1. Introduction
A growing body of evidence indicates dietary polyphenols to be efficient antioxidants counteracting negative effects of oxidative stress accompanying many diseases such as cardiovascular diseases, inflammation, and also cancer [
1,
2,
3,
4,
5,
6]. In line with previous reports, many of these compounds may act as chemopreventive or even chemotherapeutic agents [
5,
7,
8,
9]. Specifically to malignant cells, cinnamic acid (CinA) and its hydroxy derivatives have been shown to exhibit both antioxidant a antineoplastic activity, with their cytostatic effect being largely related to their structural characteristics [
10,
11,
12,
13]. However, the mode of action of CinA and its derivatives is still incompletely determined, but may involve scavenging of reactive oxygen species, modulation of gene expression, activation of xenobiotics metabolism-related enzymes, and regulation of signal transduction pathways essential for tumor cell growth and progression [
14].
Despite their natural origin and many promising biological effects, bioavailability of hydroxycinnamic acids presents certain limitations. Although working well in aqueous media, their hydrophilic nature is usually a restriction for lipophilic system protection [
15]. Lipophilicity is a major physicochemical property of chemical substances, which affects their biological activities. It is known to be important for describing both pharmacodynamic and pharmacokinetic aspects of drug action. In biological systems, lipophilicity largely determines key properties of potential pharmacological agents, such as solubility of substances in biological fluids, penetration through the biological membranes, rate of absorption, affinity to plasma and tissue proteins, and distribution into the specific body compartments [
16]. Lipophilicity is commonly expressed by the logarithm of n-octanol/water partition coefficient (log
P) for ionizable compounds of a neutral form of compounds. Initially, log
P was considered important in drug and pesticide discovery and design, but now it is an essential characteristic of all chemicals. This is because log
P largely determines chemicals fate both inside a living organism and in the environment. Log
P values are typically between −3 (very hydrophilic) and +10 (extremely hydrophobic) [
17]. For ionizable forms of compounds, the distribution coefficient (log
D) at a specific pH is also often used. As opposed to log
P, which is only valid for a single electrical species, log
D represents the pH-dependent mixture of all electrical species occurring at given pH [
18].
Based on the observation that most medication drugs are relatively small and lipophilic molecules, Lipinski et al. formulated “the rule of five” (Ro5) [
19]. This is a rule of thumb in determining if a pharmacologically/biologically active chemical compound, a candidate for a drug, would be potentially bioavailable via oral administration in humans [
20]. According to Ro5, chemicals are less prone to adsorb on the cell membrane and more likely to permeate the bilayer when their calculated n-octanol/water partition coefficient (
clog
P) ≥ 5, they have ≥10 H-bond acceptors, ≥5 H-bond donors, and their molecular weight (
MWT) exceeds 500 g/mol [
19]. Because each threshold is a multiple of 5, the rule was called Ro5. Molecules not complying with more than one of these rules may have problems with bioavailability [
21].
To obtain lipophilicity parameters of significant pharmacokinetic and pharmacodynamic relevance, partition coefficients between (mostly but not exclusively) artificial membranes and water have been tested recently [
22]. Monolayers and bilayers have been used for several decades as models of biomembranes to study solute/biological membranes interactions. A variety of lipids and their mixtures may serve as components to prepare both types of these membrane models. Nevertheless, phospholipids tend to be the most widely exploited as they are easily reproducible and have well standardized systems [
23]. These lipids are either negatively charged or zwitterionic (electrically neutral due to an equal number of positive and negative charges). Phospholipids are amphipathic and occur naturally in all living organisms as the major components of cell membranes. Most biological membranes are characterized by the asymmetrical distribution of phospholipids within the inner and outer leaflets. The inner leaflet of the bilayer mainly consists of negatively charged lipids, such as phosphatidylserine (PS), while electrically neutral lipids, such as phosphatidylcholine (PC) and phosphatidylethanolamine (PE), are mostly located in the outer layer [
24]. At physiological pH, negative charge of the outer leaflet lipids is due to low values of acid dissociation constant (p
Ka) of the phosphate moieties of the lipid head group [
25].
Our understanding of the properties, function and structure of biological membranes has benefitted a lot from the experimental and theoretical studies of the electrical properties of lipid bilayers. Therefore, evaluation of the parameters such as surface charge density (σ) of the membrane is critical for determination of the electrostatic interactions between membranes and their surrounding solutes. The surface charge of a lipid membrane, which may change with pH, depends also on the lipids present in the outer layer and can be quantified by zeta-potential measurements using electrochemical light scattering (ELS) technique. Zeta potential depends on a lots of parameters, including temperature, pH, conductivity (ionic strength), and solvent (viscosity). Small changes in any of these parameters can potentially dramatically affect the zeta potential values [
26].
In order to best mimic electrochemical conditions observed in natural membranes, model systems of artificial bilayers ought to possess similar values of capacitance (
Cm) and resistance (
Rm) to those observed in biological membranes. In this respect, the parallel plate capacitor model may serve as an estimator of the lipid bilayer capacitance. Assuming that typical hydrophobic thickness of a membrane equals 4 nm and a relative permittivity of ranges from 2 to 4, the capacitance of the bilayer is supposed to be 0.5–1.0 μF/cm
2. In fact,
Cm values placing within this range have been already confirmed in experimental research [
27,
28,
29]. Typical values of bilayer resistance are in the range 10
4−10
7 Ω cm
2 [
30,
31]. The measurement of lipid membrane resistance may sometimes be difficult and unreproducible. These variations are most likely a result of a leakage at the bilayer support and/or trapped emulsified droplets [
32]. Luckily, membrane capacitance and resistance can be easily measured by electrochemical impedance spectroscopy (EIS). EIS is a technique that enables to extract the information not only about the bulk phase of tested material (e.g., dielectric constant and conductivity) but also about their inner and outer interfaces (e.g., interfacial region capacitance and derived quantities) [
33].
