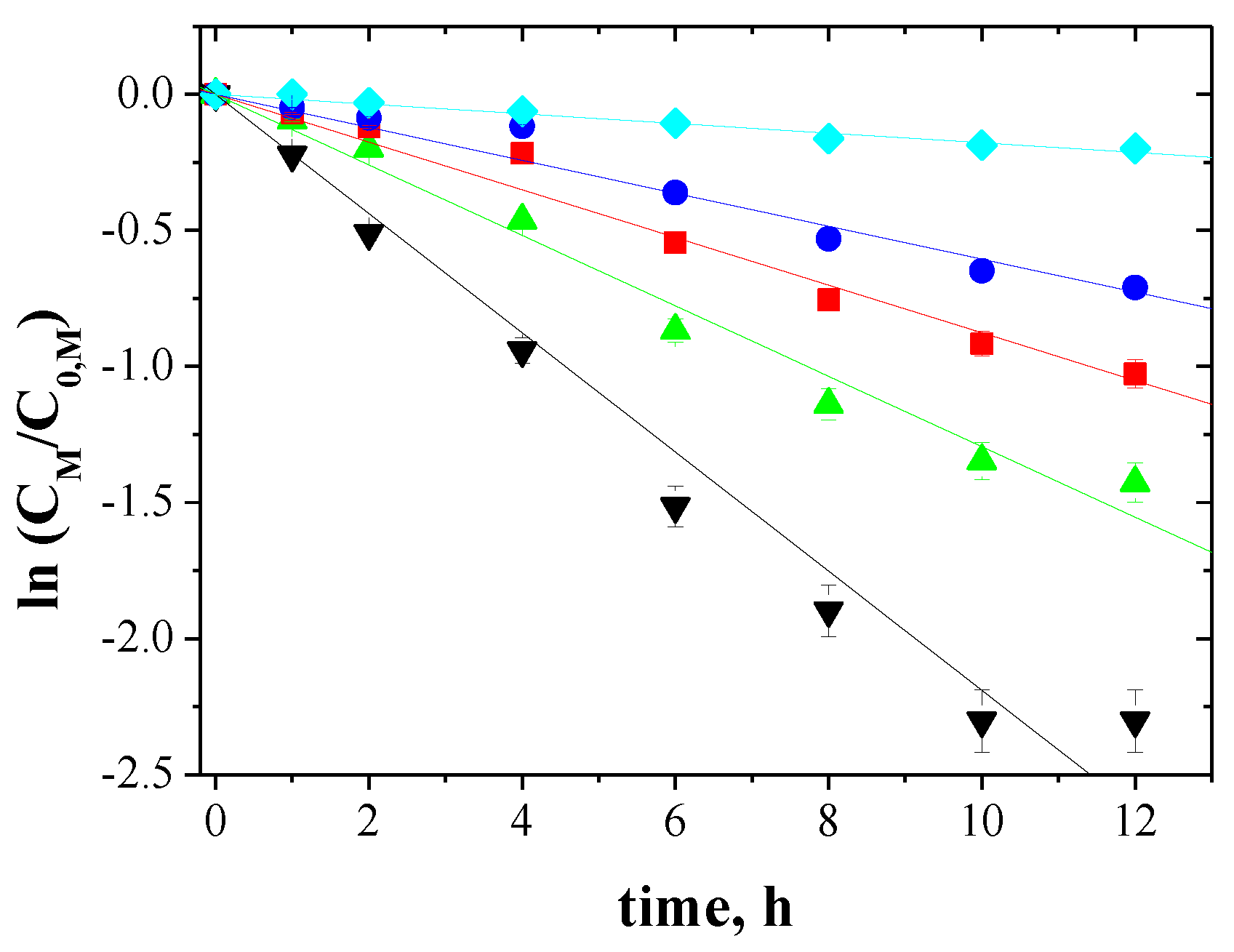
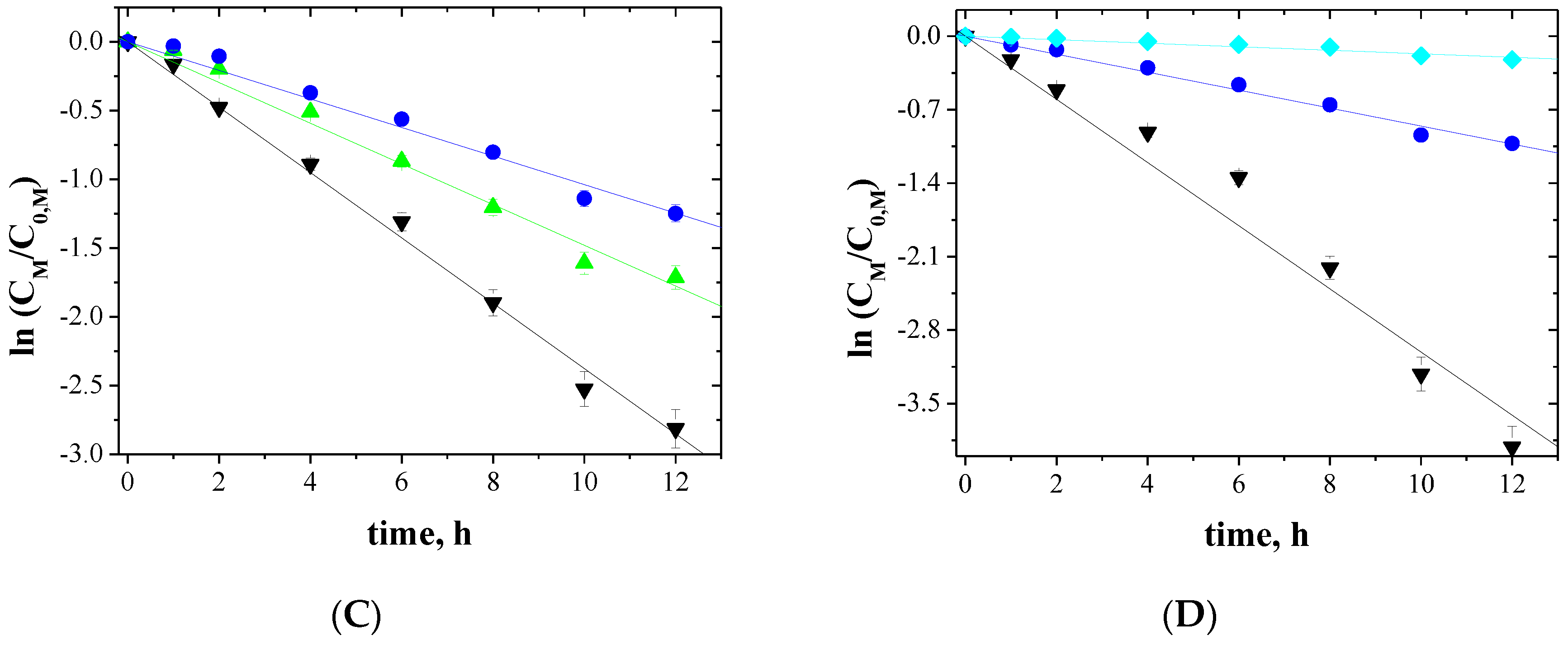
3.4. Transport across PIMs
3.5. Initial Fluxes, Order and Separation Coefficients for Non-Ferrous Metal Transport across PIMs
3.6. Recovery of Metal
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Loon, G.W.; Duffy, S.J. Environmental Chemistry—In a Global Perspective; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Wang, L.K.; Chen, Y.-T.; Hung, N.; Shammas, K. Heavy Metals in the Environment; CRC Press, Taylor & Francis Goup: Boca Raton, FL, USA, 2009. [Google Scholar]
- Sorme, L.; Lagerkvist, R. Sources of heavy metals in urban wastewater in Stockholm. Sci. Total Environ. 2002, 298, 131–145. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Szyczewski, P.; Siepak, J.; Niedzielski, P.; Sobczynski, T. Research on heavy metals in Poland. Pol. J. Environ. Stud. 2009, 18, 755–768. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Netw. 2011, 1–20. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Witt, K. The application of acetylacetone for the separation of heavy metals in roadside soil belts by extraction methods. Desalin. Water Treat. 2020, 186, 191–198. [Google Scholar] [CrossRef]
- Kentish, S.E.; Stevens, G.W. Innovations in separations technology for the recycling and re-use of liquid waste streams. Chem. Eng. J. 2001, 84, 149–159. [Google Scholar] [CrossRef]
- Silva, J.E.; Paiva, A.P.; Soares, D.; Labrincha, A.; Castro, F. Solvent extraction applied to the recovery of heavy metals from galvanic sludge. J. Hazard. Mat. 2005, 120, 113–118. [Google Scholar] [CrossRef]
- Kislik, V.S. Solvent Extraction: Classical and Novel Approaches; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 978-0-444-53778-2. [Google Scholar]
- Zhang, J.; Hu, B. Liquid-Liquid Extraction (LLE). In Separation and Purification Technologies in Biorefineries; Ramaswamy, S., Huang, H.-J., Ramarao, B.V., Eds.; Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Alguacil, F.J. Recent trends in metal extraction. Rev. Metal. 2013, 49, 292–316. [Google Scholar] [CrossRef]
- Witt, K.; Radzyminska-Lenarcik, E. The recovery and the separation of metal ions from galvanic wastewaters. Desalin. Water Treat. 2018, 128, 148–154. [Google Scholar] [CrossRef]
