Hydrogen Purification from Compact Palladium Membrane Module Using a Low Temperature Diffusion Bonding Technology
Abstract
1. Introduction
2. Materials and Method
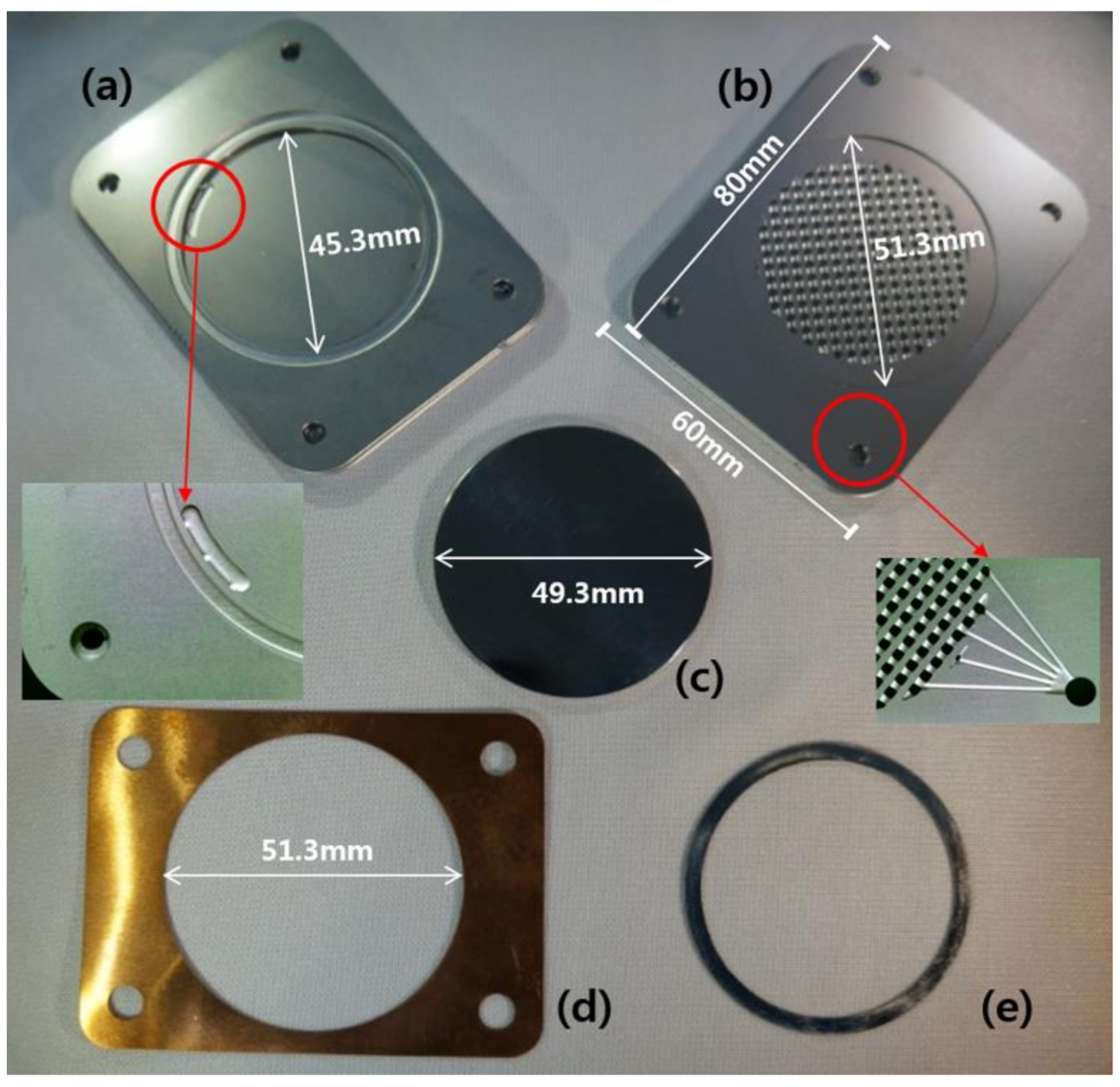
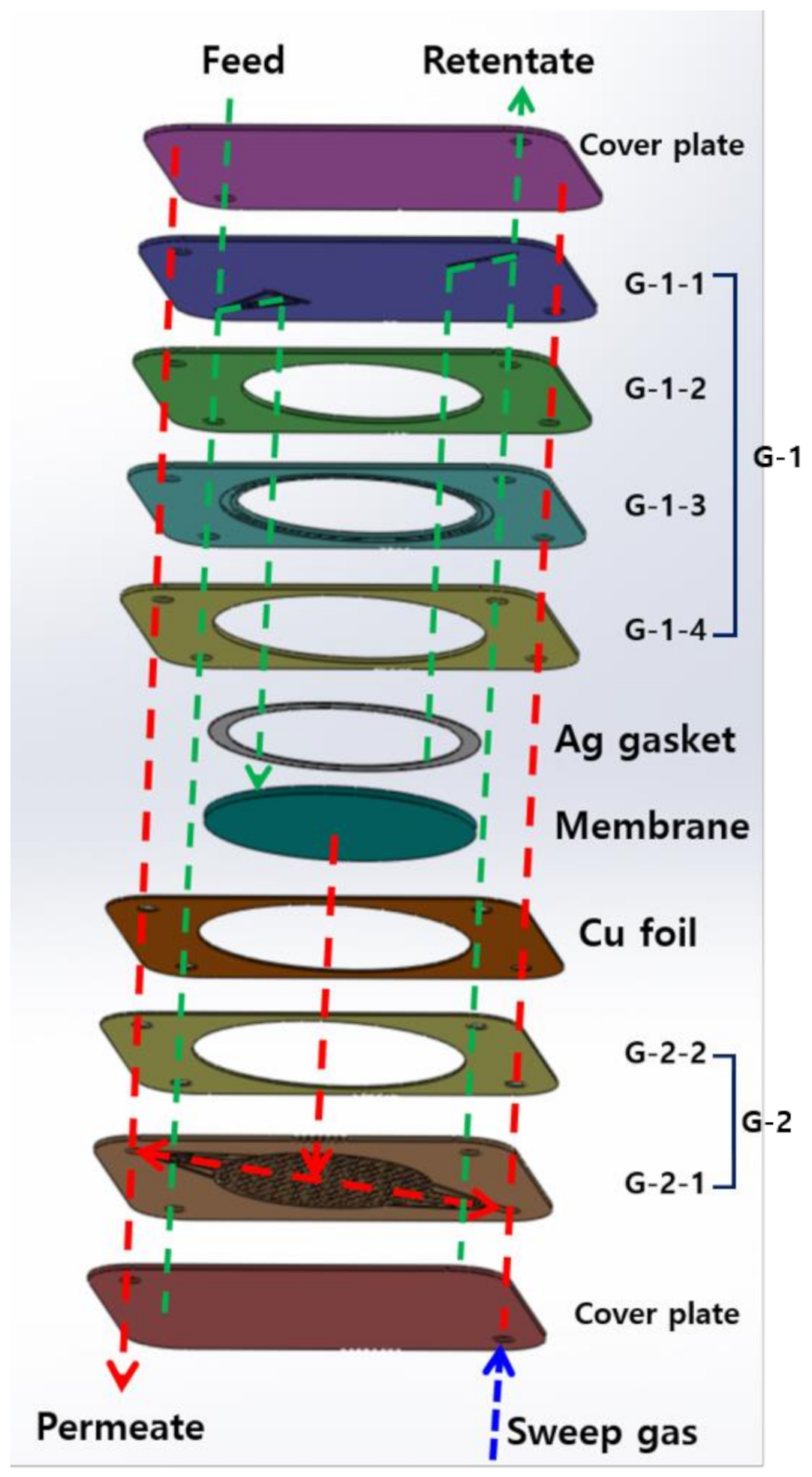
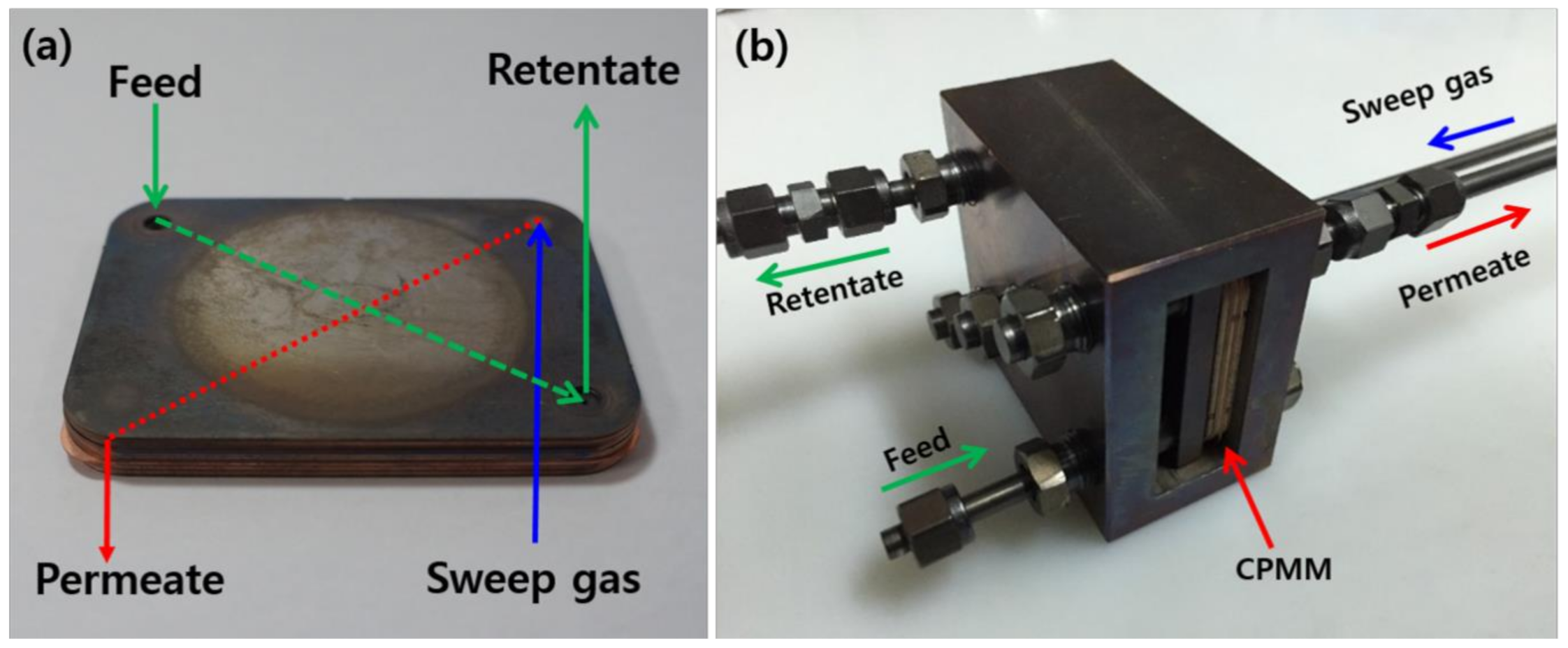
