Clarification of 1,3-Propanediol Fermentation Broths by Using a Ceramic Fine UF Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Fermentation
2.2. Ultrafiltration Unit
2.3. Operating Conditions and Procedure of Membrane Cleaning
2.4. Analytical Methods
2.5. Resistance-in-Series Analysis
3. Results
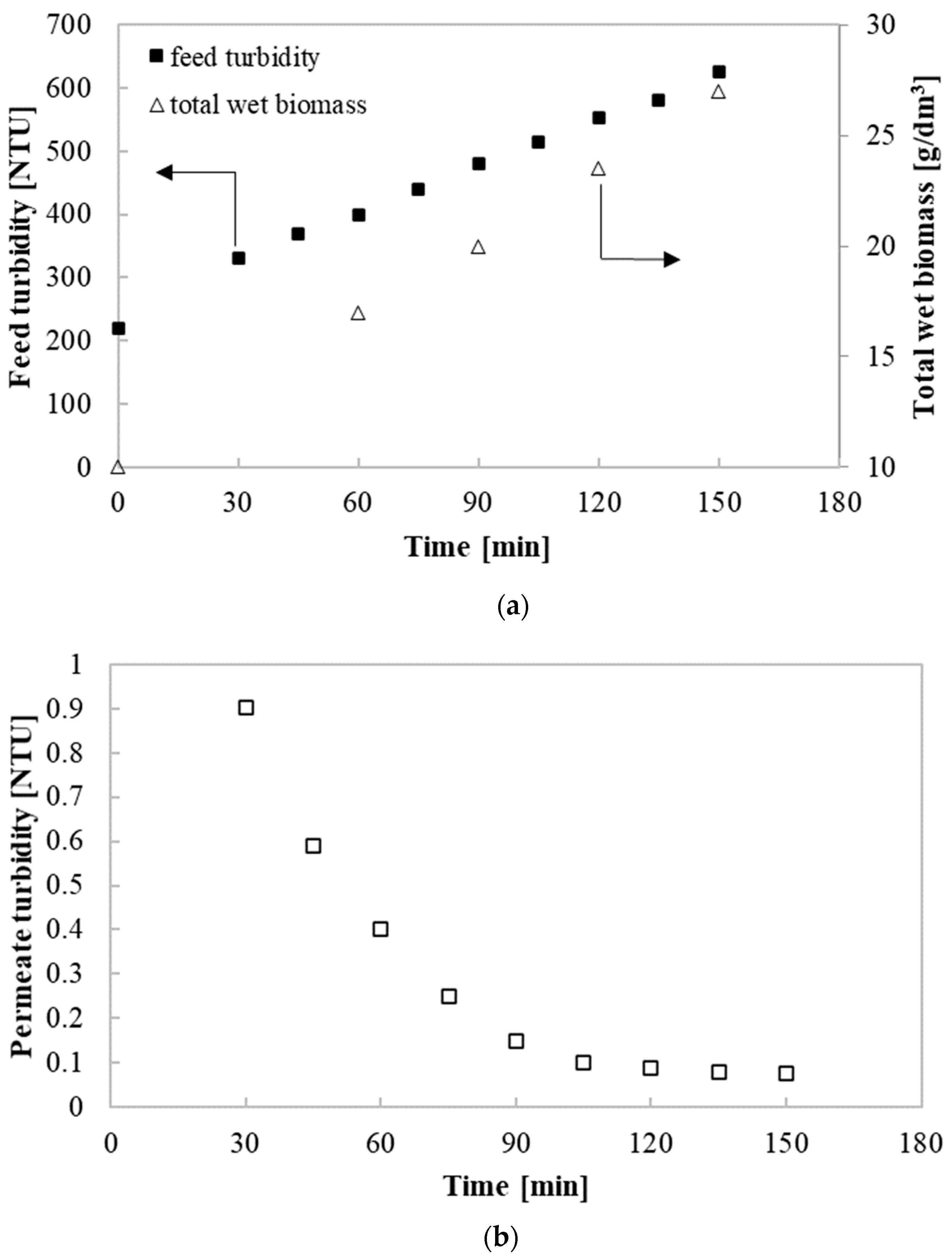
3.1. Physico-Chemical Characterzization of the Post-Fermentation Solutions
3.2. Purification Efficiency
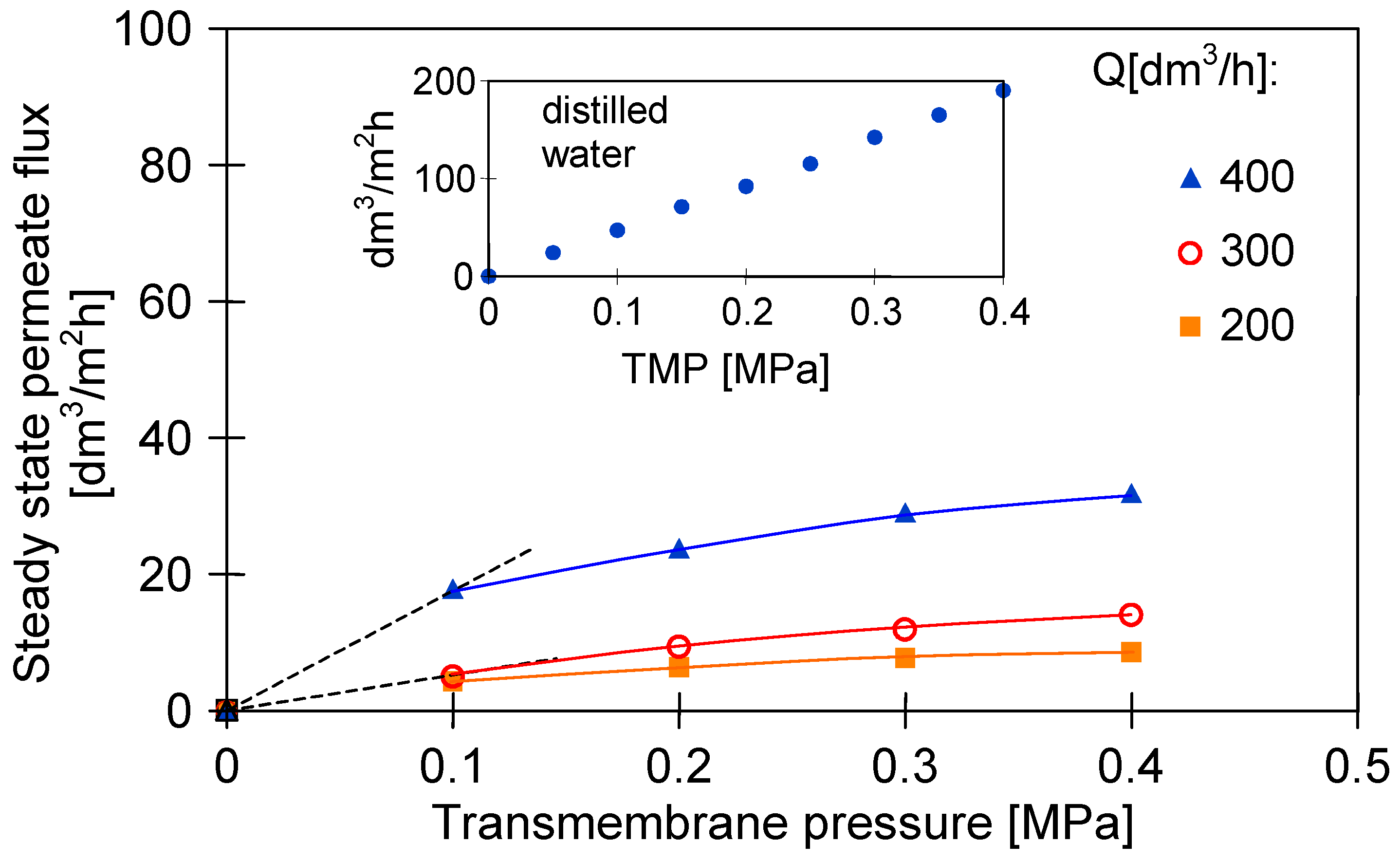
3.3. Impact of Transmembrane Pressure and Feed Flow Rate on the Permeate Flux
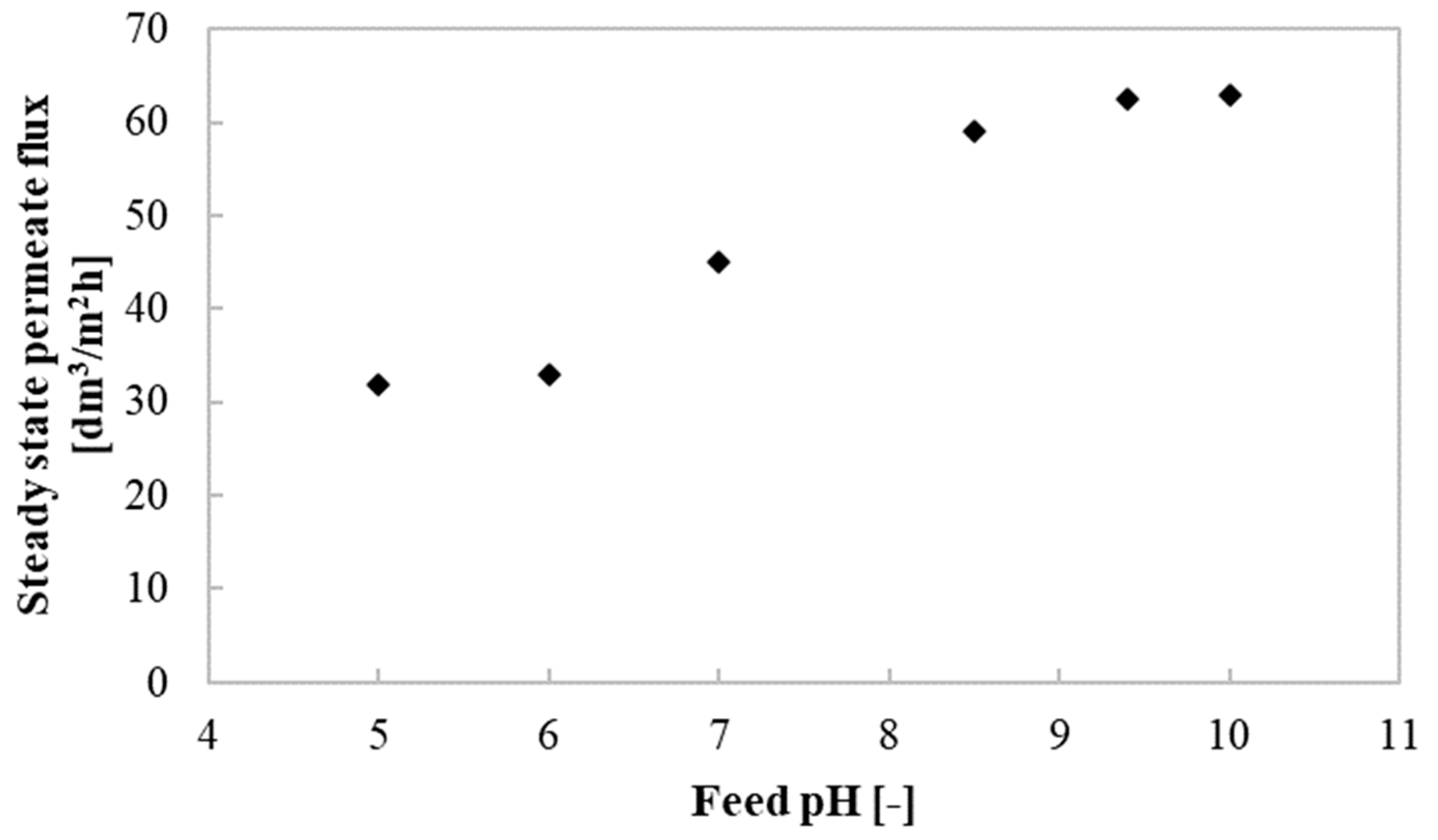
3.4. Impact of Feed pH on the Permeate Flux
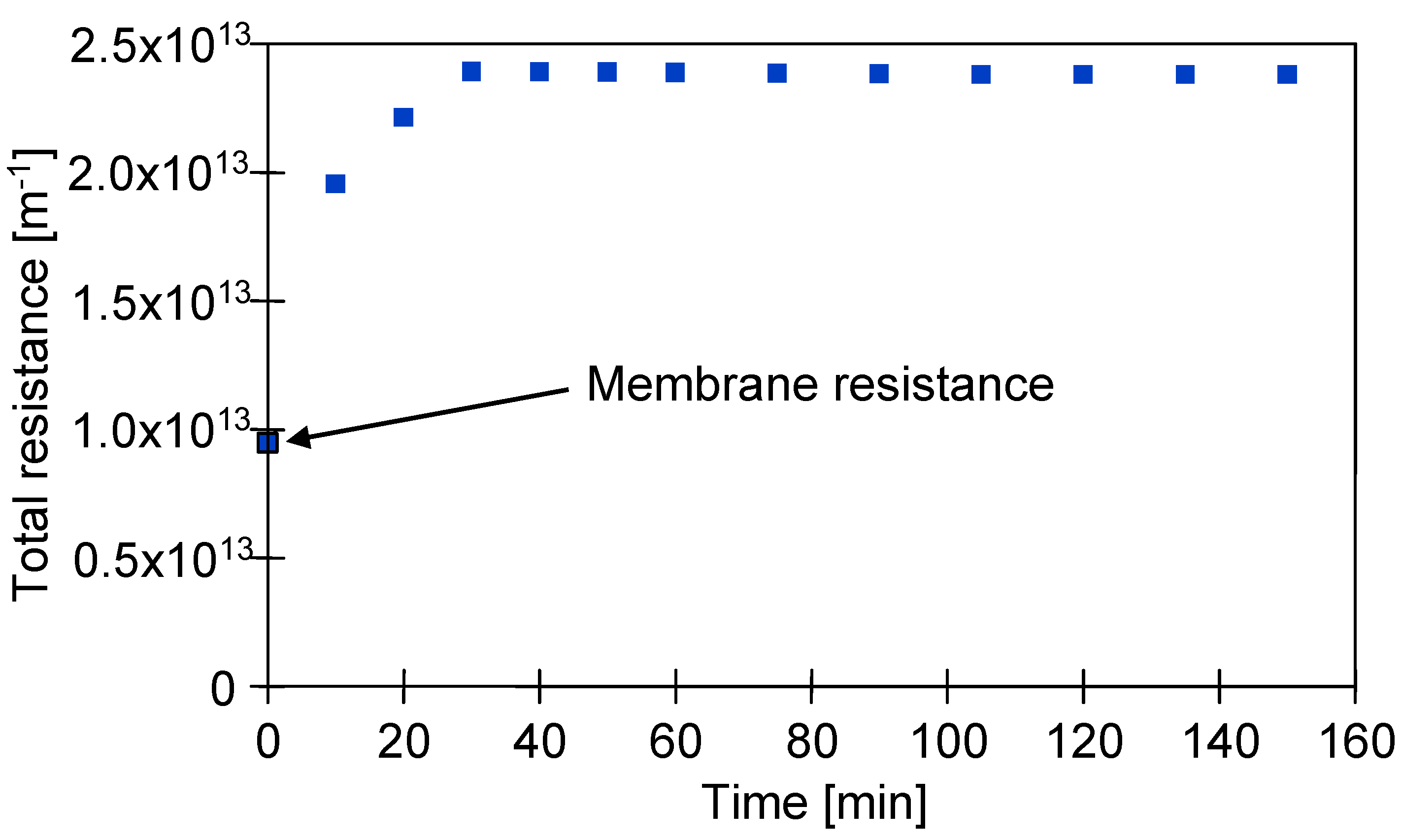
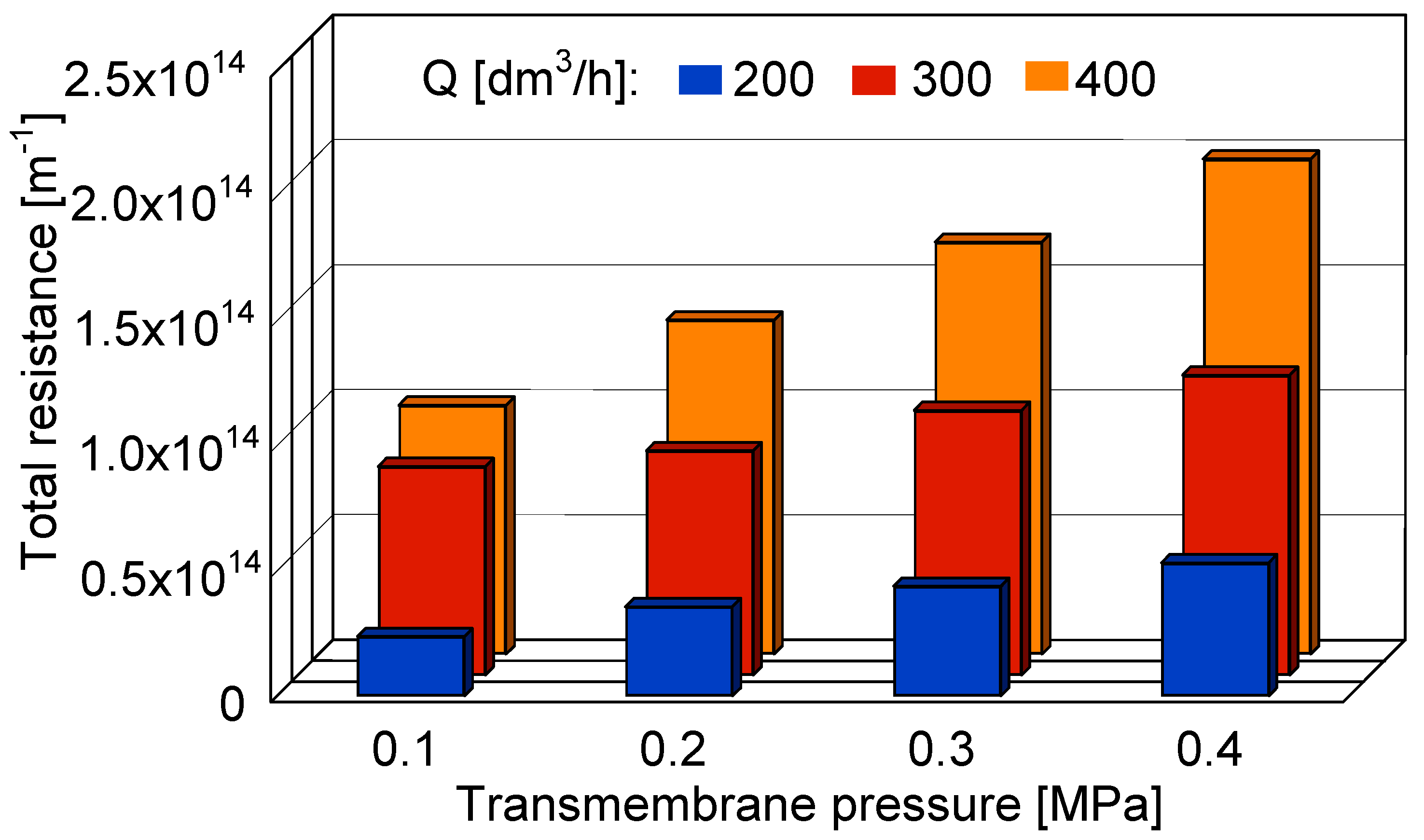
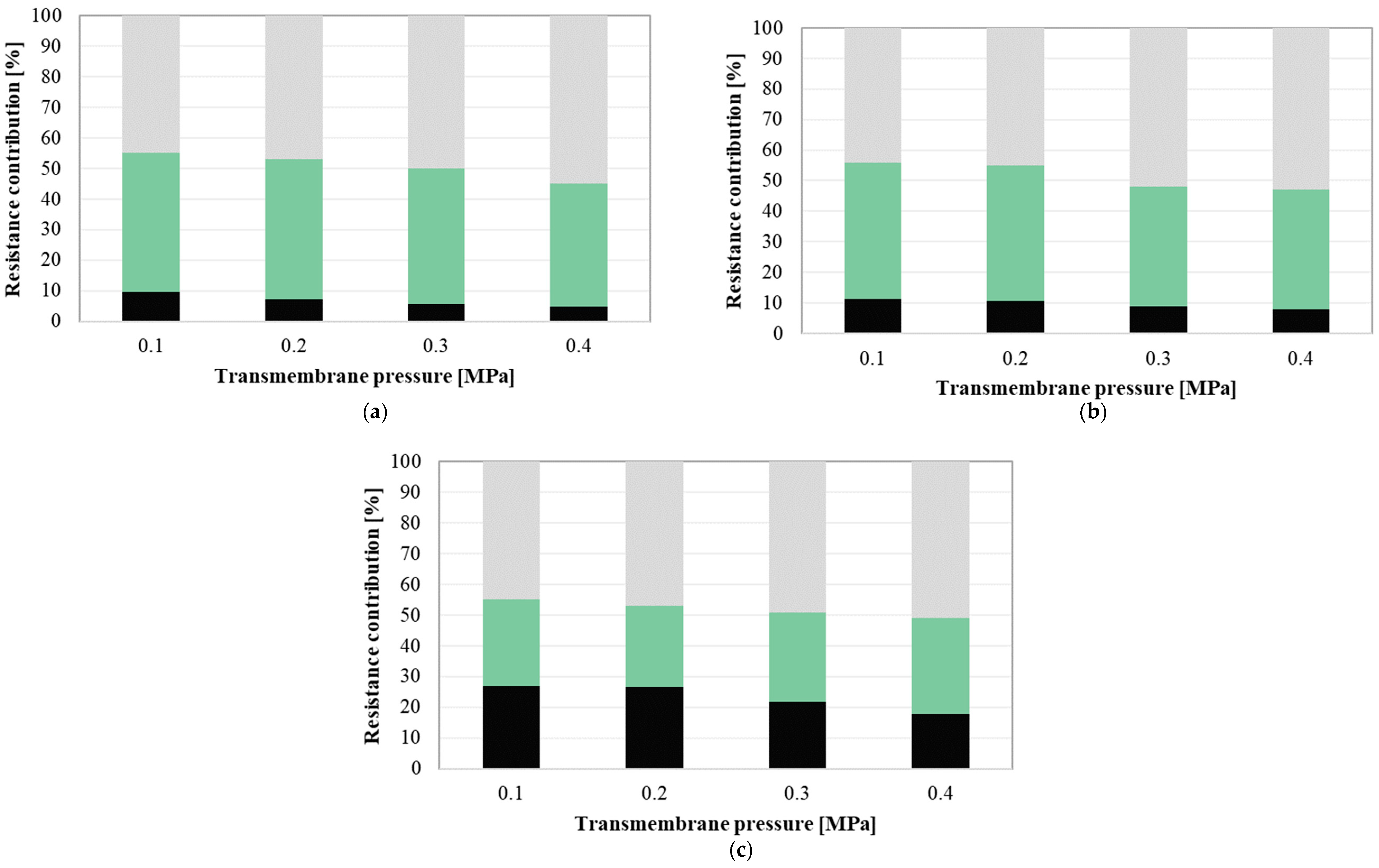
3.5. Resistance Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Fermentation Broth Characteristic | Membrane Characteristic | UF Process | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Microorganism | Main product | pH | Module | Material | MWCO (kDa) | Surface Area (m2) | Resistance (m−1) | T (°C) | TMP (MPa) | u (m/s) or Q (dm3/h) | |
| Bacillus subtilis ATCC 21332 | surfactin | NI | cross-flow | RC, PES | 10 | 0.0050 | NI | room | 0.20 | NI | [41] |
| Bacillus subtilis ATCC 21332 | surfactin | NI | flat-plate | PES and CE | 100 | 0.0065 | NI | 25 | 0.02–0.10 | u: 0.16–0.48 m/s | [42] |
| Bacillus subtilis ATCC 21332 | surfactin | 7.0 | flat-plate | PES | 100 | 0.0065 | 2.88 × 1012 | 25 | 0.02–0.10 | 0.32 | [43] |
| Bacillus subtilis ATCC 21332 | surfactin | 7.0 | cross-flow | PES, HT | 10 | 0.0200 | NI | room | 0.05–0.20 | NI | [44] |