- Radzymińska-Lenarcik, E.; Ulewicz, R.; Ulewicz, M. Zinc recovery from model and waste solutions using polymer inclusion membranes (PIMs) with 1-octyl-4-methylimidazole. Desalin. Water Treat. 2018, 102, 211–219. [Google Scholar] [CrossRef]
- Witt, K.; Radzyminska-Lenarcik, E. Study on effectiveness of PVC/ß-diketone sorbent in removing residue of Zn(II), Cr(III) and Ni(II) from post-galvanic wastewater. Desalin. Water Treat. 2020, 186, 199–205. [Google Scholar] [CrossRef]
- Elhalawany, N.; Baseer, R.A.; Mostafa, A.B.; Rabei, A.G. New efficient chelating polymers based on plastic waste for removal of toxic heavy metal pollutants. J. Elastom. Plast. 2017, 49, 481–497. [Google Scholar] [CrossRef]
- Cote, G. Hydrometallurgy of strategic metals. Solv. Extr. Ion Exch. 2000, 18, 703–727. [Google Scholar] [CrossRef]
- Kołtuniewicz, A.B.; Drioli, E. Membranes in Clean Technologies; Wiley-VchVerlag GmBH: Weinheim, Germany, 2008; ISBN 978-3-527-32007-3. [Google Scholar]
- Kislik, V.S. (Ed.) Liquid Membranes: Principles and Applications in Chemical Separations and Wastewater Treatment; Elsevier: Burlington, VT, USA, 2010; ISBN 978-0-444-53218-3. [Google Scholar]
- Way, J.D.; Noble, R.D. Facilitated Transport in: Membrane Handbook; Springer Science & Business Media: New York, NY, USA, 1992. [Google Scholar]
- Zawierucha, I.; Kozłowski, C.A.; Malina, G. Removal of toxic metal ions from landfill leachate by complementary sorption and transport across polymer inclusion membranes. Waste Manag. 2013, 33, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Costache, L.N.; Szczepanski, P.; Olteanu, C.; Lica, C.G.; Teodorescu, S.; Orbeci, C. Bulk liquid membrane separation of different cations using D2EHPA and Cyanex 302 as carriers. Rev. Chim. 2014, 65, 26–32. [Google Scholar]
- De Gyves, J.; de San Miguel, E.R. Metal ion separations by supported liquid membranes. Ind. Eng. Chem. Res. 1999, 38, 2182–2202. [Google Scholar]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Zulkefeli, N.S.W.; Weng, S.K.; Halim, N.S.A. Removal of Heavy Metals by Polymer Inclusion Membranes. Curr. Pollut. Rep. 2018, 4, 84–92. [Google Scholar] [CrossRef]
