Microfluidics Used as a Tool to Understand and Optimize Membrane Filtration Processes
Abstract
1. Introduction
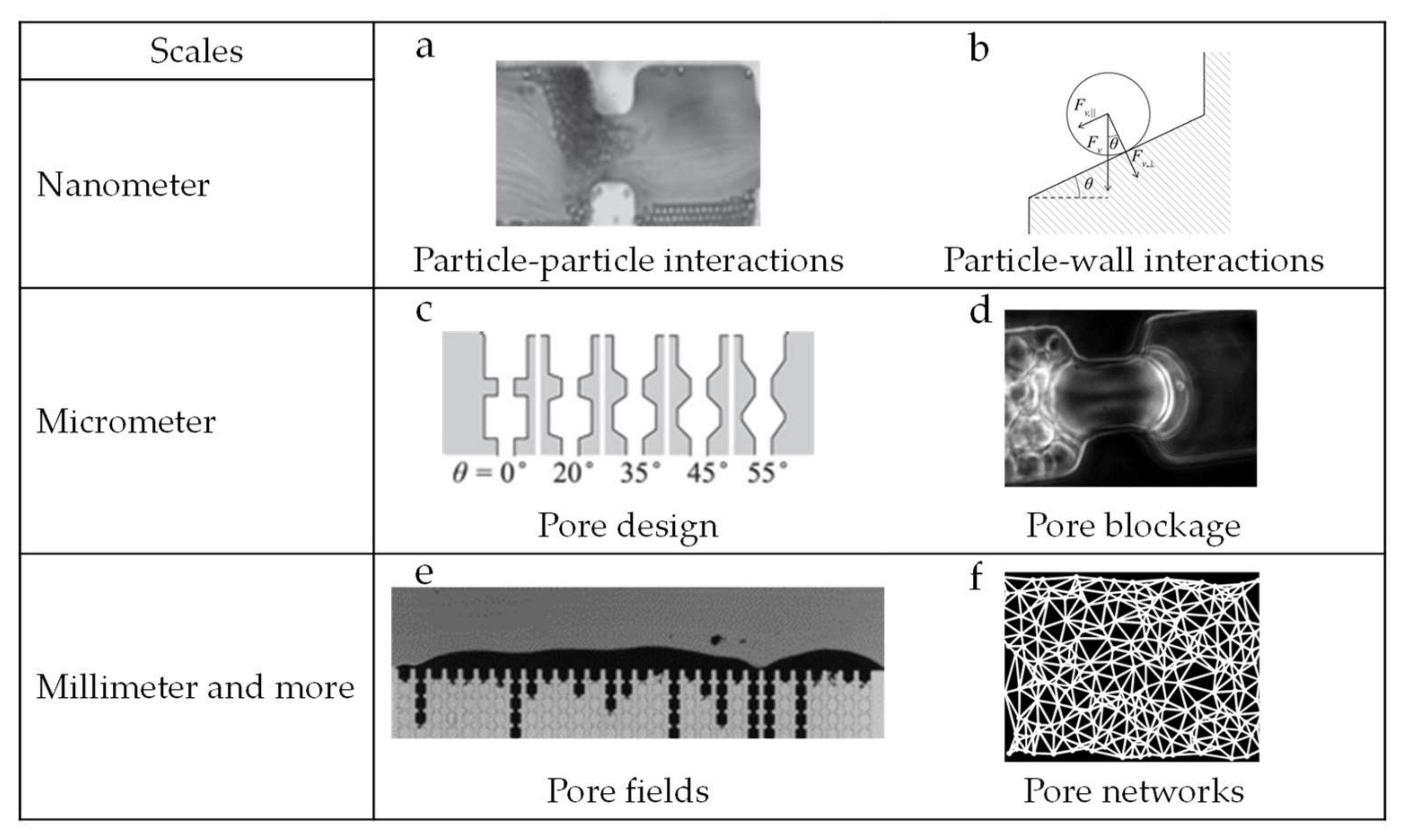
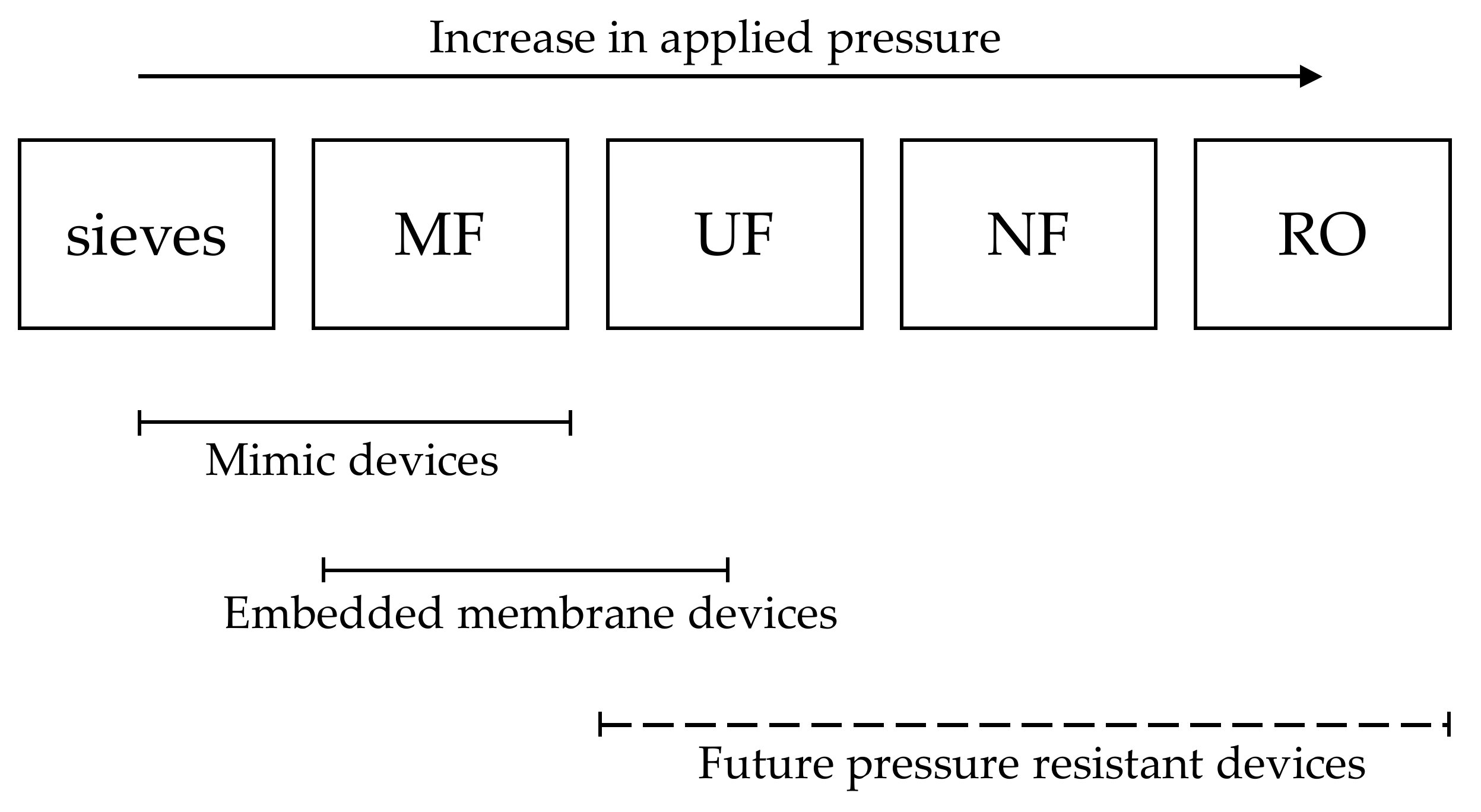
2. Microfluidic Devices as Tools
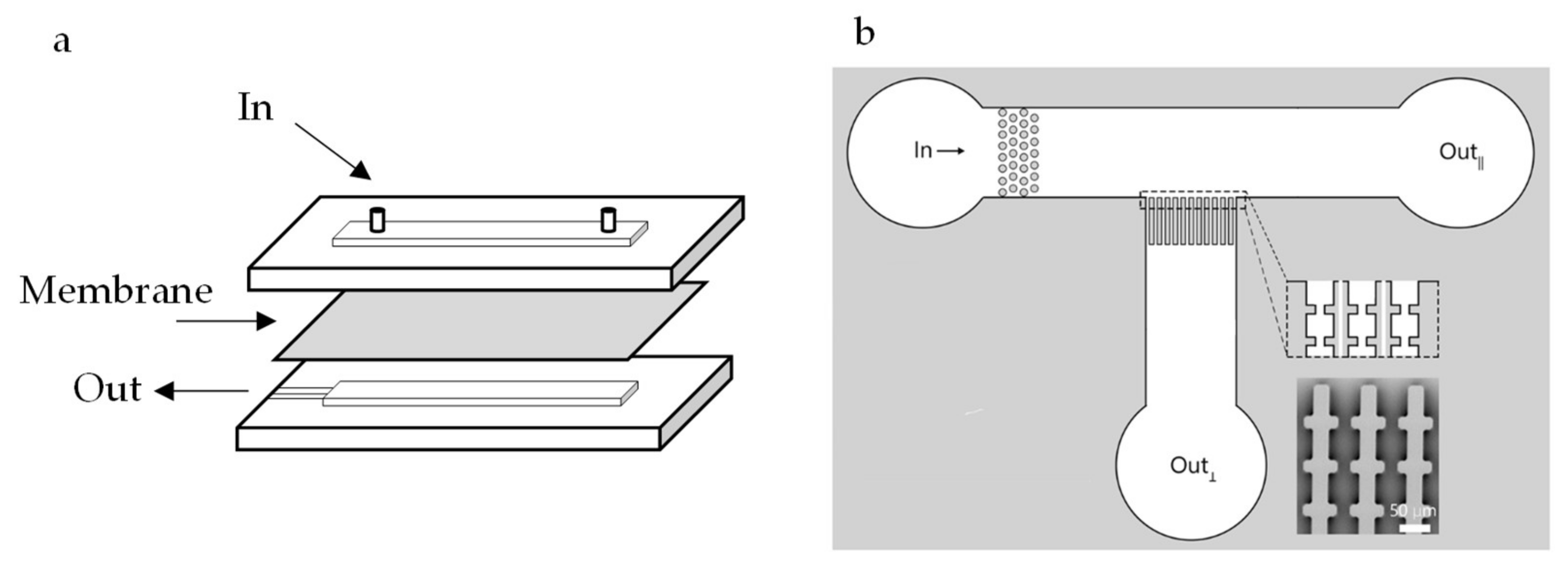
2.1. Structure
2.2. Foulants
3. Understanding Current Challenges in Membrane Processes
3.1. Flux Loss/Decrease
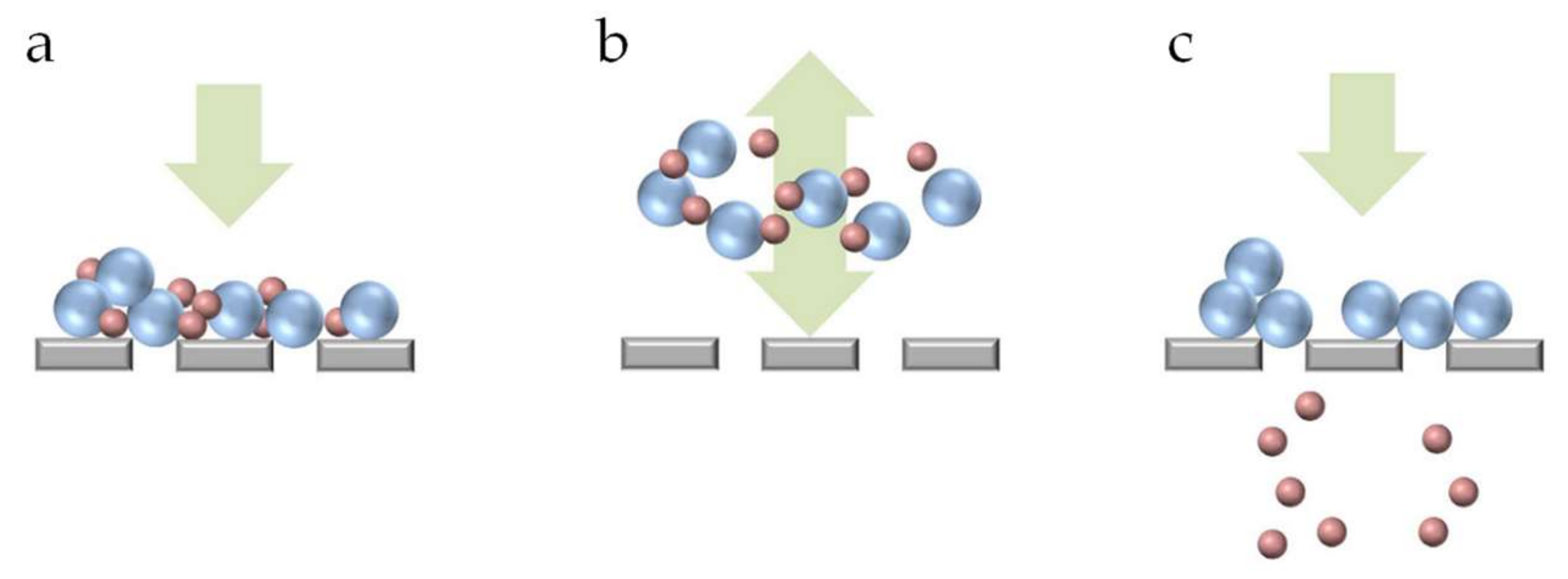
3.2. Pore Blocking Mechanisms
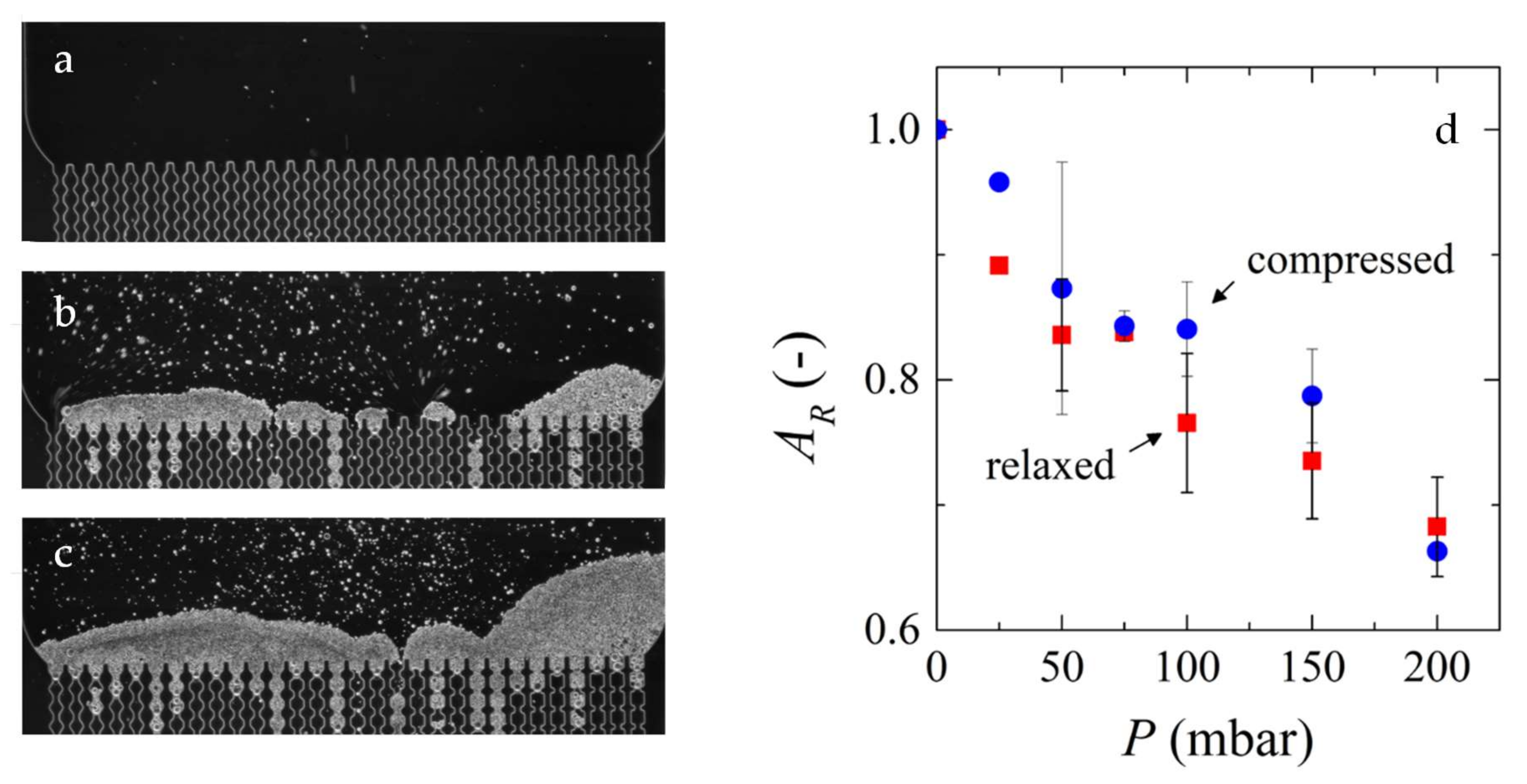
3.3. Deposit Layer Formation/Cake formation/Kinetics of Deposition
3.4. Flux Decrease Mittigating Measures
3.5. Biofilms
4. Optimization of Existing Membrane Processes
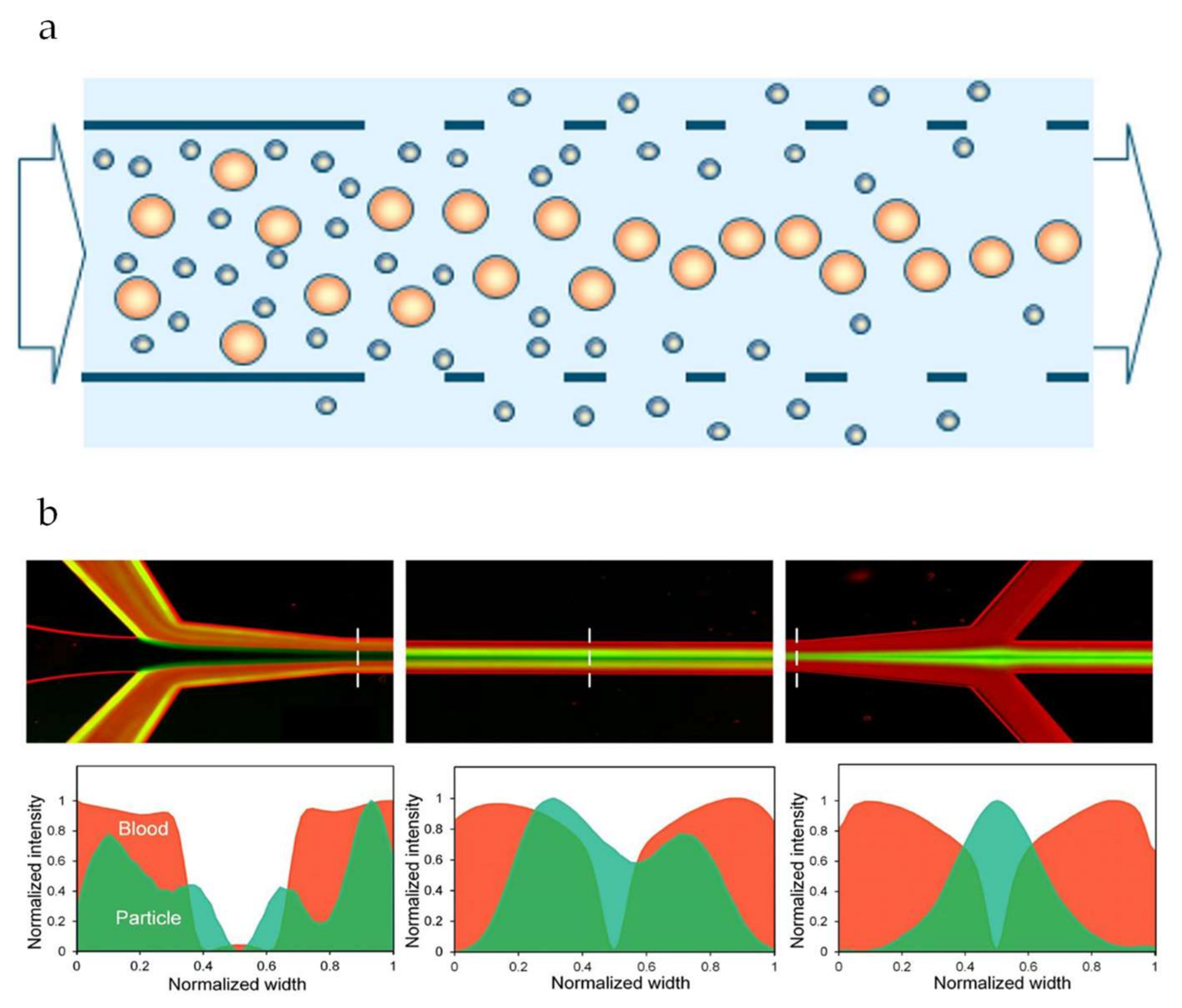
4.1. Improving Flux and Selectivity
4.2. Surface Modification
4.3. Pore Design
4.4. Determination of Particle Properties (Auxiliary Techniques)
5. Outlook
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iulianelli, A.; Drioli, E. Membrane engineering: Latest advancements in gas separation and pre-treatment processes, petrochemical industry and refinery, and future perspectives in emerging applications. Fuel Process. Technol. 2020, 206, 106464. [Google Scholar] [CrossRef]
- Bassyouni, M.; Abdel-Aziz, M.; Zoromba, M.S.; Abdel-Hamid, S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Jun, B.-M.; Al-Hamadani, Y.A.; Son, A.; Park, C.M.; Jang, M.; Jang, A.; Kim, N.C.; Yoon, Y. Applications of metal-organic framework based membranes in water purification: A review. Sep. Purif. Technol. 2020, 247, 116947. [Google Scholar] [CrossRef]