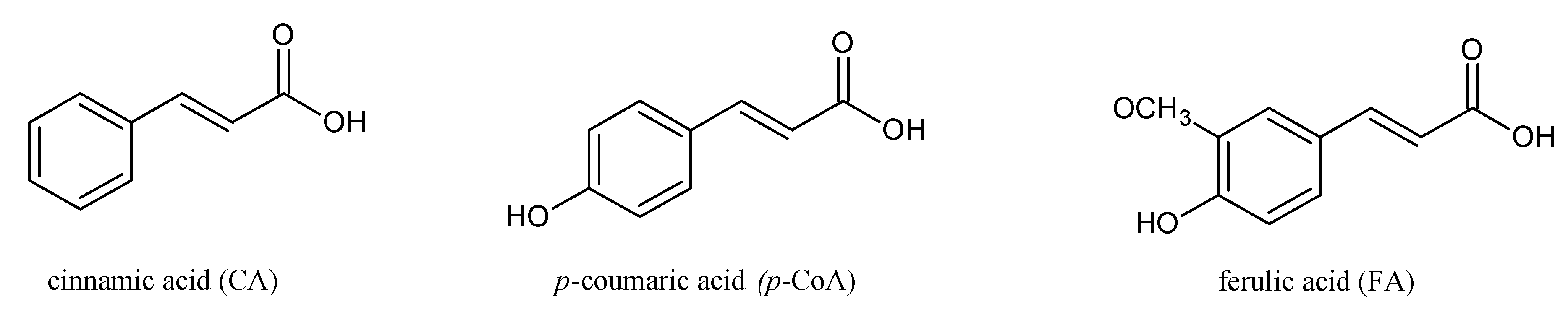
Herein, we report on the modulation of the electrical properties of model cell membranes by cinnamic acid and its two naturally occurring hydroxy derivatives:
p-coumaric acid (
p-CoA) and ferulic acid (FA). The selected phenolic acids are widely distributed in plants, fungi and algae and recognized as privileged structures for the development of bioactive compounds with therapeutic potential. These compounds are structurally related (
Figure 1) and a correlation between their structures and behavior in the surrounding solution seems to warrant further investigations. Although a considerable amount of data concerning hydroxycinnamic acids interactions with membranes exists, still little is known about the electrical properties of bilayer lipid membranes modified by CinA,
p-CoA or FA. Therefore, this paper is focused on the effect of cinnamic acid and its derivatives on the resistance, capacitance, and the surface charge density of model membranes (spherical lipid bilayers and liposomes).
We previously described changes in the electrical parameters caused by e.g., membrane compositions in lipid–lipid [
34], lipid–sterol [
35], lipid–fatty acid [
36], or lipid-carotenoid membranes [
37]. The investigations reported in this paper are a continuation of the studies on the interaction of model membranes of increasing complexity with naturally occurring phenolic compounds. The bilayers were formed from 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-diacyl-sn-glycero-3-phospho-l-serine (PS) or from DOPC-PS mixture in concentrations of 9:1 and 8:2 mol%, respectively, which corresponds to PS content in the human cerebral cortex [
38]. Next, phospholipid membranes were modified by CinA,
p-CoA or FA in the concentrations determined on the basis of MTT analysis shown in previous studies on human glioblastoma cell lines. Afterwards, we analyzed the influence of the pH of the electrolyte solution and the composition of membranes on their surface charge to better describe interactions in model membranes modified by the phenolic acids. We also analyzed if CinA,
p-CoA and FA were capable of altering the electrical resistance and capacitance of the bilayers. The results of our research should give useful indications in understanding the role of chemical structure of hydroxycinnamic acids in determining their interactions with the microenvironment of model lipid bilayer and, thereby, allow us to speculate about the in vivo bioavailability of the investigated compounds.
4. Discussion
Cinnamic acid and its derivatives have attracted the attention of more and more researchers due to the broad variety of pharmaceutical and biological activities and due to the wide technological or industrial applications [
50]. Polyphenols are important primarily because of their antioxidant property, antitumor activity or hypertension-preventing effect. Interactions of these phenolic acids with cell membranes play an essential role in their transport, distribution, selectivity, activity, and toxicity.
Small organic acids such as hydroxycinnamic acids have been postulated to interact with/and penetrate into biological membranes, however, their ability to demonstrate such an interaction with lipid membranes can be greatly influenced by the type of substituents attached to the main structure. Therefore, structurally similar compounds may differently interact with biological membranes, because of the complex relationship (which can be modulated by pH changes) between liposolubility and permeability [
51]. The dissociation constant is a very important parameter describing solubility, lipophilicity, and permeability properties of compounds. Phenolic acids in aqueous solutions exhibit protonated and deprotonated forms. Analyzing the values of dissociation constants of the carboxyl and hydroxyl groups of the studied phenolic acids (
Table 7), it can be stated that their molecular and monodeprotonated forms prevail within the range of physiological pH values. Moreover, the corresponding dissociation constant values are all similar to each other, which demonstrates that they are not a main factor determining the activity of the hydroxycinnamic acids. However, these data could be useful in studies concerning bioavailability and pharmacokinetics of potential pharmacological agents [
52].
The positive values of the calculated log
P of CinA,
p-CoA and FA may indicate that these acids are partitioning in the octanol phase. Nevertheless, only lipophilicity of uncharged molecules can be determined by this method, thus it is difficult to apply to these phenolic acids, which at physiological conditions are rather negatively charged. Analysis of
Table 7 revealed that there are relevant differences between
clog
P and log
D coefficient partitions for each acid. These findings suggest that the drug distribution between the aqueous solution and the lipid membrane is regulated by the ionization state of the molecule. All three compounds tested here are ionizable molecules, and it is already widely recognized that ionizable drugs partition into the lipid membrane to a high extent thanks to electrostatic interactions and formation of hydrogen bonds with polar groups of the phospholipids [
18]. As a matter of fact, several reports inform about different experimental log
D values from the predicted log
P values calculated exclusively for the neutral species of drugs [
58,
59].
Rocher et al. reported the percentage of the undissociated forms of i.e., CinA,
p-CoA and FA in pulvinar cells of
Mimosa pudica L. at pH 5.2, which amounted to 70.4%, 58.6%, and 52.7% of the total pool of these acids respectively [
18]. Considering the fact that only lipophilic neutral forms are able to cross the plasma membrane by diffusion, this parameter bears important information on this family of carboxylic acids [
60].