- Kolev, S.D.; Almeida, M.I.G.S.; Cattrall, R.W. Polymer Inclusion Membranes, Smart Materials for Sensing and Separation. In Handbook of Smart Materials in Analytical Chemistry; de la Guardia, M., Esteve-Turrillas, F.A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Bringas, E.; Tan, N.R.; Ortiz, I.; Ghahramani, M.; Shahmirzadi, M.A.A. Recent progress in development of high performance polymeric membranes and materials for metal plating wastewater treatment: A review. J. Water Proc. Eng. 2016, 9, 78–110. [Google Scholar] [CrossRef]
- Drioli, E.; Romano, M. Progress and new perspectives on integrated membrane operations for sustainable industrial growth. Ind. Eng. Chem. Res. 2001, 40, 1277–1300. [Google Scholar] [CrossRef]
- Agreda, D.D.; Garcia-Diaz, I.; López, F.A.; Alguacil, F.J. Supported liquid membranes technologies in metals removal from liquid effluents. Rev. Metal. 2011, 47, 146–168. [Google Scholar]
- Rynkowska, E.; Fatyeyeva, K.; Kujawski, W. Application of polymer-based membranes containing ionic liquids in membrane separation processes: A critical review. Rev. Chem. Eng. 2018, 34, 341–363. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Polymer inclusion membranes (PIMs) in chemical analysis—A review. Anal. Chim. Acta 2017, 987, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Staniszewski, B.; Urbaniak, W. A simple and efficient synthesis of 3-substituted derivatives of pentane-2,4-dione. Chem. Pap. 2009, 63, 212–216. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Cobo, A. Solvent extraction with LIX 973N for selective separation of copper and nickel. J. Chem. Technol. Biotechnol. 1999, 74, 467–471. [Google Scholar] [CrossRef]
- Ochromowicz, K.; Jeziorek, M.; Wejman, K. Copper(II) extraction from ammonia leach solution. Physicochem. Probl. Miner. Process. 2014, 50, 327–335. [Google Scholar] [CrossRef]
- De San Miguel, E.R.; Hernández-Andaluz, A.M.; Bañuelos, J.G.; Saniger, J.M.; Aguilar, J.C.; de Gyves, J. LIX®-loaded polymer inclusion membrane for copper(II) transport: 1. Composition–performance relationships through membrane characterization and solubility diagrams. Mater. Sci. Eng. A 2006, 434, 30–38. [Google Scholar] [CrossRef]
- De Gyves, J.; Hernández-Andaluz, A.M.; de San Miguel, E.R. LIX®-loaded polymer inclusion membrane for copper (II) transport: 2. Optimization of the efficiency factors (permeability, selectivity, and stability) for LIX® 84-I. J. Membr. Sci. 2006, 268, 142–149. [Google Scholar] [CrossRef]