| Bacillus subtilis ATCC 21332 | surfactin | NI | hollow fiber | cellulose | 1–100 | NI | NI | NI | 0.07–0.21 | NI | [45] |
| NI | clavulanic acid | 4.5 | tubular | ceramic | 15; 150 | NI | NI | 15 | 0.20 | NI | [46] |
| NI | clavulanic acid | 4.5 | flat-plate | organic | 5; 20 | NI | NI | 15 | 0.20 | NI | [46] |
| Serratia marcescens SMΔR | prodigiosin | NI | disc | YM | 1; 10 | 0.0048 | 3.06 × 1012 | 25 | 0.07–0.21 | NI | [47] |
| Serratia marcescens SMΔR | prodigiosin | NI | disc | PES | 1; 10 | 0.0048 | 3.11 × 1011 | 25 | 0.07–0.21 | NI | [47] |
| Lactococcus lactis spp. lactis ATCC 19 435 | lactic acid | 6.0 | cross-flow | PES | 25 | 0.0500 | NI | 30 | 0.08 | NI | [48] |
| Lactococcus lactis spp. lactis ATCC 19 435 | lactic acid | 6.0 | cross-flow | cellulose | 10; 20; 30 | 0.0500 | NI | 30 | 0.08 | NI | [48] |
| Bifidobacteria longum | lactic acid | 6.5 | cross-flow | PES | 5; 20 | 0.0200 | NI | 21; 37 | 0.07–0.42 | u: 1.00; 2.00 m/s | [49] |
| Lactobacillus delbrueckii ssp. lactis | lactic acid | 6.2 | tubular | ceramic | 300 | NI | 4.86 × 1011 | NI | NI | NI | [50] |
| Actinobacillus succinogenes BE-1 | succinic acid | NI | flat | PES | 10; 30; 100 | 0.0045 | NI | room | 0.20 | NI | [51] |
| Actinobacillus succinogenes BE-1 | succinic acid | NI | flat | RC | 10 | 0.0045 | NI | room | 0.20 | NI | [51] |
| Streptomyces aureofaciens | demethylchlortetracycline | 6.16 | plate and frame | fluoro polymer | 100 | 0.1800 | NI | 15 | 0.3–0.4 | NI | [52] |
