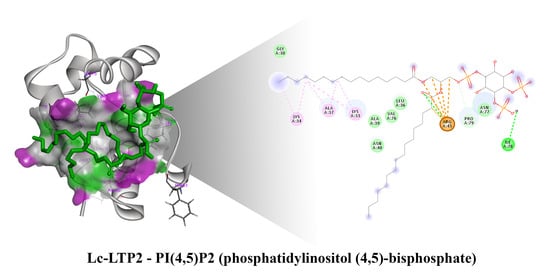
Interaction between the Lentil Lipid Transfer Protein Lc-LTP2 and Its Novel Signal Ligand PI(4,5)P2
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lipid Binding Assays
2.3. Bioinformatic Approaches to Study Interaction of PI(4,5)P2 with Lc-LTP2 Protein Variants
2.4. Calcein Release Assay
3. Results
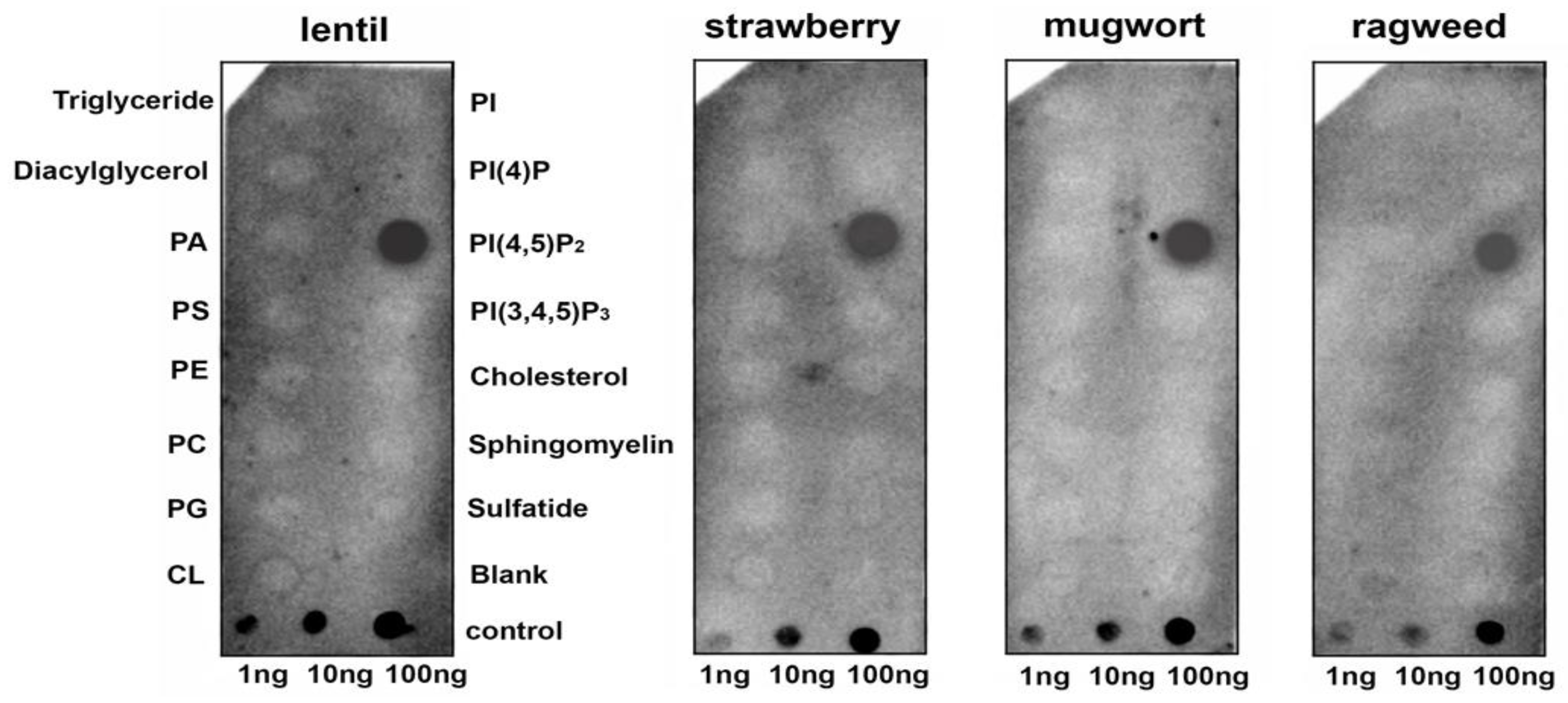
3.1. Binding of Lipids
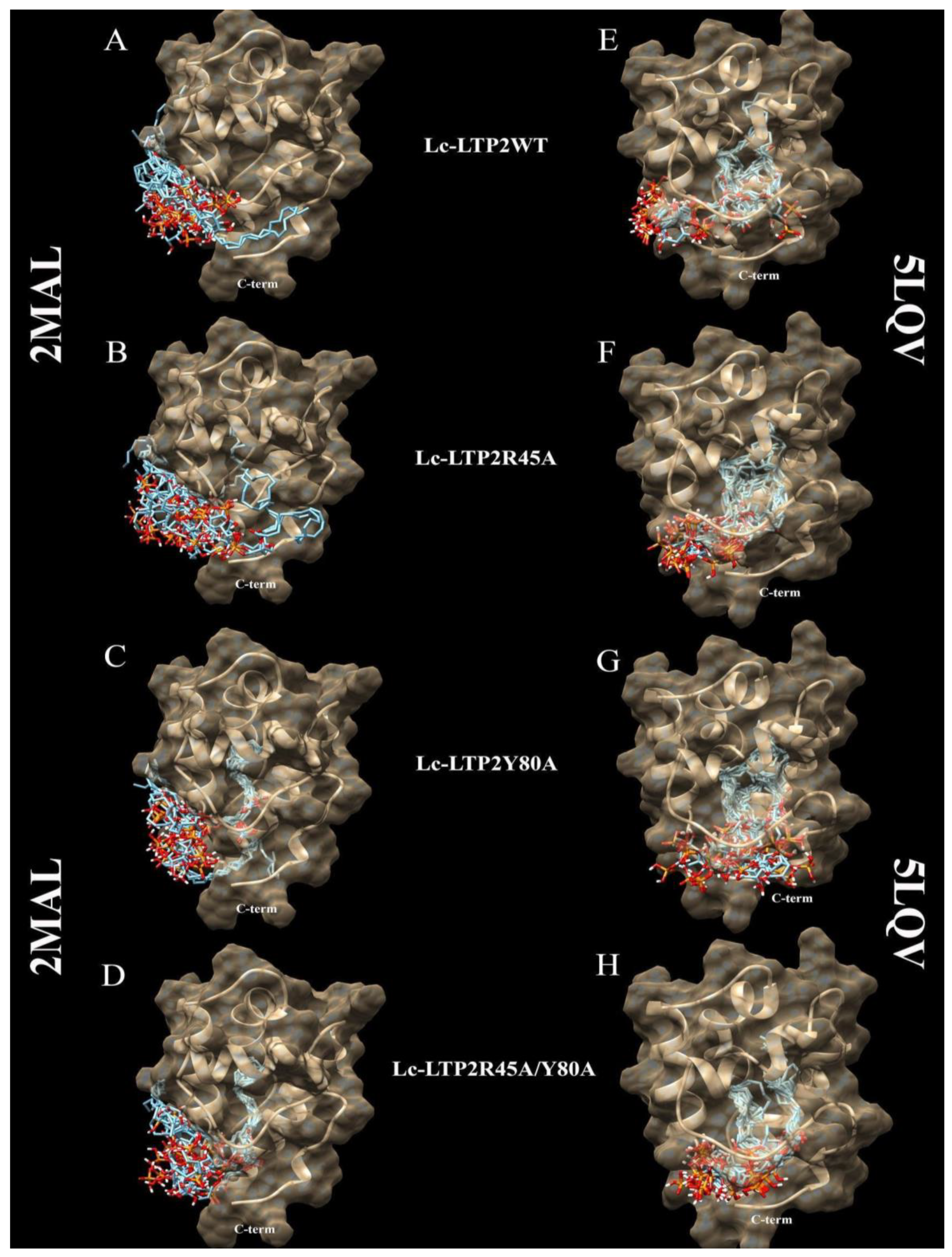
3.2. Bioinformatic Approaches to Study of the Protein-Ligand Interactions
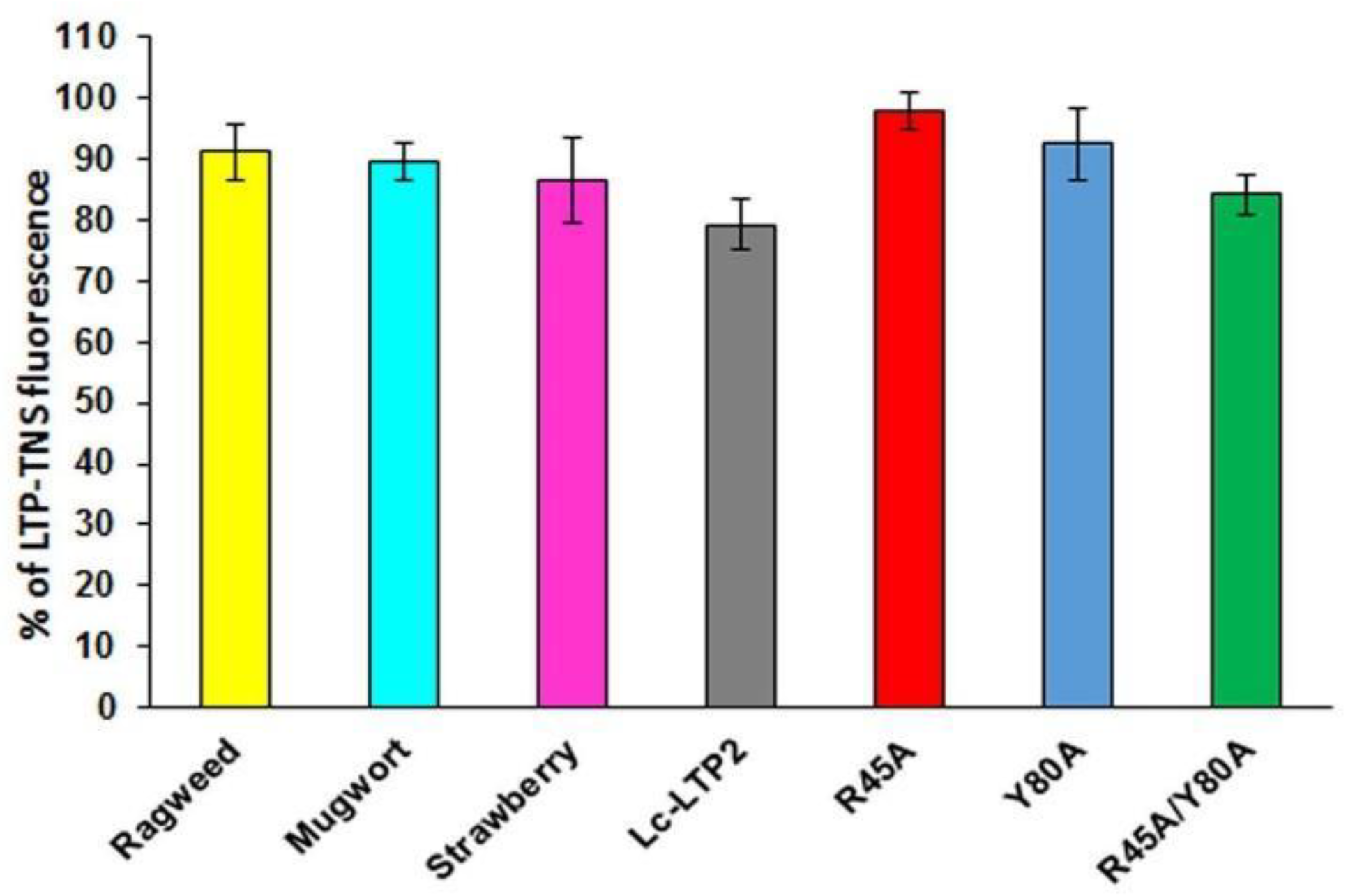
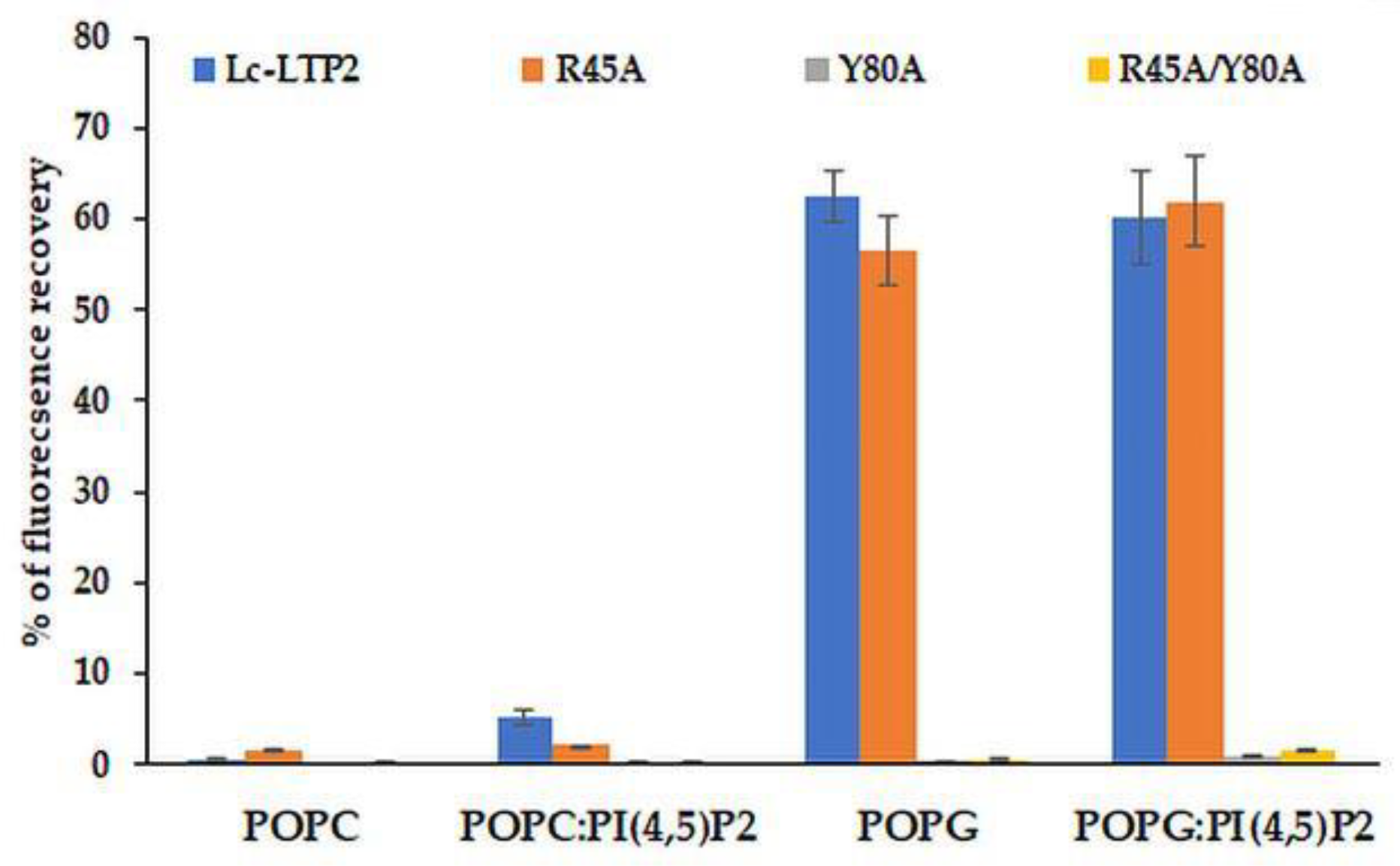
3.3. Effect of Lc-LTP2 and Its Mutant Analogs on SUVs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DHPC | 1,2-dihexanoyl-snglycero-3-phosphocholine |
| DMPG | 1,2-dimyristoyl-sn-glycero-3-phosphoglycerol |
| FA | fatty acid |
| LPPC | lyso-palmitoyl phosphatidylcholine |
| LPPG | lyso-palmitoyl phosphatidylglycerol |
| LTP | lipid transfer protein |
| PI(4,5)P2 | phosphatidylinositol (4,5)-bisphosphate |
| POPC | 1-palmitoyl-2-oleoyl-snglycero-3-phosphocholine |
| POPG | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol |
| TNS | 2-p-toluidinonaphthalene-6-sulphonate |
| SUV | small unilamellar vesicle |
References
- Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Ovchinnikova, T.V. Lipid Transfer Proteins As Components of the Plant Innate Immune System: Structure, Functions, and Applications. Acta Nat. 2016, 8, 47–61. [Google Scholar] [CrossRef]
- Edqvist, J.; Blomqvist, K.; Nieuwland, J.; Salminen, T.A. Plant lipid transfer proteins: Are we finally closing in on the roles of these enigmatic proteins? J. Lipid Res. 2018, 59, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Fleury, C.; Gracy, J.; Gautier, M.-F.; Pons, J.-L.; Dufayard, J.-F.; Labesse, G.; Ruiz, M.; De Lamotte, F. Comprehensive classification of the plant non-specific lipid transfer protein superfamily towards its sequence-structure-function analysis. PeerJ 2019, 7, e7504. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, D.N.; Finkina, E.I.; Bogdanov, I.V.; Ovchinnikova, T.V. Plant Pathogenesis-Related Proteins Binding Lipids and Other Hydrophobic Ligands. Russ. J. Bioorgan. Chem. 2018, 44, 586–594. [Google Scholar] [CrossRef]
- Cubells-Baeza, N.; Gómez-Casado, C.; Tordesillas, L.; Ramírez-Castillejo, C.; Garrido-Arandia, M.; González-Melendi, P.; Herrero, M.; Pacios, L.F.; Díaz-Perales, A. Identification of the ligand of Pru p 3, a peach LTP. Plant Mol. Biol. 2017, 94, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Bakan, B.; Hamberg, M.; LaRue, V.; Prangé, T.; Marion, D.; Lascombe, M.