- Dziwinski, E.J.; Szymanowski, J. Composition of copper extractant LIX 54-100. Solv. Extr. Ion Exch. 1996, 14, 219–226. [Google Scholar] [CrossRef]
- Mickler, W.; Reich, A.; Uhlemann, E.; Bart, H.J. Liquid membrane permeation of zinc, cadmium and nickel with 4-acyl-5-pyrazolones and β-diketones. J. Membr. Sci. 1996, 119, 91–97. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Witt, K. Solvent extraction of copper ions by 3-substituted derivatives of β-diketones. Sep. Sci. Technol. 2018, 53, 1223–1229. [Google Scholar] [CrossRef]
- Witt, K.; Radzyminska-Lenarcik, E.; Urbaniak, W. Selective transport of zinc ions through a novel polymer inclusion membranes (PIMs) containing β-diketone derivatives as a carrier reagents. Sep. Sci. Technol. 2016, 51, 2620–2627. [Google Scholar] [CrossRef]
- Radzymińska-Lenarcik, E.; Witt, K.; Bożejewiecz, D. Selective transport of copper(II) ions across polymer inclusion membrane with aromatic ß-diketones as carriers. Physicochem. Probl. Miner. Process. 2018, 54, 741–750. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Ulewicz, M. Separation of Zn(II), Cr(III), and Ni(II) ions using the polymer inclusion membranes containing acetylacetone derivative as the carrier. Membranes 2020, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Böttcher, A.; Quezada, C.M.; Meade, T.J.; Gray, H.B. Inhibition of thermolysin and human -thrombin by cobalt(III) Schiff base complexes. Bioorganic Med. Chem. 1999, 7, 815–819. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of Hydrophobic Alkylimidazoles in the Separation of Non-Ferrous Metal Ions across Plasticised Membranes—A Review. Membranes 2020, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Radzyminska-Lenarcik, E.; Ulewicz, M. The Application of Polymer Inclusion Membranes Based on CTA with 1-alkylimidazole for the Separation of Zinc(II) and Manganese(II) Ions from Aqueous Solutions. Polymers 2019, 11, 242. [Google Scholar] [CrossRef]
- Braibanti, A.; Ostacoli, G.; Paoletti, P.; Pettit, L.D.; Sammartano, S. Potentiometric apparatus and technique for the pH-metric measurement of metal-complex equilibrium constants. Pure Appl. Chem. 1987, 59, 1721–1728. [Google Scholar] [CrossRef]