| Streptomyces aureofaciens | demethylchlortetracycline | 6.16 | tubular | PVDF | 100 | 0.9000 | NI | 15 | 0.3–0.4 | NI | [52] |
| Streptococcus zooepidemicus | hyaluronic acid | 4.0–7.0 | flat-plate | PVDF | 100; 300 | 0.0010 | NI | 25 | 0.05–0.20 | 0.25–2.50 | [53] |
| Xanthomonas campestris NRRL B-1459 | xanthan | 4.0–10.0 | tutbular | PS | 500 | 0.0650 | NIa | 30 | 0.07; 0.14; 0.19 | Q: 6–60 dm3/h | [54] |
| Citrobacter freundii | 1,3-propanediol | NI | tubular | ceramic | 8 | 0.0038 | 2.62 × 109 | 35 | 0.10–0.25 | u: 3.64–6.80 m/s | [55] |
| Bacillus thuringiensis | - | NI | plate and frame | PES | 6; 10 | NI | NI | NI | 0.07–0.21 | Q: 40–120 dm3/h | [57] |
References
- Ahmed, H.; Naouel, O. 1,3-Propanediol production by Clostridium Butyricum in various fed- batch feeding strategies. J. Biotechnol. Biomater. 2012, 2, 134. [Google Scholar] [CrossRef]
- Dobson, R.; Gray, V.; Rumbold, K. Microbial utilization of crude glycerol for the production of value-added products. J. Ind. Microbiol. Biotechnol. 2012, 39, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Homann, T.; Tag, C.; Biebl, H.; Deckwer, W.-D.; Schink, B. Fermentation of glycerol to 1,3-propanediol by Klebsiella and Citrobacter strains. Appl. Microbiol. Biotechnol. 1990, 33, 121–126. [Google Scholar] [CrossRef]
- Lee, C.S.; Aroua, M.K.; Daud, W.M.A.W.; Cognet, P.; Pérès-Lucchese, Y.; Fabre, P.-L.; Reynes, O.; Latapie, L. A review: Conversion of bioglycerol into 1,3-propanediol via biological and chemical method. Renew. Sustain. Energy Rev. 2015, 42, 963–972. [Google Scholar] [CrossRef]