- Ausri, I.R.; Feygin, E.M.; Cheng, C.Q.; Wang, Y.; Lin, Z.Y.W.; Tang, X.S. A highly efficient and antifouling microfluidic platform for portable hemodialysis devices. MRS Commun. 2018, 8, 474–479. [Google Scholar] [CrossRef]
- Snisarenko, D.; Pavlenko, D.; Stamatialis, D.; Aimar, P.; Causseranda, C.; Bacchin, P. Insight into the transport mechanism of solute removed in dialysis by a membrane with double functionality. Chem. Eng. Res. Des. 2017, 126, 97–108. [Google Scholar] [CrossRef]
- Nazir, A.; Khan, K.; Maan, A.; Zia, R.; Giorno, L.; Schroën, K. Membrane separation technology for the recovery of nutraceuticals from food industrial streams. Trends Food Sci. Technol. 2019, 86, 426–438. [Google Scholar] [CrossRef]
- Brans, G.; Schroën, C.; Van Der Sman, R.; Boom, R. Membrane fractionation of milk: State of the art and challenges. J. Membr. Sci. 2004, 243, 263–272. [Google Scholar] [CrossRef]
- Huang, Y.; MacKenzie, A.; Meteer, L.; Hofmann, R. Evaluation of phosphorus removal from a lake by two drinking water treatment plants. Environ. Technol. 2018, 41, 863–869. [Google Scholar] [CrossRef]
- Robinson, S.; Bérubé, P. Membrane ageing in full-scale water treatment plants. Water Res. 2019, 169, 115212. [Google Scholar] [CrossRef]