-B. The crystal structure of oxylipin-conjugated barley LTP1 highlights the unique plasticity of the hydrophobic cavity of these plant lipid-binding proteins. Biochem. Biophys. Res. Commun. 2009, 390, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Shenkarev, Z.; Melnikova, D.N.; Finkina, E.I.; Sukhanov, S.V.; Boldyrev, I.A.; Gizatullina, A.K.; Mineev, K.S.; Arseniev, A.S.; Ovchinnikova, T.V. Ligand Binding Properties of the Lentil Lipid Transfer Protein: Molecular Insight into the Possible Mechanism of Lipid Uptake. Biochemistry 2017, 56, 1785–1796. [Google Scholar] [CrossRef]
- Melnikova, D.; Bogdanov, I.; Ignatova, A.; Ovchinnikova, T.; Finkina, E. New insights into ligand binding by plant lipid transfer proteins: A case study of the lentil Lc-LTP2. Biochem. Biophys. Res. Commun. 2020, 528, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Gizatullina, A.K.; Finkina, E.I.; Mineev, K.S.; Melnikova, D.N.; Bogdanov, I.V.; Telezhinskaya, I.N.; Balandin, S.V.; Shenkarev, Z.; Arseniev, A.S.; Ovchinnikova, T.V. Recombinant production and solution structure of lipid transfer protein from lentil Lens culinaris. Biochem. Biophys. Res. Commun. 