- da Silva Ruy, A.D.; de Brito Alves, R.M.; Reis Hewer, T.L.; de Aguiar Pontes, D.; Gomes Teixeira, L.S.; Magalhães Pontes, L.A. Catalysts for glycerol hydrogenolysis to 1,3-propanediol: A review of chemical routes and market. Catal. Today 2020. [Google Scholar] [CrossRef]
- Sivasankaran, C.; Ramanujam, P.K.; Balasubramanian, B.; Mani, J. Recent progress on transforming crude glycerol into high value chemicals: A critical review. Biofuels 2019, 10, 309–314. [Google Scholar] [CrossRef]
- Deckwer, W.-D. Microbial conversion of glycerol to 1,3-propanediol. FEMS Microbiol. Rev. 1995, 16, 143–149. [Google Scholar] [CrossRef]
- Jolly, J.; Hitzmann, B.; Ramalingam, S.; Ramachandran, K.B. Biosynthesis of 1,3-propanediol from glycerol with Lactobacillus reuteri: Effect of operating variables. J. Biosci. Bioeng. 2014, 118, 188–194. [Google Scholar] [CrossRef]
- Ju, J.-H.; Wang, D.; Heo, S.-Y.; Kim, M.-S.; Seo, J.-W.; Kim, Y.-M.; Kim, D.-H.; Kang, S.-A.; Kim, C.-H.; Oh, B.-R. Enhancement of 1,3-propanediol production from industrial by-product by Lactobacillus reuteri CH53. Microb. Cell Factories 2020, 19, 6. [Google Scholar] [CrossRef]
- Mendes, F.S.; González-Pajuelo, M.; Cordier, H.; François, J.M.; Vasconcelos, I. 1,3-Propanediol production in a two-step process fermentation from renewable feedstock. Appl. Microbiol. Biotechnol. 2011, 92, 519–527. [Google Scholar] [CrossRef]
- Markets and MarketsTM. Available online: https://www.globenewswire.com/news-release/2019/12/10/1958828/0/en/1-3-Propanediol-PDO-Markets-Global-Analysis-Insights-Trends-and-Opportunities-to-2024.html (accessed on 28 October 2020).