- Khalid, A.; Aslam, M.; Qyyum, M.A.; Faisal, A.; Khan, A.L.; Ahmed, F.; Lee, M.; Kim, J.; Jang, N.; Chang, I.S.; et al. Membrane separation processes for dehydration of bioethanol from fermentation broths: Recent developments, challenges, and prospects. Renew. Sustain. Energy Rev. 2019, 105, 427–443. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Li, Q.; Elimelech, M. In situ monitoring techniques for concentration polarization and fouling phenomena in membrane filtration. Adv. Colloid Interface Sci. 2004, 107, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Keil, F.J. Process intensification. Rev. Chem. Eng. 2018, 34, 135–200. [Google Scholar] [CrossRef]
- Schroën, K.; De Ruiter, J.; Berton-Carabin, C.C. Microtechnological Tools to Achieve Sustainable Food Processes, Products, and Ingredients. Food Eng. Rev. 2020, 12, 101–120. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, W.; Soladoye, O.P. Towards potato protein utilisation: Insights into separation, functionality and bioactivity of patatin. Int. J. Food Sci. Technol. 2019, 55, 2314–2322. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, P.; Jaimni, S.; Kehinde, B.A.; Kaur, S. Recent developments in purification techniques and industrial applications for whey valorization: A review. Chem. Eng. Commun. 2019, 207, 123–138. [Google Scholar] [CrossRef]
- Kashani, M.N.; Kriel, F.H.; Binder, C.; Priest, C. Analysis of co-flowing immiscible liquid streams and their interfaces in a high-throughput solvent extraction chip. Microfluid. Nanofluidics 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Browne, C.A.; Shih, A.; Datta, S.S. Pore-Scale Flow Characterization of Polymer Solutions in Microfluidic Porous Media. Small 2019, 16, e1903944. [Google Scholar] [CrossRef]