2013, 439, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, I.V.; Finkina, E.I.; Balandin, S.V.; Melnikova, D.N.; Stukacheva, E.A.; Ovchinnikova, T.V. Structural and Functional Characterization of Recombinant Isoforms of the Lentil Lipid Transfer Protein. Acta Nat. 2015, 7, 65–73. [Google Scholar] [CrossRef]
- DeLano, W.L. PyMol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar]
- O’Boyle, N.M.; Banck, M.; A James, C.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Dassault Systèmes BIOVIA. Discovery Studio Visualizer; v20.1.0.19295; Dassault Systèmes: San Diego, CA, USA, 2020. [Google Scholar]
- Colin, L.A.; Jaillais, Y. Phospholipids across scales: Lipid patterns and plant development. Curr. Opin. Plant Biol. 2020, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Deeken, R.; Saupe, S.; Klinkenberg, J.; Riedel, M.; Leide, J.; Hedrich, R.; Mueller, T.D. The Nonspecific Lipid Transfer Protein AtLtpI-4 Is Involved in Suberin Formation of Arabidopsis thaliana Crown Galls. Plant Physiol. 2016, 172, 1911–1927. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, Y.; Zhao, F.; Zhang, T.; Yu, X. An improved protein lipid overlay assay for studying lipid-protein interactions. Plant Methods 