- Yang, X.; Kim, D.S.; Choi, H.S.; Kim, C.K.; Thapa, L.P.; Park, C.; Kim, S.W. Repeated batch production of 1,3-propanediol from biodiesel derived waste glycerol by Klebsiella pneumoniae. Chem. Eng. J. 2017, 314, 660–669. [Google Scholar] [CrossRef]
- Zhu, C.; Fang, B.; Wang, S. Effects of culture conditions on the kinetic behavior of 1,3-propanediol fermentation by Clostridium butyricum with a kinetic model. Bioresour. Technol. 2016, 212, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yun, J.; Zhang, H.; Magocha, H.Z.; Zabed, H.; Xue, Y.; Fokum, E.; Sun, W.; Qi, X. Genetically engineered strains: Application and advances for 1,3-Propanediol production from glycerol. Food Technol. Biotechnol. 2018, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lama, S.; Sankaranarayanan, M.; Park, S. Metabolic engineering of Pseudomonas denitrificans for the 1,3-propanediol production from glycerol. Bioresour. Technol. 2019, 292, 121933. [Google Scholar] [CrossRef]
- Crosse, A.J.; Brady, D.; Zhou, N.; Rumbold, K. Biodiesel’s trash is a biorefineries’ treasure: The use of “dirty” glycerol as an industrial fermentation substrate. World J. Microbiol. Biotechnol. 2020, 36, 2. [Google Scholar] [CrossRef] [PubMed]
- Boenigk, R.; Bowien, S.; Gottschalk, G. Fermentation of glycerol to 1,3-propanediol in continuous cultures of Citrobacter freundii. Appl. Microbiol. Biotechnol. 1993, 38, 453–457. [Google Scholar] [CrossRef]
- Celińska, E.; Drożdżyńska, A.; Jankowska, M.; Białas, W.; Czaczyk, K.; Grajek, W. Genetic engineering to improve 1,3-propanediol production in an isolated Citrobacter freundii strain. Process Biochem. 2015, 50, 48–60. [Google Scholar] [CrossRef]
- Metsoviti, M.; Zeng, A.-P.; Koutinas, A.A.; Papanikolaou, S. Enhanced 1,3-propanediol production by a newly isolated Citrobacter freundii strain cultivated on biodiesel-derived waste glycerol through sterile and non-sterile bioprocesses. J. Biotechnol. 2013, 163, 408–418. [Google Scholar] [CrossRef]
- Maina, S.; Kachrimanidou, V.; Ladakis, D.; Papanikolaou, S.; de Castro, A.M.; Koutinas, A. Evaluation of 1,3-propanediol production by twoCitrobacter freundiistrains using crude glycerol and soybean cake hydrolysate. Environ. Sci. Pollut. Res. 2019, 26, 35523–35532. [Google Scholar] [CrossRef]
- Laura, M.; Monica, T.; Dan-Cristian, V. The effect of crude glycerol impurities on 1,3-propanediol biosynthesis by Klebsiella pneumoniae DSMZ 2026. Renew. Energy 2020, 153, 1418–1427. [Google Scholar] [CrossRef]
- Durgapal, M.; Kumar, V.; Yang, T.H.; Lee, H.J.; Seung, D.; Park, S. Production of 1,3-propanediol from glycerol using the newly isolated Klebsiella pneumoniae J2B. Bioresour. Technol. 2014, 159, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Srivastava, A.K.; Chand, S. Determination of kinetic parameters of 1,3-propanediol fermentation by Clostridium diolis using statistically optimized medium. Bioprocess Biosyst. Eng. 2012, 35, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska-Powałowska, D.; Białas, W. Scale-up of anaerobic 1,3-propanediol production by Clostridium butyricum DSP1 from crude glycerol. BMC Microbiol. 2014, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Pflügl, S.; Marx, H.; Mattanovich, D.; Sauer, M. 1,3-Propanediol production from glycerol with Lactobacillus diolivorans. Bioresour. Technol. 2012, 119, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.A.; Russo, A.; Pisano, I.; Palmieri, L.; de Angelis, M.; Agrimi, G. Improved 1,3-Propanediol synthesis from glycerol by the robust Lactobacillus reuteri strain DSM 20016. J. Microbiol. Biotechnol. 2015, 25, 893–902. [Google Scholar] [CrossRef]
- Vivek, N.; Pandey, A.; Binod, P. Biological valorization of pure and crude glycerol into 1,3-propanediol using a novel isolate Lactobacillus brevis N1E9.3.3. Bioresour. Technol. 2016, 213, 222–230. [Google Scholar] [CrossRef]
- Barbirato, F.; Camarasa-Claret, C.; Grivet, J.P.; Bories, A. Glycerol fermentation by a new 1,3-propanediol-producing microorganism:Enterobacter agglomerans. Appl. Microbiol. Biotechnol. 1995, 43, 786–793. [Google Scholar] [CrossRef]
- Barbirato, F.; Grivet, J.P.; Soucaille, P.; Bories, A. 3-Hydroxypropionaldehyde, an inhibitory metabolite of glycerol fermentation to 1,3-propanediol by enterobacterial species. Appl. Environ. Microbiol. 1996, 62, 1448–1451. [Google Scholar] [CrossRef]
- Gryta, M.; Tomczak, W. Microfiltration of post-fermentation broth with backflushing membrane cleaning. Chem. Pap. 2015, 69, 544–552. [Google Scholar] [CrossRef]
- Gryta, M.; Bastrzyk, J.; Tomczak, W. The application of membrane processes for separation of post-fermentation glycerol solutions. In Membranes and Membrane Processes in Environmental Protection. Monographs of the Environmental Engineering Committee; Polish Academy of Sciences: Gliwice, Poland, 2014; Volume 119, pp. 241–250. ISBN 978-83-63714-18-5. [Google Scholar]
- Li, Z.; Jiang, B.; Zhang, D.; Xiu, Z. Aqueous two-phase extraction of 1,3-propanediol from glycerol-based fermentation broths. Sep. Purif. Technol. 2009, 66, 472–478. [Google Scholar] [CrossRef]
- Saxena, R.K.; Anand, P.; Saran, S.; Isar, J. Microbial production of 1,3-propanediol: Recent developments and emerging opportunities. Biotechnol. Adv. 2009, 27, 895–913. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Q.; Shen, J.-T.; Yan, L.; Zhou, J.-J.; Jiang, L.-L.; Chen, Y.; Yuan, J.-L.; Feng, E.; Xiu, Z.-L. Advances in bioconversion of glycerol to 1,3-propanediol: Prospects and challenges. Process Biochem. 2018, 71, 134–146. [Google Scholar] [CrossRef]
- Xiu, Z.-L.; Zeng, A.-P. Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol. Appl. Microbiol. Biotechnol. 2008, 78, 917–926. [Google Scholar] [CrossRef]