- Linkhorst, J.; Rabe, J.; Hirschwald, L.T.; Kuehne, A.J.C.; Wessling, M. Direct Observation of Deformation in Microgel Filtration. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- De Aguiar, I.B.; Van De Laar, T.; Meireles, M.; Bouchoux, A.; Sprakel, J.; Schroën, K. Deswelling and deformation of microgels in concentrated packings. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Pappas, D. Microfluidics and cancer analysis: Cell separation, cell/tissue culture, cell mechanics, and integrated analysis systems. Analyst 2016, 141, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Ma, C.; Zhao, L.; Wang, Y.; Wang, J.-C.; Xu, J.; Li, T.; Pang, L.; Shen, S. High-throughput rare cell separation from blood samples using steric hindrance and inertial microfluidics. Lab Chip 2014, 14, 2525–2538. [Google Scholar] [CrossRef] [PubMed]
- Dressaire, E.; Sauret, A. Clogging of microfluidic systems. Soft Matter 2017, 13, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chueh, B.-H.; Wu, H.; Hall, E.W.; Li, C.-W.; Schirhagl, R.; Lin, J.-M.; Zare, R.N. Particle sorting using a porous membrane in a microfluidic device. Lab Chip 2011, 11, 238–245. [Google Scholar] [CrossRef]
- Warkiani, M.E.; Wicaksana, F.; Fane, A.G.; Gong, H.-Q. Investigation of membrane fouling at the microscale using isopore filters. Microfluid. Nanofluidics 2014, 19, 307–315. [Google Scholar] [CrossRef]