2020, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, D.N.; Mineev, K.S.; Finkina, E.I.; Arseniev, A.S.; Ovchinnikova, T.V. A novel lipid transfer protein from the dillAnethum graveolensL.: Isolation, structure, heterologous expression, and functional characteristics. J. Pept. Sci. 2016, 22, 59–66. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikova, D.; Bogdanov, I.; Ovchinnikova, T.; Finkina, E. Interaction between the Lentil Lipid Transfer Protein Lc-LTP2 and Its Novel Signal Ligand PI(4,5)P2. Membranes 2020, 10, 357. https://doi.org/10.3390/membranes10110357
Melnikova D, Bogdanov I, Ovchinnikova T, Finkina E. Interaction between the Lentil Lipid Transfer Protein Lc-LTP2 and Its Novel Signal Ligand PI(4,5)P2. Membranes. 2020; 10(11):357. https://doi.org/10.3390/membranes10110357
Chicago/Turabian StyleMelnikova, Daria, Ivan Bogdanov, Tatiana Ovchinnikova, and Ekaterina Finkina. 2020. "Interaction between the Lentil Lipid Transfer Protein Lc-LTP2 and Its Novel Signal Ligand PI(4,5)P2" Membranes 10, no. 11: 357. https://doi.org/10.3390/membranes10110357
APA StyleMelnikova, D., Bogdanov, I., Ovchinnikova, T., & Finkina, E. (2020). Interaction between the Lentil Lipid Transfer Protein Lc-LTP2 and Its Novel Signal Ligand PI(4,5)P2. Membranes, 10(11), 357. https://doi.org/10.3390/membranes10110357