- Bastrzyk, J.; Gryta, M. Separation of post-fermentation glycerol solution by nanofiltration membrane distillation system. Desalin. Water Treat. 2015, 53, 319–329. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, L.; Dai, H.; Zeng, X.; Fang, B. Highly pure 1,3-propanediol: Separation and purification from crude glycerol-based fermentation. Eng. Life Sci. 2015, 15, 788–796. [Google Scholar] [CrossRef]
- Anand, P.; Saxena, R.K.; Marwah, R.G. A novel downstream process for 1,3-propanediol from glycerol-based fermentation. Appl. Microbiol. Biotechnol. 2011, 90, 1267–1276. [Google Scholar] [CrossRef]
- Cho, M.-H.; Joen, S.I.; Pyo, S.-H.; Mun, S.; Kim, J.-H. A novel separation and purification process for 1,3-propanediol. Process Biochem. 2006, 41, 739–744. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and tight ultrafiltration membranes for the recovery of polyphenols from agro-food by-products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef] [PubMed]
- Isa, M.H.M.; Frazier, R.A.; Jauregi, P. A further study of the recovery and purification of surfactin from fermentation broth by membrane filtration. Sep. Purif. Technol. 2008, 64, 176–182. [Google Scholar] [CrossRef]
- Chen, H.-L.; Chen, Y.-S.; Juang, R.-S. Flux decline and membrane cleaning in cross-flow ultrafiltration of treated fermentation broths for surfactin recovery. Sep. Purif. Technol. 2008, 62, 47–55. [Google Scholar] [CrossRef]
- Juang, R.; Chen, H.; Chen, Y. Resistance-in-series analysis in cross-flow ultrafiltration of fermentation broths of Bacillus subtilis culture. J. Membr. Sci. 2008, 323, 193–200. [Google Scholar] [CrossRef]
- Mubarak, M.Q.E.; Isa, M.H.M.; Hassan, A.R. Single-step cross-flow ultrafiltration for recovery and purification of surfactin produced by Bacillus subtilis ATCC 21332. In Proceedings of the International Conference on Chemical, Environment & Biological Sciences (CEBS-2014), Kuala Lumpur, Malaysia, 17–18 September 2014; International Institute of Chemical, Biological & Environmental Engineering: Kuala Lumpur, Malaysia, 2014. [Google Scholar] [CrossRef][Green Version]
- Lin, S.-C.; Jiang, H.-J. Recovery and purification of the lipopeptide biosurfactant of Bacillus subtilis by ultrafiltration. Biotechnol. Tech. 1997, 11, 413–416. [Google Scholar] [CrossRef]
- Alves, A.M.B.; Morão, A.; Cardoso, J.P. Isolation of antibiotics from industrial fermentation broths using membrane technology. Desalination 2002, 148, 181–186. [Google Scholar] [CrossRef]
- Juang, R.-S.; Chen, H.-L.; Lin, Y.-C. Ultrafiltration of coagulation-pretreated Serratia marcescens fermentation broth: Flux characteristics and prodigiosin recovery. Sep. Sci. Technol. 2012, 47, 1849–1856. [Google Scholar] [CrossRef]
- Toräng, A.; Jönsson, A.-S.; Zacchi, G. Separation of cells and proteins from fermentation broth in a shear-enhanced cross-fow ultrafiltration module as the first step in the refinement of lactic acid. Appl. Biochem. Biotechnol. 1999, 76, 143–158. [Google Scholar] [CrossRef]
- Li, Y.; Shahbazi, A.; Kadzere, C.T. Separation of cells and proteins from fermentation broth using ultrafiltration. J. Food Eng. 2006, 75, 574–580. [Google Scholar] [CrossRef]
- Milcent, S. Clarification of lactic acid fermentation broths. Sep. Purif. Technol. 2001, 22–23, 393–401. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Tang, H.; Zhou, W.; Yan, D.; Xing, J.; Wan, Y. Clarification of succinic acid fermentation broth by ultrafiltration in succinic acid bio-refinery: Clarification of succinic acid fermentation broth by ultrafiltration. J. Chem. Technol. Biotechnol. 2013, 88, 444–448. [Google Scholar] [CrossRef]
- Morão, A. Ultrafiltration of demethylchlortetracycline industrial fermentation broths. Sep. Purif. Technol. 2001, 22–23, 459–466. [Google Scholar] [CrossRef]
- Zhou, H.; Ni, J.; Huang, W.; Zhang, J. Separation of hyaluronic acid from fermentation broth by tangential flow microfiltration and ultrafiltration. Sep. Purif. Technol. 2006, 52, 29–38. [Google Scholar] [CrossRef]
- Lo, Y.-M.; Yang, S.-T.; Min, D.B. Kinetic and feasibility studies of ultrafiltration of viscous xanthan gum fermentation broth. J. Membr. Sci. 1996, 117, 237–249. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. The application of ultrafiltration for separation of glycerol solution fermented by bacteria. Pol. J. Chem. Technol. 2013, 15, 115–120. [Google Scholar] [CrossRef]
- Schiraldi, C.; Carcarino, I.L.; Alfano, A.; Restaino, O.F.; Panariello, A.; De Rosa, M. Purification of chondroitin precursor from Escherichia coli K4 fermentation broth using membrane processing. Biotechnol. J. 2011, 6, 410–419. [Google Scholar] [CrossRef]
- Marzban, R.; Saberi, F.; Shirazi, M.M.A. Microfiltration and ultrafiltration of Bacillus thuringiensis fermentation broth: Membrane performance and spore-crystal recovery approaches. Braz. J. Chem. Eng. 2016, 33, 783–791. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Li, J.; Fu, X.; Wickramasinghe, R.; Chen, J. Chemical cleaning of PS ultrafilters fouled by the fermentation broth of glutamic acid. Sep. Purif. Technol. 2005, 42, 181–187. [Google Scholar] [CrossRef]
- Keller, K.; Friedmann, T.; Boxman, A. The bioseparation needs for tomorrow. Trends Biotechnol. 2001, 19, 438–441. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Y.; Lin, H.; Zhao, L.; Shen, L.; Li, R.; Xu, Y.; Hong, H.; He, Y. Membrane fouling caused by biological foams in a submerged membrane bioreactor: Mechanism insights. Water Res. 2020, 181, 115932. [Google Scholar] [CrossRef]
- Rao, L.; Tang, J.; Hu, S.; Shen, L.; Xu, Y.; Li, R.; Lin, H. Inkjet printing assisted electroless Ni plating to fabricate nickel coated polypropylene membrane with improved performance. J. Colloid Interface Sci. 2020, 565, 546–554. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, L.; Lin, H.; Yu, W.; Xu, Y.; Li, R.; Sun, T.; He, Y. A novel strategy based on magnetic field assisted preparation of magnetic and photocatalytic membranes with improved performance. J. Membr. Sci. 2020, 612, 118378. [Google Scholar] [CrossRef]