- Ngene, I.S.; Lammertink, R.G.; Wessling, M.; Van Der Meer, W.G.J. A microfluidic membrane chip for in situ fouling characterization. J. Membr. Sci. 2010, 346, 202–207. [Google Scholar] [CrossRef]
- Alfadhel, K.A.; Kothare, M.V. Microfluidic modeling and simulation of flow in membrane microreactors. Chem. Eng. Sci. 2005, 60, 2911–2926. [Google Scholar] [CrossRef]
- Linkhorst, J.; Beckmann, T.; Go, D.; Kuehne, A.J.C.; Wessling, M. Microfluidic colloid filtration. Sci. Rep. 2016, 6, 22376. [Google Scholar] [CrossRef]
- Bacchin, P.; Derekx, Q.; Veyret, D.; Glucina, K.; Moulin, P. Clogging of microporous channels networks: Role of connectivity and tortuosity. Microfluid. Nanofluidics 2013, 17, 85–96. [Google Scholar] [CrossRef]
- De Aguiar, I.B.; Meireles, M.; Bouchoux, A.; Schroën, K. Microfluidic model systems used to emulate processes occurring during soft particle filtration. Sci. Rep. 2019, 9, 3063. [Google Scholar] [CrossRef]
- Hu, G.; Li, D. Multiscale phenomena in microfluidics and nanofluidics. Chem. Eng. Sci. 2007, 62, 3443–3454. [Google Scholar] [CrossRef]
- Van Zwieten, R.; Van De Laar, T.; Sprakel, J.; Schroën, K. From cooperative to uncorrelated clogging in cross-flow microfluidic membranes. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Creative Commons Attribution 4.0 International License. Available online: http://creativecommons.org/licenses/by/4.0/ (accessed on 22 September 2020).