Regarding the importance of physicochemical properties of drugs, Lipinski developed Ro5 [
18]. According to this rule, candidates for efficient drugs should be characterized by log
P ≤ 5, molecular weight ≤ 500, number of hydrogen bond acceptors (O) ≤ 10, and number of hydrogen bond donors (OH, NH) ≤ 5. All phenolic acids examined herein meet the Lipinski’s rule as demonstrated in
Table 7. Thus, CinA,
p-CoA and FA display favorable drug-like properties, which certainly encourage their further examination in the in vitro and in vivo studies.
Since phenolic compounds has been demonstrated as efficient cytostatic agents against various malignancies, it seems essential to evaluate how these compounds interact with lipid membranes, to get fuller insight into the transportation and anticancer mechanisms of these natural compounds. The affinity of polyphenols to the lipid bilayer is reflected in several electrochemical parameters. The first determinant is the adsorption on the membrane surface mediated by interactions of hydrophilic parts with the polar head groups of the lipids at the water–lipid interface. Second is the absorption dependent on the partitioning of the hydrophobic parts into the nonpolar core of the membrane [
61,
62]. The mechanism of action of polyphenols is determined by the presence of different substituents in their backbone structure and the pH value of their microenvironment. If the pH of the external medium is low (acidic), phenolic acids are then able to diffuse through the membrane due to their unchanged form [
63,
64]. The ELS data presented in
Figure 2,
Figure 3 and
Figure 4 clearly show that cinnamic acid and its derivatives do not affect the surface charge density of liposomal membranes at acidic pH. Therefore, it can be assumed that at acidic pH, investigated polyphenols were able to solubilize into the membrane and to permeate it. At neutral and basic pH, the CinA was unable to considerably modify the surface charge of the model membrane due to the lack of a hydroxyl group. Whereas more hydrophilic
p-CoA and FA remained anchored at the bilayer surface without perturbing the lipidic structure but clearly affecting
σ values. These findings are in agreement with literature, where the influence of structural characteristics of cinnamic acid and its hydroxyl derivatives on their interaction with model membranes was reported [
51,
65,
66]. Similarly, the penetration of many other compounds e.g., flavonoids [
7] or non-steroidal anti-inflammatory drugs [
67] in bilayers, depends on the pH of the media.
Given the
pK
COOH values (
Table 7), at neutral and basic pH, the carboxyl group of CinA,
p-CoA and FA is most likely negatively charged, and the negative charge of their molecules is probably oriented towards the positive pole of zwitterionic DOPC headgroup. ELS measurements performed in the presence of two concentrations of phenolic acids corroborate this hypothesis given that the surface charge of the membrane was altered, becoming increasingly negative with the addition of the examined polyphenols. The same tendency was observed for other negatively charged compounds [
67]. The incorporation of cinnamic acid resulted in the slightest changes of
σ, which again suggests interactions of CinA with the interior of the membrane. It is obvious that the effects of different phenolic acids are correlated to their structural characteristics, thereby even the difference in one –OH group can be important, as well as the number of H-bonds they form. CinA can form three H-bonds, with one as a H-bond donor, and two as H-bond acceptors; its partition coefficient was the highest, and it was the most soluble of these phenolic acids in octanol (
Table 7). In other words, CinA as a non-polar substance could enter the lipid bilayer easily. Our findings are in line with the literature, where the interaction of model membranes with phenolic acids presenting different structural changes in their molecular backbone is deciphered on the basis of the shift of lipid phase transition temperature [
51,
61,
66,
68]. These authors reported that for pH below p
KCOOH values, the protonation of the carboxylic group allows the substance to penetrate the lipid bilayer. Likewise, Castelli et al. employed multilamellar or unilamellar liposomes created from synthetic L-R-dimyristoylphosphatidylcholine to check whether cinnamic acid can dissolve into lipid membranes and penetrate them by migration from the aqueous phase [
51]. This process continues until there is a constant molar fraction on the membrane surface, and then progressively inside the other internal bilayers. At the end of this process, the thermotropic behavior is close to that obtained by direct mixing of the biophenol with the lipid component during the liposomal preparation. The same researchers investigating PC liposomes containing
p-CoA at two different pHs (4 and 7) reported no shift of the calorimetric peaks toward lower values, suggesting that the –OH group influences the ability of this compound to penetrate the membrane [
51]. They did not exclude a surface interaction with the lipid layers and stated that the presence of different substituents in the backbone structure of biophenols might influence their incorporation. On the other hand, Ota et al. compared the thermograms obtained for the unilamelar large vesicles of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine in the presence of the
p-CoA or FA, and noticed that the enthalpy value of the main transition of the phospholipids decreased by 1.46 ± 0.10 kcal/mol in the presence of
p-CoA, and by 0.45 ± 0.17 kcal/mol with FA. This small but significant decrease in the enthalpy of transition implies that both acids intercalate into the acyl chain region of the bilayer [
66].
Analyzing the influence of the polar headgroup of lipids on the penetration capacity of cinnamic acid and its derivatives, we expected that phenolic acids will be less able to penetrate the negatively charged headgroup of PS lipids as compared with DOPC. Surprisingly, we have not noticed such an effect. We speculate that perhaps the acids concentrations used here were not sufficient to cause significant alterations of the membrane surface charge, or it may also be the result of certain limitations of the ELS technique per se. Likewise, Fadel et al. [
64] reported that rosmarinic acid, another compound belonging to phenolic acids, evoked a weaker effect in PS than PC membranes.
Furthermore, we analyzed the influence of pH on the size of the liposomes. Our findings showed that for neutral liposomes (DOPC), their diameter increased with increasing pH of the solution. It may suggest that in acidic pH, existing electrostatic repulsive forces dominate over hydrogen bonding affinities between neighboring lipid molecules [
69]. This might be a tentative explanation of why we observed smaller sizes of DOPC liposomes. Contrarily, for the negatively charged liposomes (DOPC/PS 9:1, DOPC/PS 8:2, PS), the diameter decreased with increasing pH. As such, at acidic pH, the extent of protonation was higher than this observed in the case of DOPC liposomes. Also, an opposite dependence, the dominance of hydrogen bonding over electrostatic repulsions, was observed. This might be the reason why the negatively charged liposomes showed larger sizes than neutral ones (DOPC). Simultaneously, in alkaline and neutral pH, reduction of the diameter of negatively charged liposomes was observed most probably due to the repulsive forces between phospholipid molecules [
70].