- Stary, J.; Liljenzin, J.O. Critical evaluation of equilibrium constants involving acetylacetone and its metal chelates. Pure Appl. Chem. 1982, 54, 2557–2592. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E. The influence of the alkyl chain length on extraction equilibrium of Cu(II) complexes with 1-alkylimidazole in aqueous solution/organic solvent system. Solv. Extr. Ion Exch. 2006, 25, 53–64. [Google Scholar] [CrossRef]
- Nicholls, D. Complexes and First-Row Transition Elements; The Macmillan Press LTD.: London, UK, 1974. [Google Scholar]
- Rzepka, M.; Kulig, J.; Lenarcik, B. Complexes of some transition cations with 7-methylpyrido [2,3-d]imidazole and 2-(2′-pyridyl)imidazole in aqueous solution. Gazz. Chim. Ital. 1992, 122, 73–77. [Google Scholar]
- Kurzak, B.; Kamecka, A.; Kurzak, K.; Jezierska, J.; Kafarski, P. Potentiometric and spectroscopic studies of the copper(II) complexes with some aminodiphosphonic acids in aqueous solution. Polyhedron 1998, 17, 4403–4413. [Google Scholar] [CrossRef]
- Lenarcik, B.; Ojczenasz, P. Investigation of the Stability Constants of Co(II) Complexes with a Homologous Series of 1-Alkylimidazoles in Aqueous Solution by Using a Partition Method with Several Solvents. Sep. Sci. Technol. 2004, 39, 199–226. [Google Scholar] [CrossRef]
- Anagnostopoulos, A.; Hadjispyrou, S. The influence of solvent polarity on the tetrahedral-octahedral equilibrium of Co(II)complexes with 3,5-dimethylpyrazole. Polyhedron 1984, 3, 251–255. [Google Scholar] [CrossRef]
- Aizawa, S.; Funahashi, S. Octahedral−Tetrahedral Equilibrium and Solvent Exchange of Cobalt(II) Ions in Primary Alkylamines. Inorg. Chem. 2002, 41, 4555–4559. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E. Effect of alkyl chain length on the extraction of Cu(II) complexes with 1-alkyl-2-methylimidazole. Sep. Sci. Technol. 2007, 42, 2661–2675. [Google Scholar] [CrossRef]
- Lenarcik, B.; Ojczenasz, P.; Kopkowski, A. The Influence of the Alkyl Chain Length and Steric Effect on Stability Constants and Extractability of Co(II) Complexes with 1-Alkyl-4(5)-methylimidazoles. Sep. Sci. Technol. 2006, 41, 1697–1724. [Google Scholar] [CrossRef]
- Lenarcik, B.; Rauckyte, T. The Influence of Alkyl Length on Extraction Equilibria of Ni(II) Complexes with 1-Alkylimidazoles in Aqueous Solution/Organic Solvent Systems. Sep. Sci. Technol. 2004, 39, 3353–3372. [Google Scholar] [CrossRef]