- Ngene, I.S.; Lammertink, R.G.H.; Wessling, M.; Van Der Meer, W.G. Visual characterization of fouling with bidisperse solution. J. Membr. Sci. 2011, 368, 110–115. [Google Scholar] [CrossRef]
- Mustin, B.; Stoeber, B. Deposition of particles from polydisperse suspensions in microfluidic systems. Microfluid. Nanofluidics 2010, 9, 905–913. [Google Scholar] [CrossRef]
- Kim, H.-S.; Michielsen, S.; Denhartog, E. New wicking measurement system to mimic human sweating phenomena with continuous microfluidic flow. J. Mater. Sci. 2020, 55, 7816–7832. [Google Scholar] [CrossRef]
- Zhang, S.; Cagney, N.; Lacassagne, T.; Balabani, S.; Naveira-Cotta, C.P.; Tiwari, M.K. Mixing in flows past confined microfluidic cylinders: Effects of pin and fluid interface offsetting. Chem. Eng. J. 2020, 397, 125358. [Google Scholar] [CrossRef]
- Dudek, M.; Fernandes, D.; Herø, E.H.; Øye, G. Microfluidic method for determining drop-drop coalescence and contact times in flow. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124265. [Google Scholar] [CrossRef]
- Hanson, C.; Sieverts, M.; Tew, K.; Dykes, A.; Salisbury, M.; Vargis, E. The use of microfluidics and dielectrophoresis for separation, concentration, and identification of bacteria. In Proceedings of the SPIE BiOS, San Fransico, CA, USA, 21 March 2016. [Google Scholar]
- Jimenez, M.; Bridle, H. Angry pathogens, how to get rid of them: Introducing microfluidics for waterborne pathogen separation to children. Lab Chip 2015, 15, 947–957. [Google Scholar] [CrossRef]
- Van De Laar, T.; Klooster, S.T.; Schroën, K.; Sprakel, J. Transition-state theory predicts clogging at the microscale. Sci. Rep. 2016, 6, 28450. [Google Scholar] [CrossRef]
- De Aguiar, I.B.; Meireles, M.; Bouchoux, A.; Schroën, K. Conformational changes influence clogging behavior of micrometer-sized microgels in idealized multiple constrictions. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Van De Laar, T. Sticky, Squishy & Stuck: A Soft Matter Approach to Membrane Failure. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, May 2018. [Google Scholar]
- Chen, X.; Shen, J. Review of membranes in microfluidics. J. Chem. Technol. Biotechnol. 2016, 92, 271–282. [Google Scholar] [CrossRef]
- De Jong, J.; Lammertink, R.G.H.; Wessling, M. Membranes and microfluidics: A review. Lab Chip 2006, 6, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Debnath, N.; Sadrzadeh, M. Microfluidic Mimic for Colloid Membrane Filtration: A Review. J. Indian Inst. Sci. 2018, 98, 137–157. [Google Scholar] [CrossRef]
- Gerami, A.; AlZahid, Y.; Mostaghimi, P.; Kashaninejad, N.; Kazemifar, F.; Amirian, T.; Mosavat, N.; Warkiani, M.E.; Armstrong, R.T. Microfluidics for Porous Systems: Fabrication, Microscopy and Applications. Transp. Porous Media 2018, 130, 277–304. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhao, C.; Kang, Y.; Yang, C. Microfluidics-based fundamental characterization of external concentration polarization in forward osmosis. Microfluid. Nanofluidics 2019, 23, 36. [Google Scholar] [CrossRef]
- Chen, V.; Li, H.; Fane, A. Non-invasive observation of synthetic membrane processes—A review of methods. J. Membr. Sci. 2004, 241, 23–44. [Google Scholar] [CrossRef]
- Agbangla, G.C.; Climent, E.; Bacchin, P. Experimental investigation of pore clogging by microparticles: Evidence for a critical flux density of particle yielding arches and deposits. Sep. Purif. Technol. 2012, 101, 42–48. [Google Scholar] [CrossRef]
- Bacchin, P.; Marty, A.; Duru, P.; Meireles, M.; Aimar, P. Colloidal surface interactions and membrane fouling: Investigations at pore scale. Adv. Colloid Interface Sci. 2011, 164, 2–11. [Google Scholar] [CrossRef]
- Maitri, R.V.; De, S.; Koesen, S.P.; Wyss, H.M.; Van Der Schaaf, J.; Kuipers, J.A.M.; Padding, J.T.; Peters, E.A.J.F. Effect of microchannel structure and fluid properties on non-inertial particle migration. Soft Matter 2019, 15, 2648–2656. [Google Scholar] [CrossRef]
- Debnath, N.; Kumar, A.; Thundat, T.; Sadrzadeh, M. Investigating fouling at the pore-scale using a microfluidic membrane mimic filtration system. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Decock, J.; Schlenk, M.; Salmon, J.-B. In situphoto-patterning of pressure-resistant hydrogel membranes with controlled permeabilities in PEGDA microfluidic channels. Lab Chip 2018, 18, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-T.; Massino, M.; Keita, C.; Salmon, J.-B. Microfluidic dialysis using photo-patterned hydrogel membranes in PDMS chips. Lab Chip 2020, 20, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cui, D.; Liu, C.; Li, H. Microfluidic chip for blood cell separation and collection based on crossflow filtration. Sens. Actuators B Chem. 2008, 130, 216–221. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, J.; Kim, S.J.; Ko, D.-H.; Lee, S.H.; Lee, S.J.; Park, J.-K.; Lee, M.-K. On-site extraction and purification of bacterial nucleic acids from blood samples using an unpowered microfluidic device. Sens. Actuators B Chem. 2020, 320, 128346. [Google Scholar] [CrossRef]