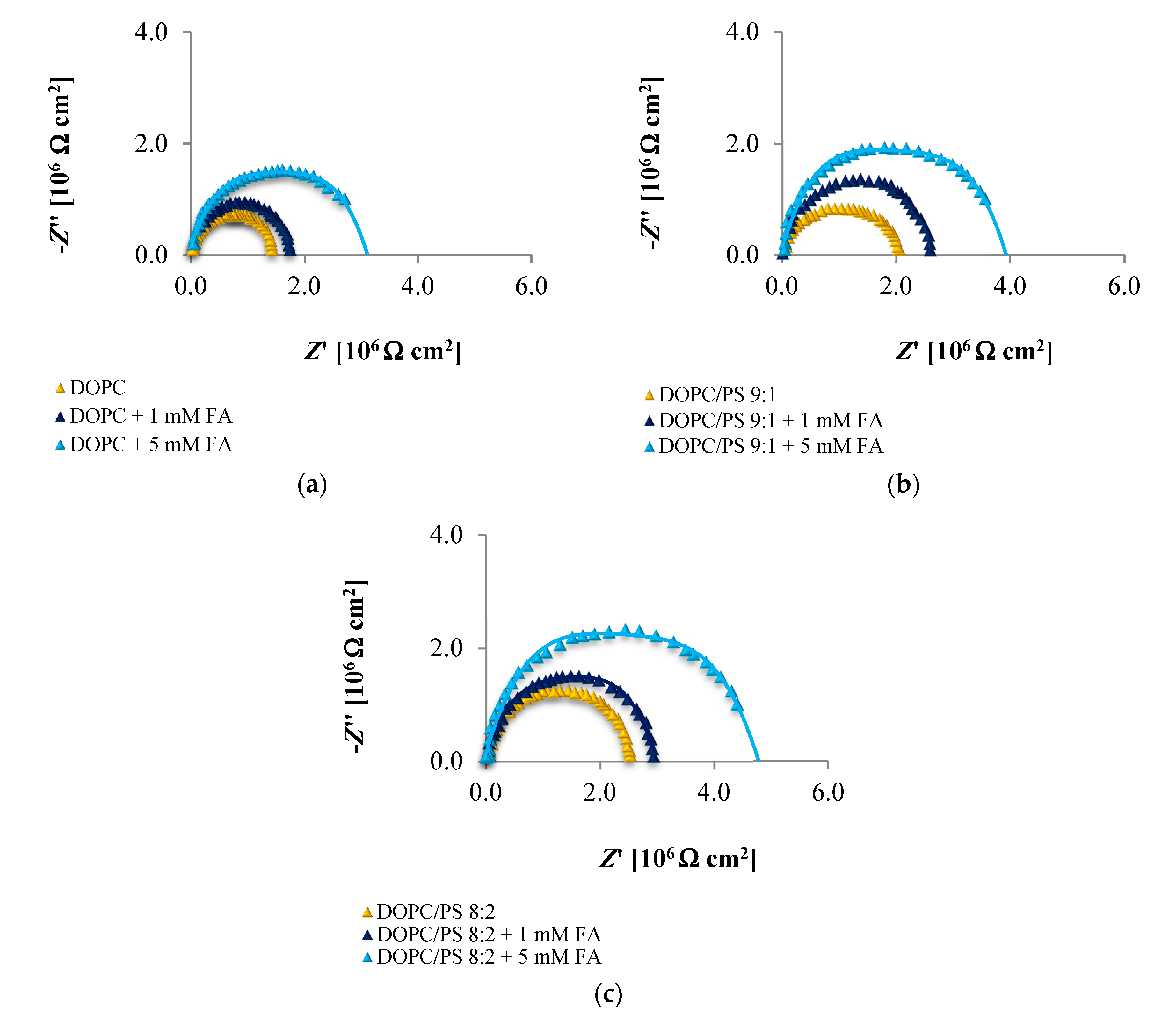
The EIS data presented in
Figure 6,
Figure 7 and
Figure 8 and collected in
Table 4,
Table 5 and
Table 6 indicate that the addition of cinnamic acid and its hydroxy derivatives to the DOPC, DOPC/PS 9:1 or DOPC/PS 8:2 membranes caused an increase in their resistance, and thus reduced conductivity. An increase in the
Rm value implies that these phenolic acids exacerbated the ordering and decreased the dynamics of the phospholipid alkyl chains of spherical bilayers. This behavior can be explained in relation to the polarities of the examined molecules, indicating to what levels they can penetrate into the lipid bilayer. CinA, the least polar acid among the tested ones, had the greatest effect on the structure of the membrane lipids (i.e., stabilizing of the structure).
p-CoA and FA are both more polar than CinA (see
Figure 1), therefore, presenting a weaker effect on the membrane resistance. Based on our experimental approach, the following order of the stabilization effect was established: cinnamic acid >
p-coumaric acid > ferulic acid. Our results are in line with previously reported data where the influence of the phenolic acids on structural properties of a model lipid membrane was investigated by differential scanning calorimetry [
51,
66,
68], fluorescence spectroscopy [
66,
68], and electron paramagnetic resonance spectroscopy [
68].
As opposed to the resistance, alterations in the capacitance seem to be less obvious in Nyquist plots, therefore, no remarkable differences between subsequent EIS measurements can be observed in
Figure 6,
Figure 7 and
Figure 8. It may be stated that the deposition of phenolic acids on the membrane was not accompanied by the appearance of any additional time constant, which was also reported not to happen in the case of protein adsorption [
71]. Consequently, the
Cm values fitted from the equivalent circuit model were in alignment with the indications from the Nyquist (
Table 4,
Table 5 and
Table 6), revealing increasing capacitance with increasing phenolic acids concentrations.
Together, the EIS data indicate that in comparison to untreated plain phospholipid bilayer, CinA-stimulated membranes showed a significant and systematic increase in bilayer resistance, which is dependent on increasing the concentration of phenolic acid. These changes were accompanied by moderate elevation of the bilayer capacitance, which can be attributed to a decrease in membrane thickness. This in turn, may indicate the ordering and stabilizing effect of CinA on the phospholipid alkyl chains of bilayers.