- Majewska-Nowak, K.M. Application of ceramic membranes for the separation of dye particles. Desalination 2010, 254, 185–191. [Google Scholar] [CrossRef]
- Ogunbiyi, O.O.; Miles, N.J.; Hilal, N. The effects of performance and cleaning cycles of new tubular ceramic microfiltration membrane fouled with a model yeast suspension. Desalination 2008, 220, 273–289. [Google Scholar] [CrossRef]
- Luque, S.; Gómez, D.; Álvarez, J.R. Industrial applications of porous ceramic membranes (pressure-driven processes). Membr. Sci. Technol. 2008, 13, 177–216. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Ashaghi, K.S.; Ebrahimi, M.; Czermak, P. Ceramic ultra- and nanofiltration membranes for oilfield produced water treatment: A mini review. Open Environ. J. 2007, 1, 1–8. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surf. Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Vladisavljević, G.T.; Vukosavljević, P.; Bukvić, B. Permeate flux and fouling resistance in ultrafiltration of depectinized apple juice using ceramic membranes. J. Food Eng. 2003, 60, 241–247. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.; Xie, T.; Wang, B.; An, L. Effect of mass transfer on heat transfer of microporous ceramic membranes for water recovery. Int. J. Heat Mass Transf. 2017, 112, 643–648. [Google Scholar] [CrossRef]
- Barredo-Damas, S.; Alcaina-Miranda, M.I.; Iborra-Clar, M.I.; Mendoza-Roca, J.A. Application of tubular ceramic ultrafiltration membranes for the treatment of integrated textile wastewaters. Chem. Eng. J. 2012, 192, 211–218. [Google Scholar] [CrossRef]
- Garcia-Ivars, J.; Durá-María, J.; Moscardó-Carreño, C.; Carbonell-Alcaina, C.; Alcaina-Miranda, M.-I.; Iborra-Clar, M.-I. Rejection of trace pharmaceutically active compounds present in municipal wastewaters using ceramic fine ultrafiltration membranes: Effect of feed solution pH and fouling phenomena. Sep. Purif. Technol. 2017, 175, 58–71. [Google Scholar] [CrossRef]
- Dashtban Kenari, S.L.; Barbeau, B. Understanding ultrafiltration fouling of ceramic and polymeric membranes caused by oxidized iron and manganese in water treatment. J. Membr. Sci. 2016, 516, 1–12. [Google Scholar] [CrossRef]
- Lobo, A.; Cambiella, Á.; Benito, J.M.; Pazos, C.; Coca, J. Ultrafiltration of oil-in-water emulsions with ceramic membranes: Influence of pH and crossflow velocity. J. Membr. Sci. 2006, 278, 328–334. [Google Scholar] [CrossRef]
- Narong, P.; James, A.E. Effect of the ζ-potential on the micro/ultra-filtration of yeast suspensions using ceramic membranes. Sep. Purif. Technol. 2006, 49, 149–156. [Google Scholar] [CrossRef]
- Árki, P.; Hecker, C.; Tomandl, G.; Joseph, Y. Streaming potential properties of ceramic nanofiltration membranes—Importance of surface charge on the ion rejection. Sep. Purif. Technol. 2019, 212, 660–669. [Google Scholar] [CrossRef]
- Kumar, C.M.; Roshni, M.; Vasanth, D. Treatment of aqueous bacterial solution using ceramic membrane prepared from cheaper clays: A detailed investigation of fouling and cleaning. J. Water Process Eng. 2019, 29, 100797. [Google Scholar] [CrossRef]
- Mestre, S.; Gozalbo, A.; Lorente-Ayza, M.M.; Sánchez, E. Low-cost ceramic membranes: A research opportunity for industrial application. J. Eur. Ceram. Soc. 2019, 39, 3392–3407. [Google Scholar] [CrossRef]
- Ng, T.C.A.; Lyu, Z.; Gu, Q.; Zhang, L.; Poh, W.J.; Zhang, Z.; Wang, J.; Ng, H.Y. Effect of gradient profile in ceramic membranes on filtration characteristics: Implications for membrane development. J. Membr. Sci. 2020, 595, 117576. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Cross-flow microfiltration of glycerol fermentation broths with Citrobacter freundii. Membranes 2020, 10, 67. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Ng, C.Y.; Lim, Y.P.; Ng, G.H. Ultrafiltration in food processing industry: Review on application, membrane fouling, and fouling control. Food Bioprocess Technol 2012, 5, 1143–1156. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Di Bella, G.; Di Trapani, D. A brief review on the resistance-in-series model in membrane bioreactors (MBRs). Membranes 2019, 9, 24. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; You, J.; Park, S.; Ahn, Y.; Jung, W.; Cho, K.H. Evaluation of fouling in nanofiltration for desalination using a resistance-in-series model and optical coherence tomography. Sci. Total Environ. 2018, 642, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, S.; Li, M.; Wang, R.; Zhao, Y. Purification of cellulase fermentation broth via low cost ceramic microfiltration membranes with nanofibers-like attapulgite separation layers. Sep. Purif. Technol. 2017, 175, 435–442. [Google Scholar] [CrossRef]
- Boyaval, P.; Lavenant, C.; Gésan, G.; Daufin, G. Transient and stationary operating conditions on performance of lactic acid bacteria crossflow microfiltration. Biotechnol. Bioeng. 1996, 49, 78–86. [Google Scholar] [CrossRef]
- Guo, H.; Huang, J.; Zhou, R.; Wu, C.; Jin, Y. Microfiltration of raw soy sauce: Membrane fouling mechanisms and characterization of physicochemical, aroma and shelf-life properties. RSC Adv. 2019, 9, 2928–2940. [Google Scholar] [CrossRef]
- Hassan, I.; Ennouri, M.; Lafforgue, C.; Schmitz, P.; Ayadi, A. Experimental study of membrane fouling during crossflow microfiltration of yeast and bacteria suspensions: Towards an analysis at the microscopic level. Membranes 2013, 3, 44–68. [Google Scholar] [CrossRef]
- Tomasula, P.M.; Mukhopadhyay, S.; Datta, N.; Porto-Fett, A.; Call, J.E.; Luchansky, J.B.; Renye, J.; Tunick, M. Pilot-scale crossflow-microfiltration and pasteurization to remove spores of Bacillus anthracis (Sterne) from milk. J. Dairy Sci. 2011, 94, 4277–4291. [Google Scholar] [CrossRef]