- Wiese, M.; Malkomes, C.; Krause, B.; Wessling, M. Flow and filtration imaging of single use sterile membrane filters. J. Membr. Sci. 2018, 552, 274–285. [Google Scholar] [CrossRef]
- Choi, G.; Nouri, R.; Zarzar, L.; Guan, W. Microfluidic deformability-activated sorting of single particles. Microsyst. Nanoeng. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- De Aguiar, I.B.; Schroën, K.; Meireles, M.; Bouchoux, A. Compressive resistance of granular-scale microgels: From loose to dense packing. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 406–416. [Google Scholar] [CrossRef]
- Schroën, K.; Van Dinther, A.; Stockmann, R. Particle migration in laminar shear fields: A new basis for large scale separation technology? Sep. Purif. Technol. 2017, 174, 372–388. [Google Scholar] [CrossRef]
- Davis, R.H. Modeling of Fouling of Crossflow Microfiltration Membranes. Sep. Purif. Methods 1992, 21, 75–126. [Google Scholar] [CrossRef]
- Cejas, C.M.; Maini, L.; Monti, F.; Tabeling, P. Deposition kinetics of bi- and tridisperse colloidal suspensions in microchannels under the van der Waals regime. Soft Matter 2019, 15, 7438–7447. [Google Scholar] [CrossRef]
- Peinemann, K.-V.; Nunes, S.P.; Giorno, L. Membranes for Food Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Lohaus, J.; Stockmeier, F.; Surray, P.; Lölsberg, J.; Wessling, M. What are the microscopic events during membrane backwashing? J. Membr. Sci. 2020, 602, 117886. [Google Scholar] [CrossRef]
- Wyss, H.M.; Blair, D.L.; Morris, J.F.; A Stone, H.; Weitz, D.A. Mechanism for clogging of microchannels. Phys. Rev. E 2006, 74, 061402. [Google Scholar] [CrossRef] [PubMed]
- Anbari, A.; Chien, H.-T.; Datta, S.S.; Deng, W.; Weitz, D.A.; Fan, J. Microfluidic Model Porous Media: Fabrication and Applications. Small 2018, 14, e1703575. [Google Scholar] [CrossRef]
- Guo, P.; Wang, M.; He, M.; Wang, Y.; Gao, K.; Gong, W. Experimental investigation on macroscopic behavior and microfluidic field of nonlinear flow in rough-walled artificial fracture models. Adv. Water Resour. 2020, 142, 103637. [Google Scholar] [CrossRef]
- Datta, S.S.; Chiang, H.; Ramakrishnan, T.S.; A Weitz, D. Spatial Fluctuations of Fluid Velocities in Flow through a Three-Dimensional Porous Medium. Phys. Rev. Lett. 2013, 111, 064501. [Google Scholar] [CrossRef] [PubMed]
- Cejas, C.M.; Monti, F.; Truchet, M.; Burnouf, J.-P.; Tabeling, P. Particle Deposition Kinetics of Colloidal Suspensions in Microchannels at High Ionic Strength. Langmuir 2017, 33, 6471–6480. [Google Scholar] [CrossRef] [PubMed]
- Di, H.; Martin, G.J.; Sun, Q.; Xie, D.; Dunstan, D.E. Detailed, real-time characterization of particle deposition during crossflow filtration as influenced by solution properties. J. Membr. Sci. 2018, 555, 115–124. [Google Scholar] [CrossRef]
- Kromkamp, J.; Van Domselaar, M.; Schroën, K.; Van Der Sman, R.G.M.; Boom, R. Shear-induced diffusion model for microfiltration of polydisperse suspensions. Desalination 2002, 146, 63–68. [Google Scholar] [CrossRef]
- Chang, H.; Liang, H.; Qu, F.; Liu, B.; Yu, H.; Du, X.; Li, G.; Snyder, S.A. Hydraulic backwashing for low-pressure membranes in drinking water treatment: A review. J. Membr. Sci. 2017, 540, 362–380. [Google Scholar] [CrossRef]
- Yoon, Y.; Kim, S.; Lee, J.; Choi, J.; Kim, R.-K.; Lee, S.-J.; Sul, O.; Lee, S.-B. Clogging-free microfluidics for continuous size-based separation of microparticles. Sci. Rep. 2016, 6, 26531. [Google Scholar] [CrossRef]
- Mansouri, J.; Harrisson, S.; Chen, V. Strategies for controlling biofouling in membrane filtration systems: Challenges and opportunities. J. Mater. Chem. 2010, 20, 4567–4586. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.-S.; Chae, S.-R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Karig, D.K.; Kumar, A.; Ardekani, A.M. Interplay of physical mechanisms and biofilm processes: Review of microfluidic methods. Lab Chip 2015, 15, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Schaule, G.; Griebe, T.; Schmitt, J.; Tamachkiarowa, A. Biofouling—The Achilles heel of membrane processes. Desalination 1997, 113, 215–225. [Google Scholar] [CrossRef]
- Nejati, S.; Mirbagheri, S.A.; Warsinger, D.M.; Fazeli, M. Biofouling in seawater reverse osmosis (SWRO): Impact of module geometry and mitigation with ultrafiltration. J. Water Process. Eng. 2019, 29, 100782. [Google Scholar] [CrossRef]
- Flemming, H.-C. Biofouling and me: My Stockholm syndrome with biofilms. Water Res. 2020, 173, 115576. [Google Scholar] [CrossRef]
- Pousti, M.; Zarabadi, M.P.; Amirdehi, M.A.; Paquet-Mercier, F.; Greener, J. Microfluidic bioanalytical flow cells for biofilm studies: A review. Analyst 2019, 144, 68–86. [Google Scholar] [CrossRef]
- Marty, A.; Roques, C.; Causserand, C.; Bacchin, P. Formation of bacterial streamers during filtration in microfluidic systems. Biofouling 2012, 28, 551–562. [Google Scholar] [CrossRef]
- Rusconi, R.; Lecuyer, S.; Guglielmini, L.; Stone, H.A. Laminar flow around corners triggers the formation of biofilm streamers. J. R. Soc. Interface 2010, 7, 1293–1299. [Google Scholar] [CrossRef]
- Sendekie, Z.B.; Gaveau, A.; Lammertink, R.G.H.; Bacchin, P. Bacteria Delay the Jamming of Particles at Microchannel Bottlenecks. Sci. Rep. 2016, 6, 31471. [Google Scholar] [CrossRef]