According to the EIS response shown in
Figure 6,
Figure 7 and
Figure 8 and data collected in
Table 4,
Table 5 and
Table 6, it is evident that
p-CoA and FA interact with the bilayer in a different way than CinA. Both of these compounds elicit significant changes in the lipid membrane capacitance, which indicate their adsorption at the bilayer interface. In contrast to CinA, changes in membrane resistance caused by increasing concentrations of
p-CoA or FA were much smaller. These observations were consistent with the ELS data, suggesting that the CinA associates strongly and penetrates deeply into the lipid membrane. Conversely,
p-CoA and FA may locate near the phospholipid headgroup, most probably via electrostatic interaction, without perturbing the lipidic structure.
Finally, if we compare the influence of the polar headgroup of lipids on the Rm and Cm values of membranes modified with CinA, p-CoA or FA, it seems that phenolic acids are less able to interact with the negatively charged headgroup of PS lipids as compared with DOPC. This result is not surprising, because the data were registered at pH equals 6.59 (pH of 155 mM/L NaCl electrolyte solution), in which all tested compounds bear a negative charge (carboxylate group) that may cause charge repulsion between the two carboxylate functions of the serine in PS. Notably, we failed to identify the differences in the interaction between phenolic acids and DOPC or PS bilayers using zeta potential analysis. This indicates that impedance measurement is a useful and effective technique worth utilizing in physicochemical studies.

 ) 0, (
) 0, ( ) 1, and (
) 1, and ( ) 5 mM/L of cinnamic acid concentration. The results represent mean values from three independent experiments run in triplicate.
) 5 mM/L of cinnamic acid concentration. The results represent mean values from three independent experiments run in triplicate.
 ) 0, (
) 0, ( ) 1, and (
) 1, and ( ) 5 mM/L of cinnamic acid concentration. The results represent mean values from three independent experiments run in triplicate.
) 5 mM/L of cinnamic acid concentration. The results represent mean values from three independent experiments run in triplicate.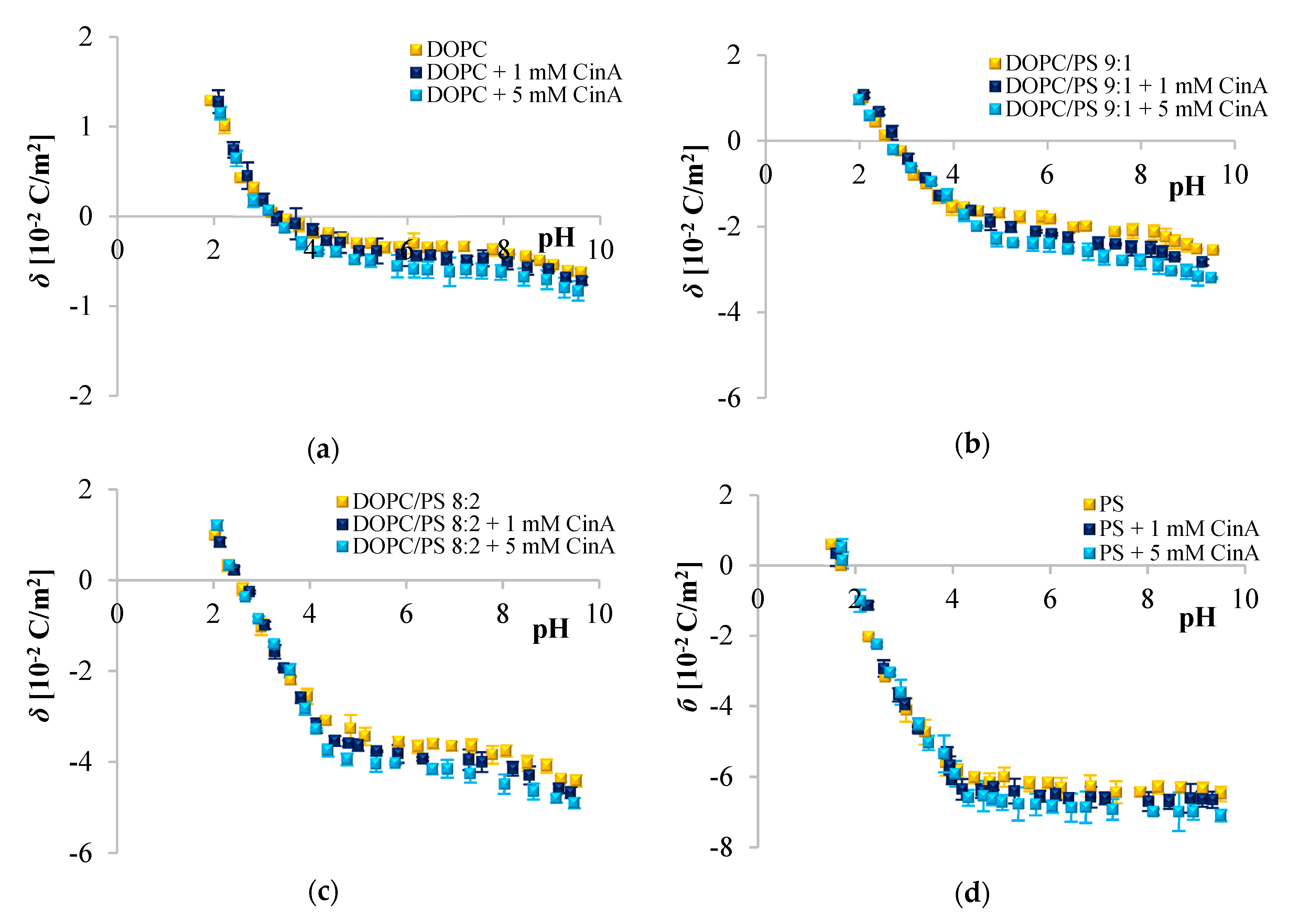
 ) 0, (
) 0, ( ) 1, and (
) 1, and ( ) and 5 mM/L of p-coumaric acid concentration. The results represent mean values from three independent experiments run in triplicate.