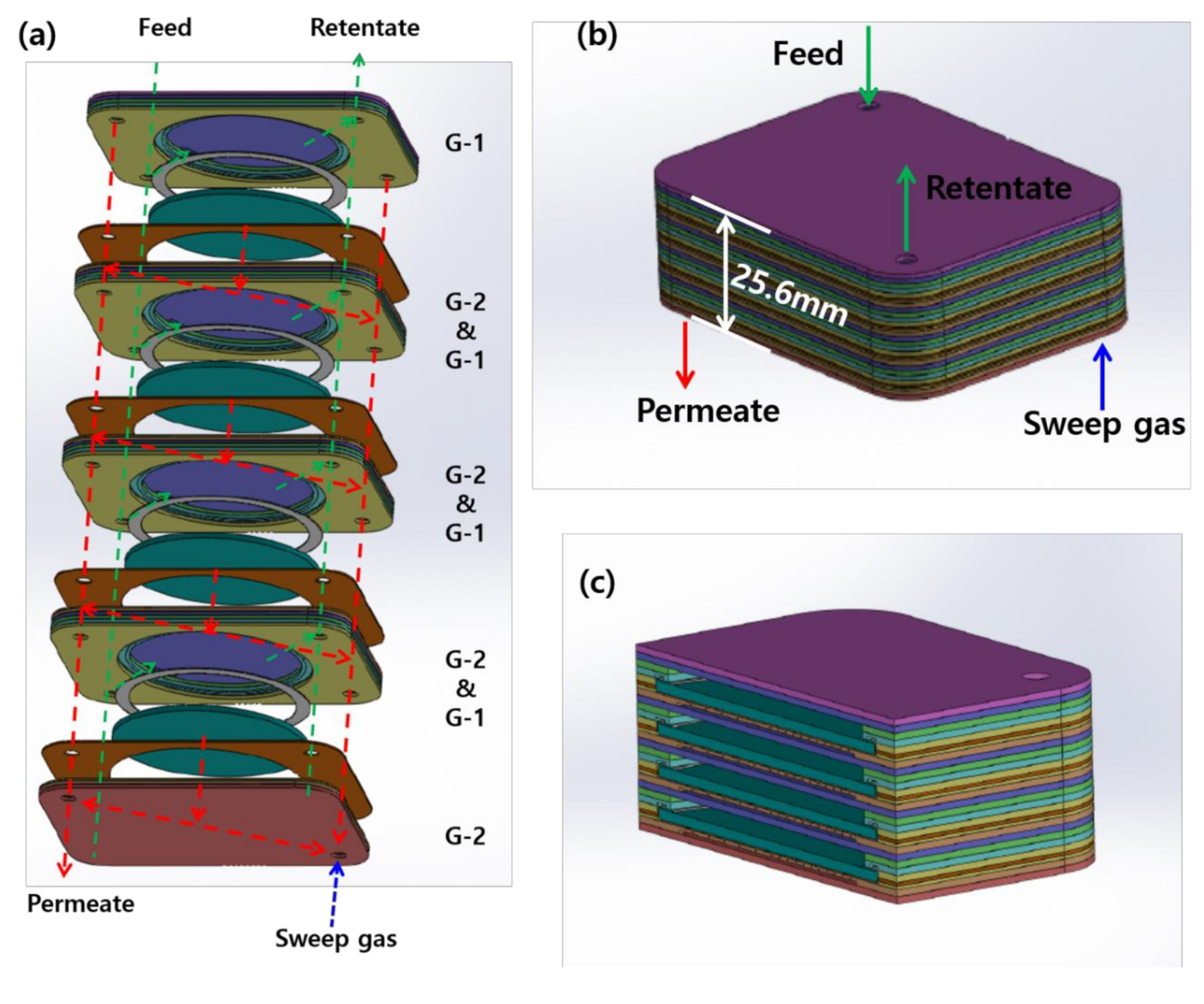
2.1. Design and Manufacture of the CPMM
- (1)
- Each G-1 and G-2 part was diffusion bonded using hot-press (Samyang Ceratech Co., Ltd., Incheon, Korea) under high vacuum (3.0 × 10−6 Torr) and 900 °C
- (2)
- The surfaces of bonded G-1 and G-2 (G-1-4 in G-1 and G-2-2 in G-2) were peened using a peening machine (IEPCO PEENMATIC 550, Swiss Instruments Limited Co., Ltd., Mississauga, Canada) to increase the surface roughness using 316L SS powder by spraying method.
- (3)
- Ni and Cu were deposited over the peened surfaces of the G-1 and G-2 using the DC magnetron sputtering method.