- Lenarcik, B.; Kierzkowska, A. The Influence of Alkyl Chain Length and Steric Effect on Extraction of Zinc(II) Complexes with 1-Alkyl-2-methylimidazoles. Solv. Extr. Ion Exch. 2006, 24, 433–445. [Google Scholar] [CrossRef]
- Lenarcik, B.; Kierzkowska, A. The Influence of Alkyl Length on Stability Constants of Zn(II) Complexes with 1-Alkylimidazoles in Aqueous Solutions and Their Partition Between Aqueous Phase and Organic Solvent. Solv. Extr. Ion Exch. 2004, 22, 449–471. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Urbaniak, W. Cadmium(II) and lead(II) extraction and transport through polymer inclusion membranes with 1-alkylimidazole. Desalin. Water Treat. 2020, in press. [Google Scholar]
- Rydberg, J.; Musakis, C.; Choppin, G.R. Principles and Practices of Solvent Extraction; Marcel Dekker, Inc.: New York, NY, USA, 1992; Volume 1. [Google Scholar]
- Rossotti, F.J.C.; Rossotti, H. The Determination of Stability Constants; McGraw-Hill: New York, NY, USA, 1961. [Google Scholar]
- Gâzo, J.; Bersuker, I.B.; Garaj, J.; Kabešová, M.; Kohout, J.; Langfelderowá, H.; Melník, M.; Serator, M.; Valach, F. Plasticity of the coordination sphere of Copper(II) complexes, its manifestation and causes. Coord. Chem. Rev. 1976, 19, 253–297. [Google Scholar] [CrossRef]
- Danesi, P.R. Separation of metal species by supported liquid membranes. Sep. Sci. Technol. 1985, 19, 857–894. [Google Scholar] [CrossRef]



| Ligand | pKa | Ref. |
|---|---|---|
| EDAB-acac | 10.67 | [This work] |
| pentane-2-dione (acac) | 8.79 | [48] |
| 3-butyl-acetylacetone | 9.60 | [40] |
| 3-allyl- acetylacetone | 9.58 | [40] |
| Ligand. | Metal ion | log β1 | log β2 | log P1 | log P2 | Ref. |
|---|---|---|---|---|---|---|
| acetylacetone | Cu(II) | 8.01 | 9.20 | 3.15 | 5.40 | [40] |
| Co(II) | 5.40 | 9.57 | [48] | |||
| Ni(II) | 5.96 | 10.54 | [48] | |||
| Cu(II) | 8.25 | 15.05 | [48] | |||
| Zn(II) | 5.03 | 8.80 | [48] | |||
| Cd(II) | 3.83 | 6.60 | [48] | |||
| 3-butyl-acetylacetonec | Cu(II) | 7.95 | 9.45 | 2.78 | 6.19 | [40] |
| 3-allyl-acetylacetone | Cu(II) | 8.32 | 10.26 | 2.96 | 7.38 | [40] |
| EDAB-acac | Cu(II) | 4.98 | 10.61 | 3.08 | 6.49 | [this work] |
| Co(II) | 5.26 | 12.73 | 4.03 | 6.82 | [this work] | |