) and 5 mM/L of p-coumaric acid concentration. The results represent mean values from three independent experiments run in triplicate.
 ) 0, (
) 0, ( ) 1, and (
) 1, and ( ) and 5 mM/L of p-coumaric acid concentration. The results represent mean values from three independent experiments run in triplicate.
) and 5 mM/L of p-coumaric acid concentration. The results represent mean values from three independent experiments run in triplicate.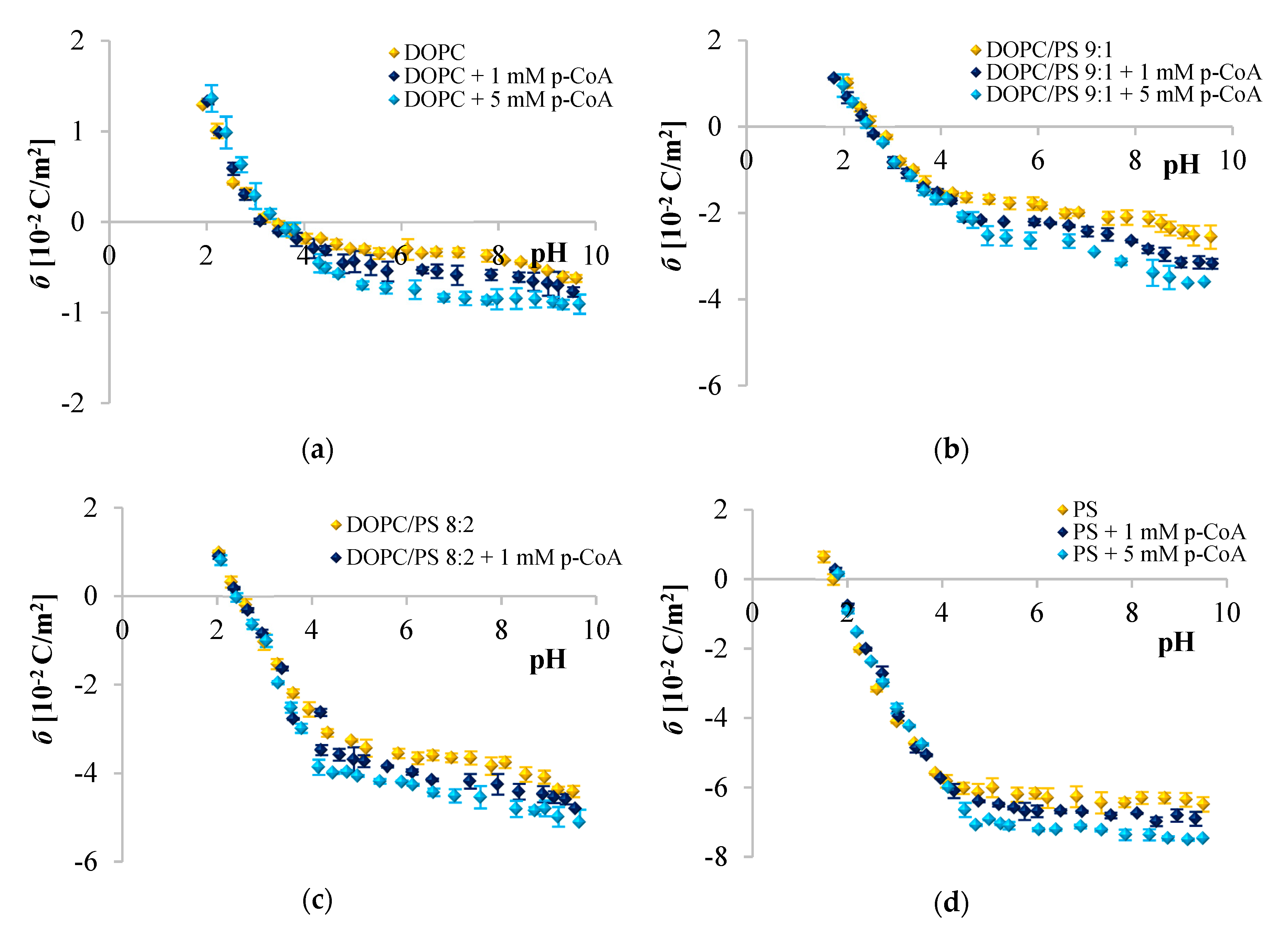
 ) 0, (
) 0, ( ) 1, and (
) 1, and ( ) 5 mM/L of ferulic acid concentration. The results represent mean values from three independent experiments run in triplicate.
) 5 mM/L of ferulic acid concentration. The results represent mean values from three independent experiments run in triplicate.
 ) 0, (
) 0, ( ) 1, and (
) 1, and ( ) 5 mM/L of ferulic acid concentration. The results represent mean values from three independent experiments run in triplicate.