- (4)
- G-1 and G-2 with Ni and Cu deposition were thermally treated under H2 condition at 700 °C for 2 h in the muffle furnace.
2.2. Permeation Test Method of Hydrogen
3. Result and Discussion
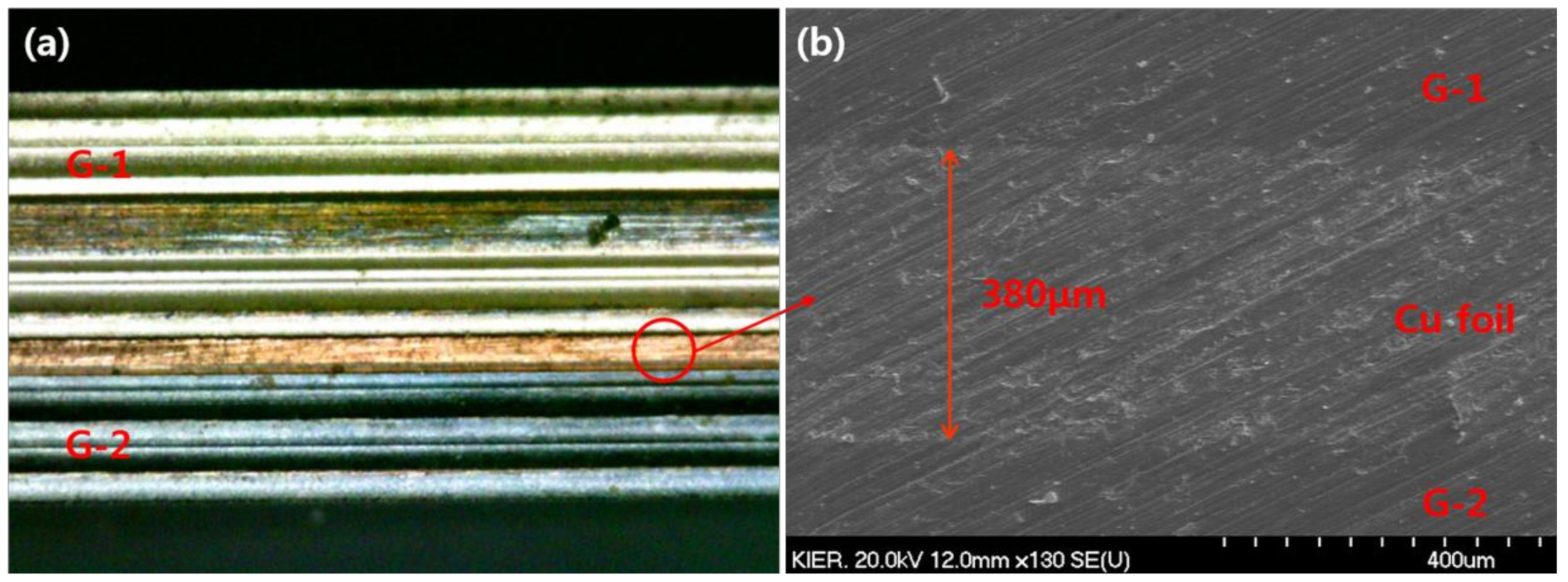
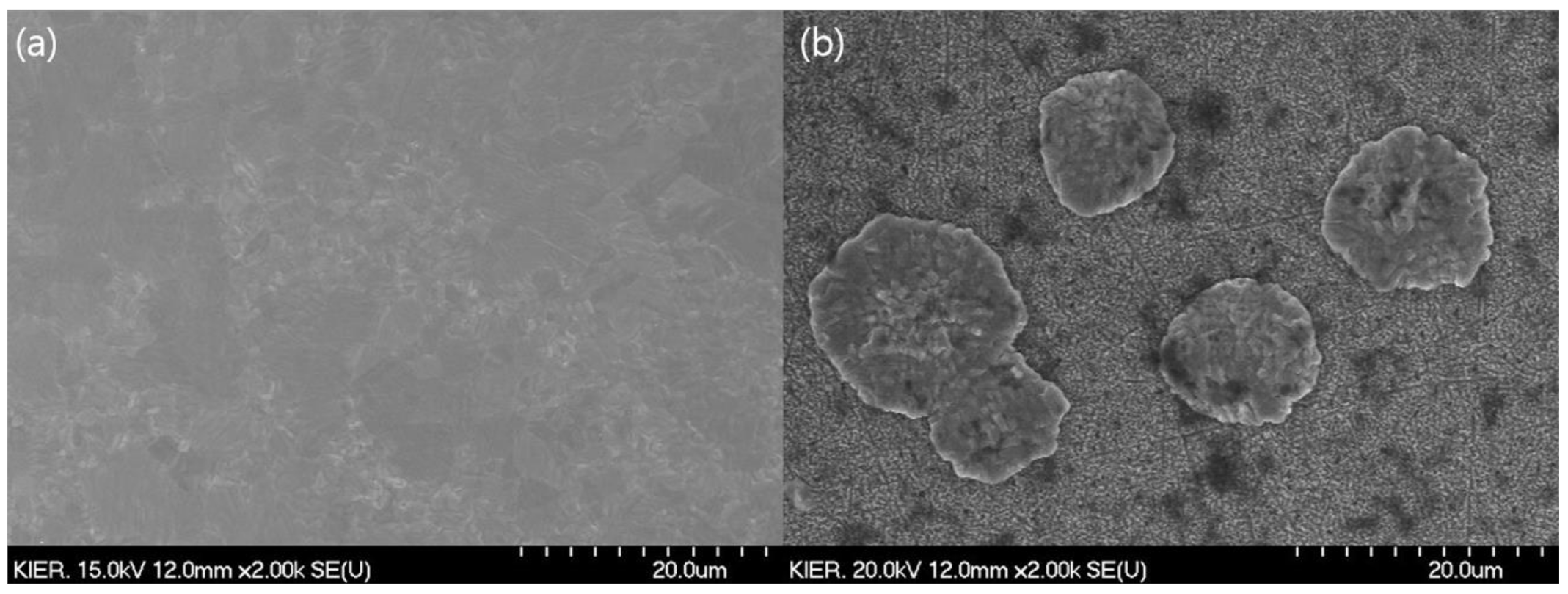
3.1. Characterization of Membrane and CPMM
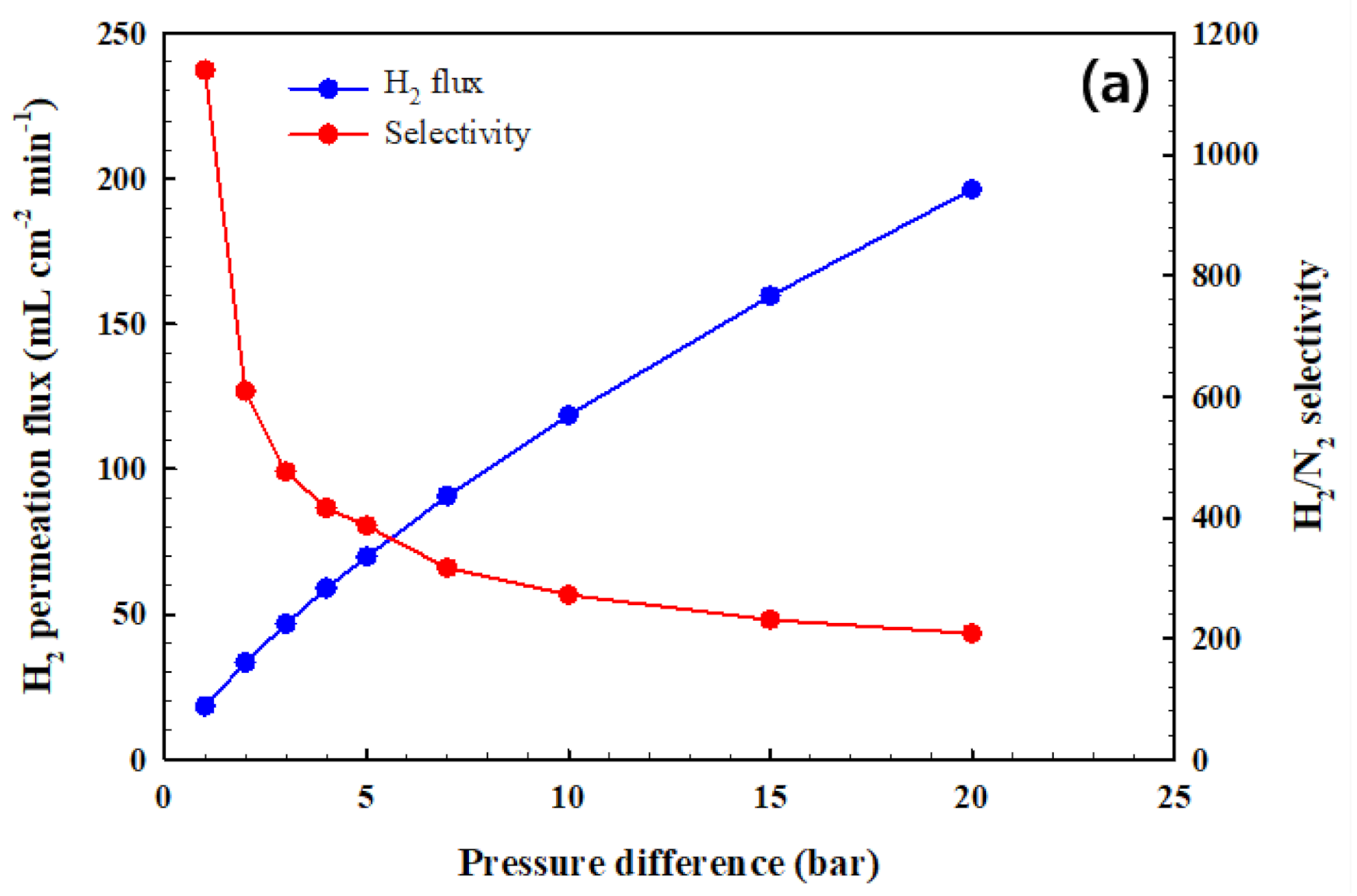
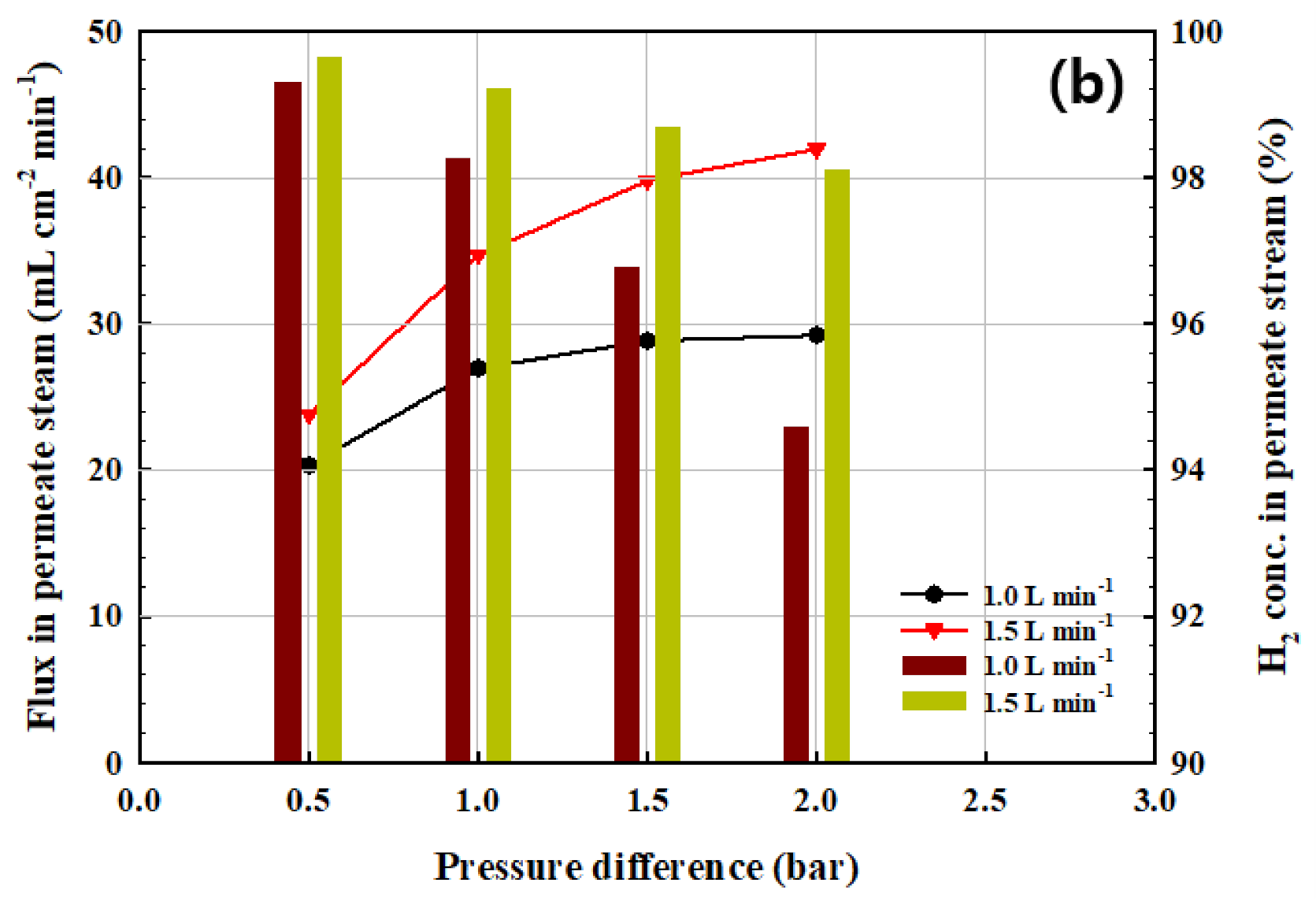
3.2. Hydrogen Permeation Test of the CPMM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiao, Y.; Chung, T.S. Grafting thermally labile molecules on cross-linkable polyimide to design membrane materials for natural gas purification and CO2 capture. Energy Environ. Sci. 2011, 4, 201–208. [Google Scholar] [CrossRef]
- Authayanun, S.; Aunsup, P.; Patcharavorachot, Y.; Arpornwichanop, A. Theoretical analysis of a biogas-fed PEMFC system with different hydrogen purifications: Conventional and membrane-based water gas shift processes. Energy Convers. Manag. 2014, 86, 60–69. [Google Scholar] [CrossRef]
- Alenazey, F.; Alyousef, Y.; Almisned, O.; Almutairi, G.; Ghouse, M.; Montinaro, D.; Ghigliazza, F. Production of synthesis gas (H2 and CO) by high-temperature Co-electrolysis of H2O and CO2. Int. J. Hydrogen Energy 2015, 40, 10274–10280. [Google Scholar] [CrossRef]