| Ni(II) | 2.15 | 8.85 | 1.44 | 4.71 | [this work] | |
| Zn(II) | 8.29 | 16.84 | 5.76 | 8.67 | [this work] | |
| Cd(II) | 5.15 | 14.50 | 4.95 | 7.12 | [this work] |
| Solutions | Metal Ions | Initial Flux, Ji µmol/m2∙s | Selectivity Order and Selectivity Coefficients |
|---|---|---|---|
| I | Zn(II) | 10.98 | - |
| II | Zn(II) Cd(II) Co(II) | 10.53 7.48 5.39 | Zn(II) > Cd(II) > Co(II) 1.4 2.0 |
| III | Zn(II) Cu(II) Ni(II) | 10.42 6.37 0.50 | Zn(II) > Cu(II) >Ni(II) 1.6 21.0 |
| IV | Zn(II) Co(II) Cu(II) | 9.87 5.61 3.90 | Zn(II) > Co(II) > Cu(II) 1.8 2.5 |
| V | Zn(II) Co(II) Ni(II) | 10.17 5.53 0.40 | Zn(II) > Co(II) > Ni(II) 1.8 25.0 |
| VI | Zn(II) Cd(II) Co(II) Ni(II) | 9.85 7.84 5.72 0.38 | Zn(II) > Cd(II) > Co(II) > Ni(II) 1.3 1.7 26.0 |
| VII | Zn(II) Co(II) Cu(II) Ni(II) | 9.94 5.89 3.61 0.40 | Zn(II) > Co(II) > Cu(II) > Ni(II) 1.7 2.8 25.0 |
| VIII | Zn(II) Cd(II) Co(II) Cu(II) Ni(II) | 9.87 7.43 5.26 3.12 0.20 | Zn(II) > Cd(II) > Co(II) > Cu(II) > Ni(II) 1.3 1.8 3.2 49.0 |
| Mixture | RF, % | |||||
|---|---|---|---|---|---|---|
| Zn | Cd | Co | Cu | Ni | ||
| I | Zn | 99 | ||||
| II | Zn-Cd-Co | 97 | 82 | 71 | ||
| III | Zn-Cu-Ni | 98 | 64 | 15 | ||
| IV | Zn-Co-Cu | 97 | 79 | 61 | ||
| V | Zn-Co-Ni | 97 | 76 | 11 | ||
| VI | Zn-Cd-Co-Ni | 93 | 83 | 75 | 10 | |
| VII | Zn-Co-Cu-Ni | 95 | 73 | 66 | 9 | |
| VIII | Zn-Cd-Co-Cu-Ni | 90 | 76 | 64 | 51 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyszka, I.; Radzyminska-Lenarcik, E. New Polymer Inclusion Membrane in the Separation of Nonferrous Metal Ion from Aqueous Solutions. Membranes 2020, 10, 385. https://doi.org/10.3390/membranes10120385
Pyszka I, Radzyminska-Lenarcik E. New Polymer Inclusion Membrane in the Separation of Nonferrous Metal Ion from Aqueous Solutions. Membranes. 2020; 10(12):385. https://doi.org/10.3390/membranes10120385
Chicago/Turabian StylePyszka, Ilona, and Elzbieta Radzyminska-Lenarcik. 2020. "New Polymer Inclusion Membrane in the Separation of Nonferrous Metal Ion from Aqueous Solutions" Membranes 10, no. 12: 385. https://doi.org/10.3390/membranes10120385
APA StylePyszka, I., & Radzyminska-Lenarcik, E. (2020). New Polymer Inclusion Membrane in the Separation of Nonferrous Metal Ion from Aqueous Solutions. Membranes, 10(12), 385. https://doi.org/10.3390/membranes10120385