) 5 mM/L of ferulic acid concentration. The results represent mean values from three independent experiments run in triplicate.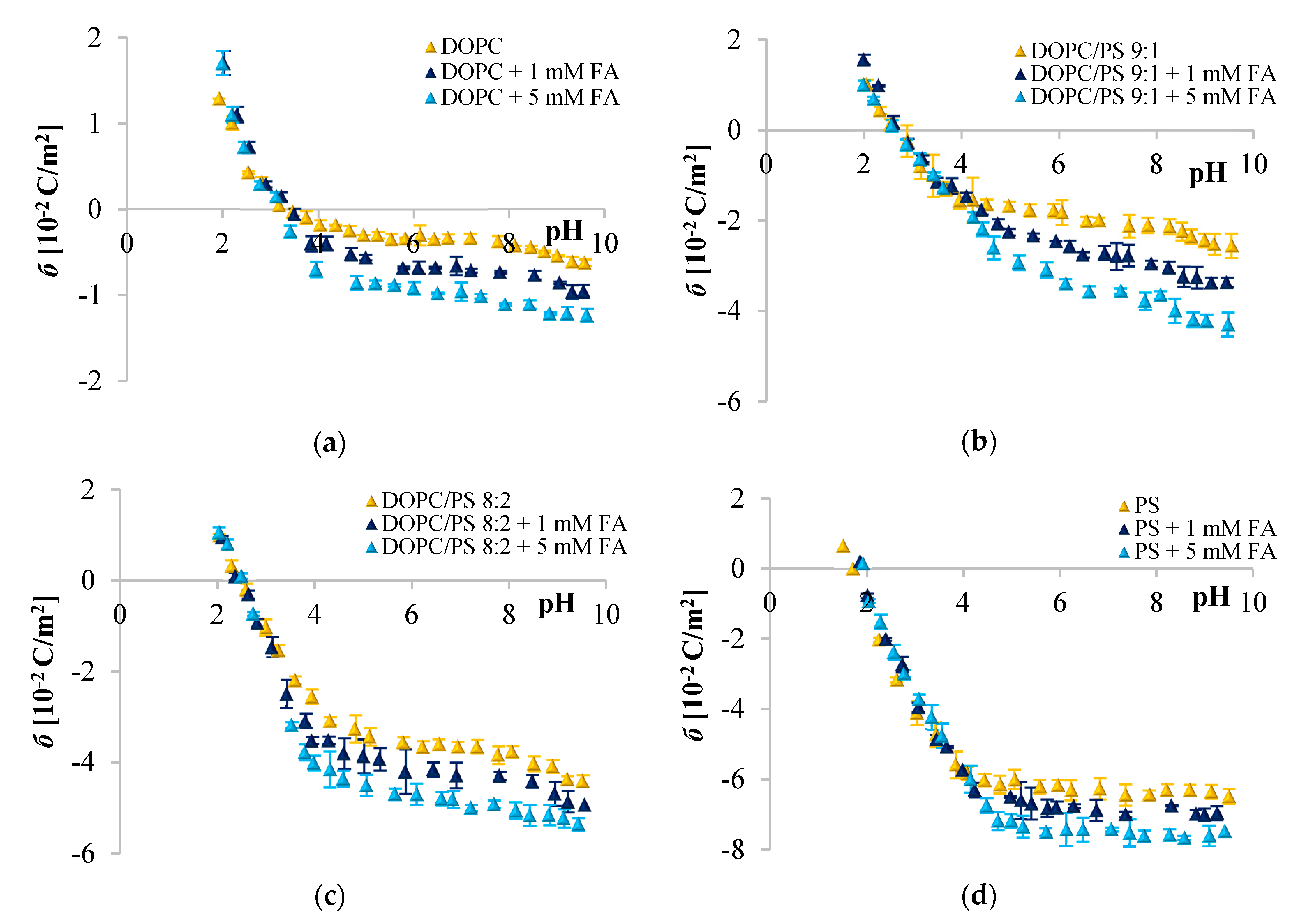
 ) and DOPC modified with 5 mM/L ferulic acid (
) and DOPC modified with 5 mM/L ferulic acid ( ). The results represent mean values from three independent experiments run in triplicate.
). The results represent mean values from three independent experiments run in triplicate.
 ) and DOPC modified with 5 mM/L ferulic acid (
) and DOPC modified with 5 mM/L ferulic acid ( ). The results represent mean values from three independent experiments run in triplicate.
). The results represent mean values from three independent experiments run in triplicate.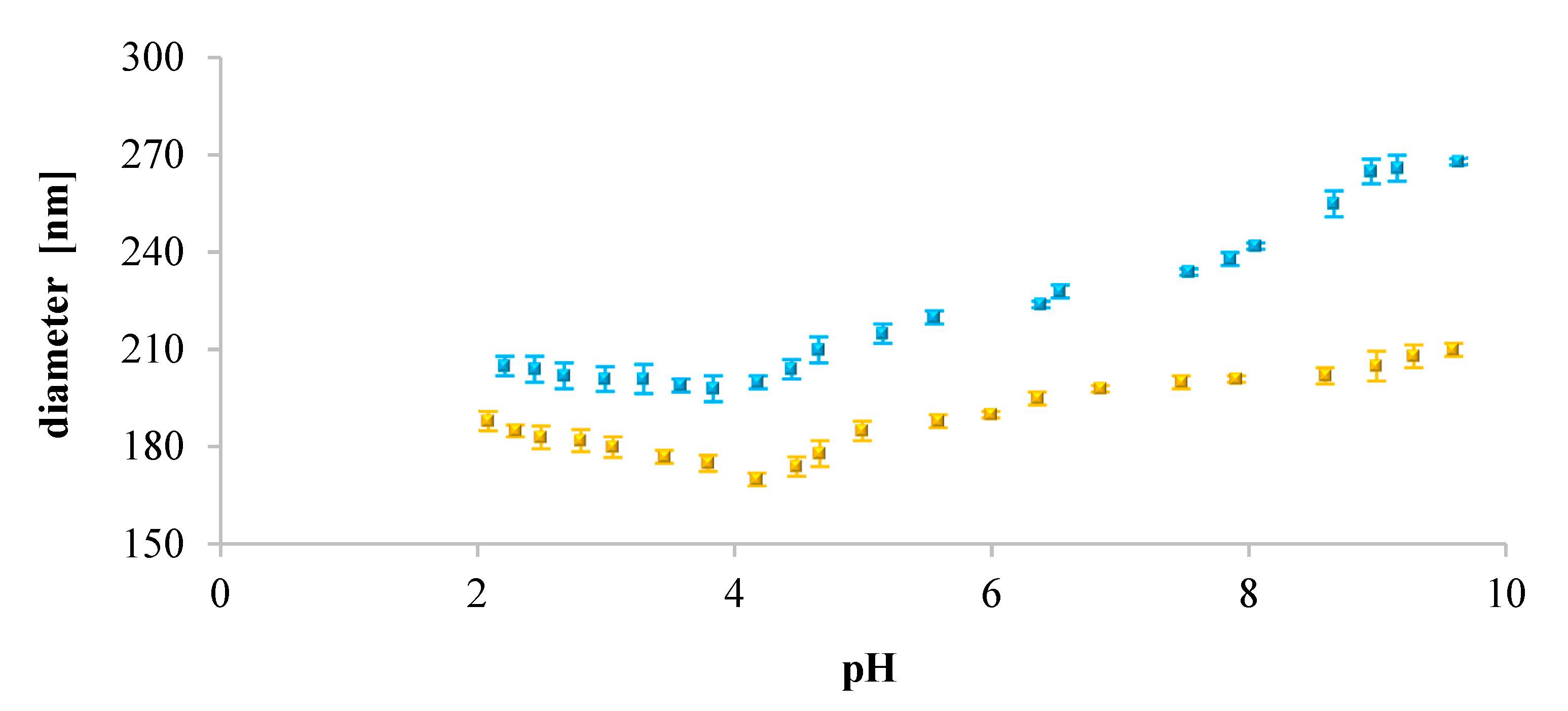
 ) 0, (
) 0, ( ) 1, and (
) 1, and ( ) 5 mM/L of cinnamic acid concentration. Experimental data points are indicated with markers; model fits are shown with lines.