- Rothenberger, K.S.; Cugini, A.V.; Howard, B.H.; Killmeyer, R.P.; Ciocco, M.V.; Morreale, B.D.; Enick, R.M.; Bustamante, F.; Mardilovich, I.P.; Ma, Y.H. High pressure hydrogen permeance of porous stainless steel coated with a thin palladium film via electroless plating. J. Membr. Sci. 2004, 244, 55–68. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The hydrogen issue. ChemSusChem 2011, 4, 21–36. [Google Scholar] [CrossRef]
- Shimura, K.; Yoshida, H. Heterogeneous photocatalytic hydrogen production from water and biomass derivatives. Energy Environ. Sci. 2011, 4, 2467–2481. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today. 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Schell, J.; Casas, N.; Marx, D.; Mazzotti, M. Precombustion CO2 capture by pressure swing adsorption (PSA): Comparison of laboratory PSA experiments and simulations. Ind. Eng. Chem. Res. 2013, 52, 8311–8322. [Google Scholar] [CrossRef]
- You, Y.W.; Lee, D.G.; Yoon, K.Y.; Moon, D.K.; Kim, S.M.; Lee, C.H. H2 PSA purifier for CO removal from hydrogen mixtures. Int. J. Hydrogen Energy 2012, 37, 18175–18186. [Google Scholar] [CrossRef]
- Chen, W.H.; Hsu, P.C. Hydrogen permeation measurements of Pd and Pd-Cu membranes using dynamic pressure difference method. Int. J. Hydrogen Energy 2011, 36, 9355–9366. [Google Scholar] [CrossRef]
- Psara, N.; Van Sint Annaland, M.; Gallucci, F. Hydrogen safety risk assessment methodology applied to a fluidized bed membrane reactor for autothermal reforming of natural gas. Int. J. Hydrogen Energy 2015, 40, 10090–10102. [Google Scholar] [CrossRef]