- Blanpain-Avet, P.; Faille, C.; Delaplace, G.; Bénézech, T. Cell adhesion and related fouling mechanism on a tubular ceramic microfiltration membrane using Bacillus cereus spores. J. Membr. Sci. 2011, 385–386, 200–216. [Google Scholar] [CrossRef]
- Al Aani, S.; Mustafa, T.N.; Hilal, N. Ultrafiltration membranes for wastewater and water process engineering: A comprehensive statistical review over the past decade. J. Water Process Eng. 2020, 35, 101241. [Google Scholar] [CrossRef]
- Field, R.W.; Wu, D.; Howell, J.A.; Gupta, B.B. Critical flux concept for microfiltration fouling. J. Membr. Sci. 1995, 100, 259–272. [Google Scholar] [CrossRef]
- Carrère, H.; Blaszkow, F. Comparison of operating modes for clarifying lactic acid fermentation broths by batch cross-flow microfiltration. Process Biochem. 2001, 36, 751–756. [Google Scholar] [CrossRef]
- Choi, H.; Zhang, K.; Dionysiou, D.D.; Oerther, D.B.; Sorial, G.A. Influence of cross-flow velocity on membrane performance during filtration of biological suspension. J. Membr. Sci. 2005, 248, 189–199. [Google Scholar] [CrossRef]
- Mullet, M.; Fievet, P.; Szymczyk, A.; Foissy, A.; Reggiani, J.-C.; Pagetti, J. A simple and accurate determination of the point of zero charge of ceramic membranes. Desalination 1999, 121, 41–48. [Google Scholar] [CrossRef]
- Bernat, X.; Pihlajamäki, A.; Fortuny, A.; Bengoa, C.; Stüber, F.; Fabregat, A.; Nyström, M.; Font, J. Non-enhanced ultrafiltration of iron(III) with commercial ceramic membranes. J. Membr. Sci. 2009, 334, 129–137. [Google Scholar] [CrossRef]
- De Angelis, L.; de Cortalezzi, M.M.F. Ceramic membrane filtration of organic compounds: Effect of concentration, pH, and mixtures interactions on fouling. Sep. Purif. Technol. 2013, 118, 762–775. [Google Scholar] [CrossRef]
- Moritz, T.; Benfer, S.; Árki, P.; Tomandl, G. Influence of the surface charge on the permeate flux in the dead-end filtration with ceramic membranes. Sep. Purif. Technol. 2001, 25, 501–508. [Google Scholar] [CrossRef]
- Xu, J.; Chang, C.-Y.; Hou, J.; Gao, C. Comparison of approaches to minimize fouling of a UF ceramic membrane in filtration of seawater. Chem. Eng. J. 2013, 223, 722–728. [Google Scholar] [CrossRef]
- Xu, J.; Chang, C.-Y.; Gao, C. Performance of a ceramic ultrafiltration membrane system in pretreatment to seawater desalination. Sep. Purif. Technol. 2010, 75, 165–173. [Google Scholar] [CrossRef]
- De la Rubia, Á.; Rodríguez, M.; Prats, D. pH, Ionic strength and flow velocity effects on the NOM filtration with TiO2/ZrO2 membranes. Sep. Purif. Technol. 2006, 52, 325–331. [Google Scholar] [CrossRef]
- Nor, M.Z.M. Separation of bromelain from crude pineapple waste mixture by a two-stage ceramic ultrafiltration process. Food Bioprod. Process. 2016, 98, 142–150. [Google Scholar] [CrossRef]
- Van Gestel, T.; Vandecasteele, C.; Buekenhoudt, A.; Dotremont, C.; Luyten, J.; Leysen, R.; Van der Bruggen, B.; Maes, G. Salt retention in nanofiltration with multilayer ceramic TiO2 membranes. J. Membr. Sci. 2002, 209, 379–389. [Google Scholar] [CrossRef]
- Labbez, C.; Fievet, P.; Szymczyk, A.; Thomas, F.; Simon, C.; Vidonne, A.; Pagetti, J.; Foissy, A. A comparison of membrane charge of a low nanofiltration ceramic membrane determined from ionic retention and tangential streaming potential measurements. Desalination 2002, 147, 223–229. [Google Scholar] [CrossRef]


| Parameter | Unit | Value |
|---|---|---|
| Number of channels | (-) | 1 |
| Cut-off | (Da) | 450 |
| External diameter | (mm) | 10 |
| Channel diameter | (mm) | 7 |
| Filtration area per 0.215 m tube | (m2) | 0.0047 |
| Inflow area per tube | (mm2) | 38.5 |
| Support material | (-) | α-Al2O3 |
| Support mean porosity, d50 | (μm) | 3 |
| Open porosity | (%) | 30–40 |
| Membrane material | (-) | TiO2 |
| Membrane mean porosity, d50 | (nm) | 0.9 |
| Symbol | Resistance | Determining Method | Formula | Comment |
|---|---|---|---|---|
| RT | total | experimental, based on steady-state flux for UF process | - | |
| Rm | clean membrane | experimental, based on water flux before UF experiments | ηw—distilled water viscosity | |
| Rirr | irreversible fouling | calculated, based on water flux after membrane rinsing | Jr—permeate flux for distilled water after membrane rinsing | |
| Rrev | reversible fouling | calculated, based on water flux after effective chemical cleaning of membrane | Rrev = RT − Rm − Rirr | - |
| Compound | 1,3-PD | Citric Acid | Lactic Acid | Acetic Acid |
|---|---|---|---|---|
| Chemical Formula | HO(CH2)3OH | HOC(COOH)(CH2COOH)2 | CH3CH(OH)COOH | CH3COOH |
| Concentration (g/L) | 9.03–12.73 | 1.83–2.22 | 0.59–0.91 | 0.38–2.59 |
| Ion | Cl− | NO3− | PO43− | SO42− | Na+ | NH4+ | K+ | Ca2+ | Mg2+ |
|---|---|---|---|---|---|---|---|---|---|
| Concentration (g/L) | 0.05–0.10 | 0.01–0.03 | 2.01–2.30 | 1.40–1.50 | 1.03–1.35 | 0.36–0.59 | 1.39–1.44 | 0.02–0.05 | 0.03–0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, W.; Gryta, M. Clarification of 1,3-Propanediol Fermentation Broths by Using a Ceramic Fine UF Membrane. Membranes 2020, 10, 319. https://doi.org/10.3390/membranes10110319
Tomczak W, Gryta M. Clarification of 1,3-Propanediol Fermentation Broths by Using a Ceramic Fine UF Membrane. Membranes. 2020; 10(11):319. https://doi.org/10.3390/membranes10110319
Chicago/Turabian StyleTomczak, Wirginia, and Marek Gryta. 2020. "Clarification of 1,3-Propanediol Fermentation Broths by Using a Ceramic Fine UF Membrane" Membranes 10, no. 11: 319. https://doi.org/10.3390/membranes10110319
APA StyleTomczak, W., & Gryta, M. (2020). Clarification of 1,3-Propanediol Fermentation Broths by Using a Ceramic Fine UF Membrane. Membranes, 10(11), 319. https://doi.org/10.3390/membranes10110319