) 5 mM/L of cinnamic acid concentration. Experimental data points are indicated with markers; model fits are shown with lines.
 ) 0, (
) 0, ( ) 1, and (
) 1, and ( ) 5 mM/L of cinnamic acid concentration. Experimental data points are indicated with markers; model fits are shown with lines.
) 5 mM/L of cinnamic acid concentration. Experimental data points are indicated with markers; model fits are shown with lines.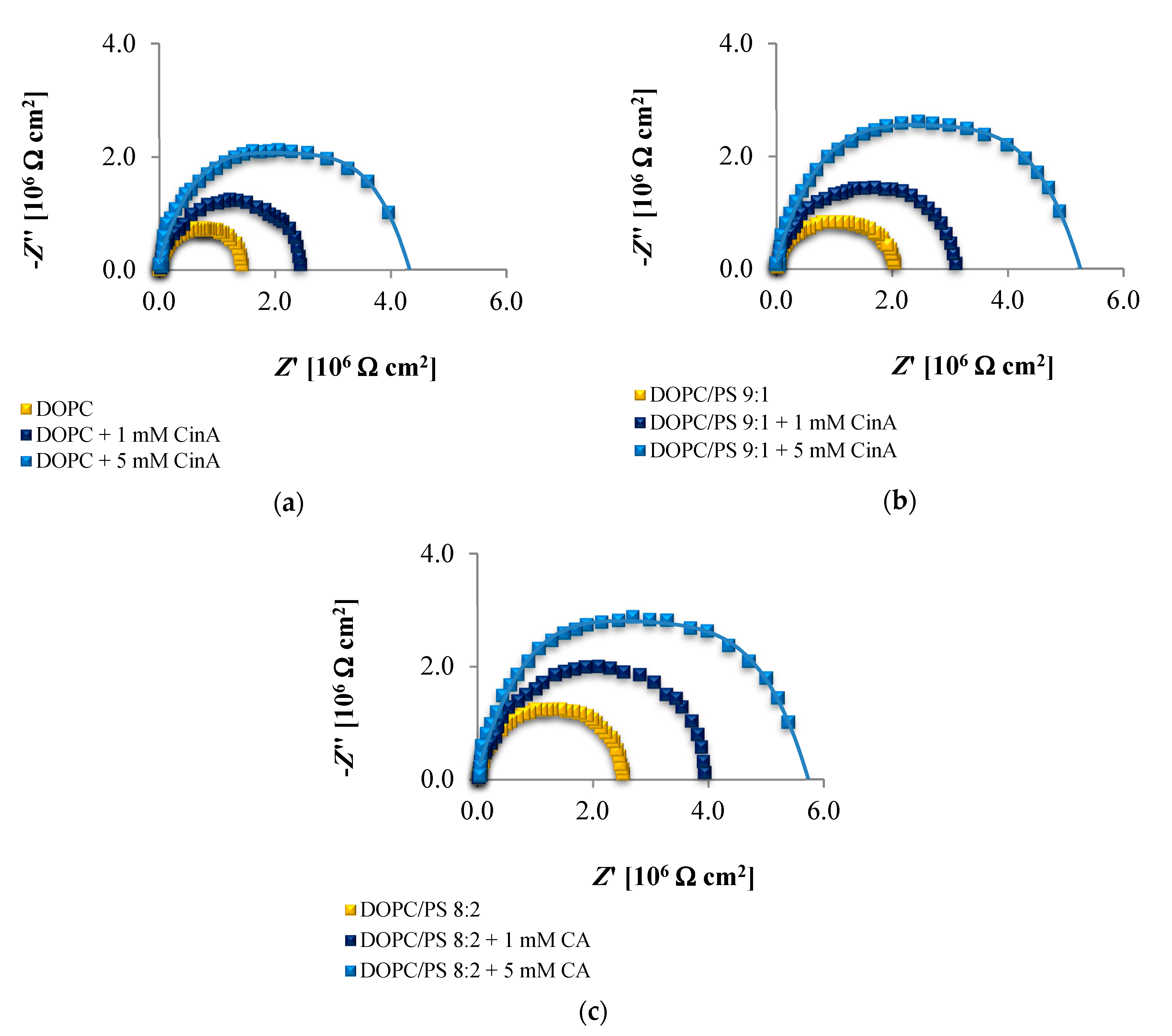
 ) 0, (
) 0, ( ) 1, and (
) 1, and ( ) 5 mM/L of p-coumaric acid concentration. Experimental data points are indicated with markers; model fits are shown with lines.
) 5 mM/L of p-coumaric acid concentration. Experimental data points are indicated with markers; model fits are shown with lines.
 ) 0, (
) 0, ( ) 1, and (
) 1, and ( ) 5 mM/L of p-coumaric acid concentration. Experimental data points are indicated with markers; model fits are shown with lines.
) 5 mM/L of p-coumaric acid concentration. Experimental data points are indicated with markers; model fits are shown with lines.
 ) 0, (
) 0, ( ) 1, and (
) 1, and ( ) 5 mM/L of ferulic acid concentration. Experimental data points are indicated with markers; model fits are shown with lines.
) 5 mM/L of ferulic acid concentration. Experimental data points are indicated with markers; model fits are shown with lines.
 ) 0, (
) 0, ( ) 1, and (
) 1, and ( ) 5 mM/L of ferulic acid concentration. Experimental data points are indicated with markers; model fits are shown with lines.
) 5 mM/L of ferulic acid concentration. Experimental data points are indicated with markers; model fits are shown with lines.