- Tong, J.; Suda, H.; Haraya, K.; Matsumura, Y. A novel method for the preparation of thin dense Pd membrane on macroporous stainless steel tube filter. J. Membr. Sci. 2005, 260, 10–18. [Google Scholar] [CrossRef]
- Mardilovich, I.P.; Castro-Dominguez, B.; Kazantzis, N.K.; Wu, T.; Ma, Y.H. A comprehensive performance assessment study of pilot-scale Pd and Pd/alloy membranes under extended coal-derived syngas atmosphere testing. Int. J. Hydrogen Energy 2015, 40, 6107–6117. [Google Scholar] [CrossRef]
- Ryi, S.K.; Park, J.S.; Kim, S.H.; Cho, S.H.; Kim, D.W. The effect of support resistance on the hydrogen permeation behavior in Pd-Cu-Ni ternary alloy membrane deposited on a porous nickel support. J. Membr. Sci. 2006, 280, 883–888. [Google Scholar] [CrossRef]
- Kurokawa, H.; Yakabe, H.; Yasuda, I.; Peters, T.; Bredesen, R. Inhibition effect of CO on hydrogen permeability of Pd-Ag membrane applied in a microchannel module configuration. Int. J. Hydrogen Energy 2014, 39, 17201–17209. [Google Scholar] [CrossRef]
- Xie, D.; Yu, J.; Wang, F.; Zhang, N.; Wang, W.; Yu, H.; Peng, F.; Park, A.H.A. Hydrogen permeability of Pd-Ag membrane modules with porous stainless steel substrates. Int. J. Hydrogen Energy 2011, 36, 1014–1026. [Google Scholar] [CrossRef]
- Ryi, S.K.; Park, J.S.; Hwang, K.R.; Lee, C.B.; Lee, S.W. The property of hydrogen separation from CO2 mixture using Pd-based membranes for carbon capture and storage (CCS). Int. J. Hydrogen Energy 2013, 38, 7605–7611. [Google Scholar] [CrossRef]
- Borgognoni, F.; Tosti, S. Multi-tube Pd-Ag membrane module for pure hydrogen production: Comparison of methane steam and oxidative reforming. Int. J. Hydrogen Energy 2013, 38, 8276–8284. [Google Scholar] [CrossRef]
- Maneerung, T.; Hidajat, K.; Kawi, S. Ultra-thin (<1 μm) internally-coated Pd-Ag alloy hollow fiber membrane with superior thermal stability and durability for high temperature H2 separation. J. Membr. Sci. 2014, 452, 127–142. [Google Scholar] [CrossRef]
- Ryi, S.K.; Park, J.S.; Choi, S.H.; Cho, S.H.; Kim, S.H. Novel micro fuel processor for PEMFCs with heat generation by catalytic combustion. Chem. Eng. J. 2005, 113, 47–53. [Google Scholar] [CrossRef]
- Li, S.X.; Xuan, F.Z.; Tu, S.T.; Yu, S.R. Microstructure evolution and interfacial failure mechanism in 316LSS diffusion-bonded joints. J. Mater. Sci. Eng. A. 2008, 491, 488–491. [Google Scholar] [CrossRef]
- Hwang, K.R.; Ryi, S.K.; Lee, C.B.; Lee, S.W.; Park, J.S. Simplified, plate-type Pd membrane module for hydrogen purification. Int. J. Hydrogen Energy 2011, 36, 10136–10140. [Google Scholar] [CrossRef]
- Lee, S.W.; Oh, D.K.; Park, J.W.; Lee, C.B.; Lee, D.W.; Park, J.S.; Kim, S.H.; Hwang, K.R. Effect of a Pt-ZrO2 protection layer on the performance and morphology of Pd-Au alloy membrane during H2S exposure. J. Alloys Compd. 2015, 641, 210–215. [Google Scholar] [CrossRef]
- Hwang, K.R.; Lee, S.W.; Ryi, S.K.; Kim, D.K.; Kim, T.H.; Park, J.S. Water-gas shift reaction in a plate-type Pd-membrane reactor over a nickel metal catalyst. Fuel Process. Technol. 2013, 106, 133–140. [Google Scholar] [CrossRef]
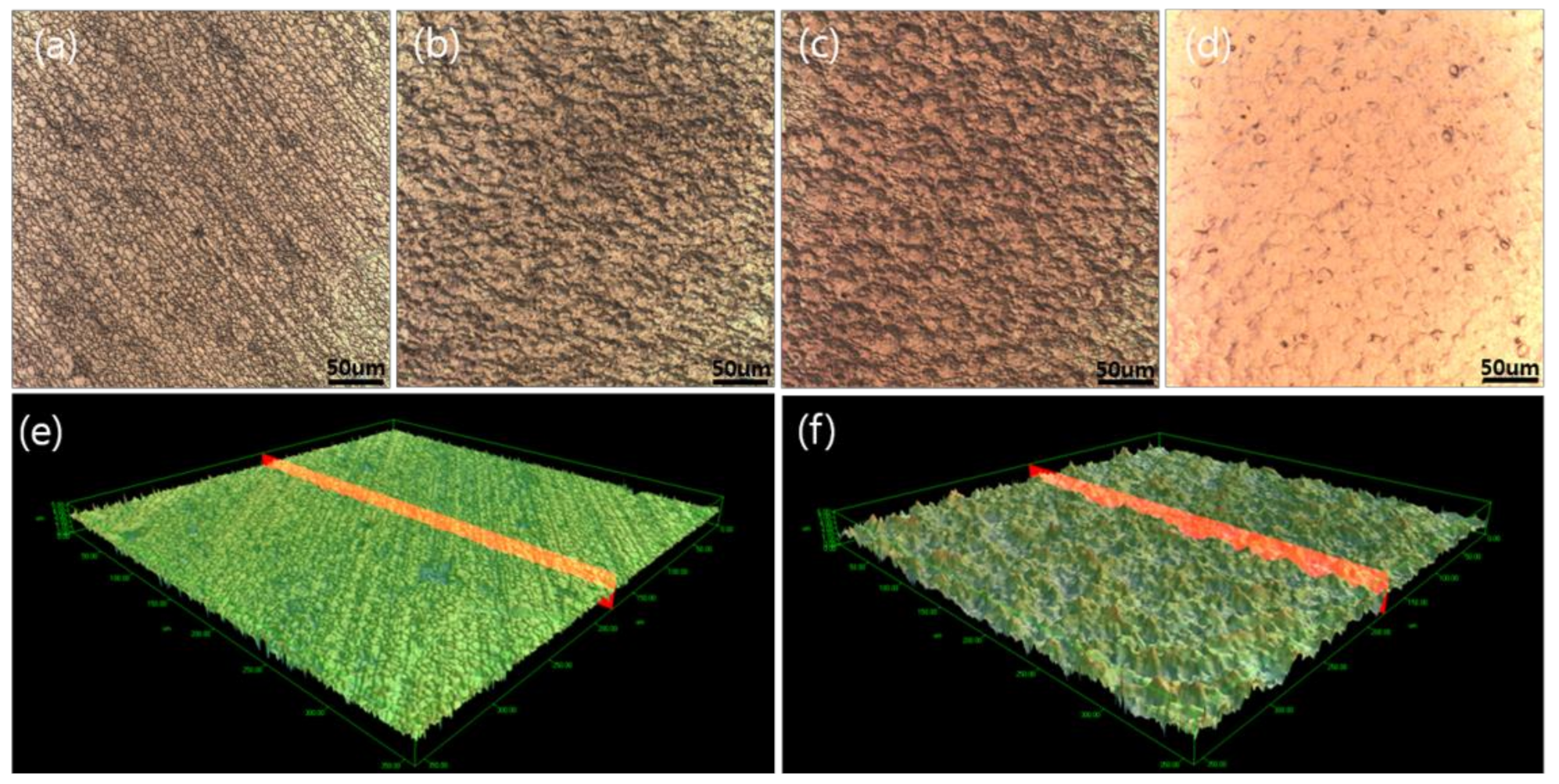
| Process | Ra (µm) | Surface Area (mm2) |
|---|---|---|
| Fresh | 0.167 | 0.128 |
| Peening | 0.446 | 0.132 |
| Ni-Cu deposition | 0.582 | 0.133 |
| Thermal treatment | 0.520 | 0.132 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, D.-K.; Lee, K.-Y.; Park, J.-S. Hydrogen Purification from Compact Palladium Membrane Module Using a Low Temperature Diffusion Bonding Technology. Membranes 2020, 10, 338. https://doi.org/10.3390/membranes10110338
Oh D-K, Lee K-Y, Park J-S. Hydrogen Purification from Compact Palladium Membrane Module Using a Low Temperature Diffusion Bonding Technology. Membranes. 2020; 10(11):338. https://doi.org/10.3390/membranes10110338
Chicago/Turabian StyleOh, Duck-Kyu, Kwan-Young Lee, and Jong-Soo Park. 2020. "Hydrogen Purification from Compact Palladium Membrane Module Using a Low Temperature Diffusion Bonding Technology" Membranes 10, no. 11: 338. https://doi.org/10.3390/membranes10110338
APA StyleOh, D.-K., Lee, K.-Y., & Park, J.-S. (2020). Hydrogen Purification from Compact Palladium Membrane Module Using a Low Temperature Diffusion Bonding Technology. Membranes, 10(11), 338. https://doi.org/10.3390/membranes10110338