- Sauret, A.; Barney, E.C.; Perro, A.; Villermaux, E.; Stone, H.A.; Dressaire, E. Clogging by sieving in microchannels: Application to the detection of contaminants in colloidal suspensions. Appl. Phys. Lett. 2014, 105, 074101. [Google Scholar] [CrossRef]
- Dijkshoorn, J.; Schutyser, M.; Wagterveld, R.M.; Schroën, K.; Boom, R. A comparison of microfiltration and inertia-based microfluidics for large scale suspension separation. Sep. Purif. Technol. 2017, 173, 86–92. [Google Scholar] [CrossRef]
- Syed, M.S.; Rafeie, M.; Vandamme, D.; Asadnia, M.; Henderson, R.; Taylor, R.A.; Warkiani, M.E. Selective separation of microalgae cells using inertial microfluidics. Bioresour. Technol. 2018, 252, 91–99. [Google Scholar] [CrossRef]
- Li, M.; Muñoz, H.E.; Goda, K.; Di Carlo, D. Shape-based separation of microalga Euglena gracilis using inertial microfluidics. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Tasadduq, B.; Lam, W.; Alexeev, A.; Sarioglu, A.F.; Sulchek, T. Enhancing size based size separation through vertical focus microfluidics using secondary flow in a ridged microchannel. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kulrattanarak, T.; Van Der Sman, R.G.M.; Schroën, K.; Boom, R. Classification and evaluation of microfluidic devices for continuous suspension fractionation. Adv. Colloid Interface Sci. 2008, 142, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Van Dinther, A.M.C.; Schroën, K.; Imhof, A.; Vollebregt, H.M.; Boom, R.M. Flow-induced particle migration in microchannels for improved microfiltration processes. Microfluid. Nanofluidics 2013, 15, 451–465. [Google Scholar] [CrossRef]
- Van De Laar, T.; Schroën, K.; Sprakel, J. Cooperativity and segregation in confined flows of soft binary glasses. Phys. Rev. E 2015, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tu, C.; Liang, Y.; Huang, B.; Fang, Y.; Liang, X.; Papautsky, I.; Ye, X. Isolation of cells from whole blood using shear-induced diffusion. Sci. Rep. 2018, 8, 9411. [Google Scholar] [CrossRef]
- Dow, P.; Kotz, K.; Gruszka, S.; Holder, J.; Fiering, J. Acoustic separation in plastic microfluidics for rapid detection of bacteria in blood using engineered bacteriophage. Lab Chip 2018, 18, 923–932. [Google Scholar] [CrossRef]
- Fornell, A.; Cushing, K.; Nilsson, J.; Tenje, M. Binary particle separation in droplet microfluidics using acoustophoresis. Appl. Phys. Lett. 2018, 112, 063701. [Google Scholar] [CrossRef]
- Ince, G.O.; Coclite, A.-M.; Gleason, K.K. CVD of polymeric thin films: Applications in sensors, biotechnology, microelectronics/organic electronics, microfluidics, MEMS, composites and membranes. Rep. Prog. Phys. 2011, 75, 016501. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Zhong, W.; Meng, S.; Kong, J.; Yang, P.; Liu, B. Construction of a Biomimetic Surface on Microfluidic Chips for Biofouling Resistance. Anal. Chem. 2006, 78, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- Brans, G.; Kromkamp, J.; Pek, N.; Gielen, J.; Heck, J.; Van Rijn, C.J.M.; Van Der Sman, R.G.M.; Schroën, K.; Boom, R. Evaluation of microsieve membrane design. J. Membr. Sci. 2006, 278, 344–348. [Google Scholar] [CrossRef]
- Massenburg, S.S.; Amstad, E.; Weitz, D.A. Clogging in parallelized tapered microfluidic channels. Microfluid. Nanofluidics 2016, 20, 94. [Google Scholar] [CrossRef]
- Cappello, J.; D’Herbemont, V.; Lindner, A.; Du Roure, O. Microfluidic In-Situ Measurement of Poisson’s Ratio of Hydrogels. Micromachines 2020, 11, 318. [Google Scholar] [CrossRef]
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-printed microfluidic devices: Fabrication, advantages and limitations—A mini review. Anal. Methods 2016, 8, 6005–6012. [Google Scholar] [CrossRef]
- Miranda, J.M.; Campos, J. Numerical study of a hybrid membrane cell with semi and fully permeable membrane sub-sections. Chem. Eng. Sci. 2007, 62, 1215–1229. [Google Scholar] [CrossRef]
- Kromkamp, J.; Bastiaanse, A.; Swarts, J.; Brans, G.; Van Der Sman, R.G.M.; Boom, R. A suspension flow model for hydrodynamics and concentration polarisation in crossflow microfiltration. J. Membr. Sci. 2005, 253, 67–79. [Google Scholar] [CrossRef]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the future of membranes: Perspectives for advanced and new membrane materials and manufacturing processes. J. Membr. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- Ben Hassan, I.; Lafforgue, C.; Ayadi, A.; Schmitz, P. In situ 3D characterization of monodispersed spherical particle deposition on microsieve using confocal laser scanning microscopy. J. Membr. Sci. 2014, 454, 283–297. [Google Scholar] [CrossRef]
- Kaade, W.; Ferrando, M.; Khanmohammed, A.; Torras, C.; De Lamo-Castellví, S.; Güell, C. Low-energy high-throughput emulsification with nickel micro-sieves for essential oils encapsulation. J. Food Eng. 2019, 263, 326–336. [Google Scholar] [CrossRef]
- Brans, G.; Van Der Sman, R.G.M.; Schroën, K.; Van Der Padt, A.; Boom, R. Optimization of the membrane and pore design for micro-machined membranes. J. Membr. Sci. 2006, 278, 239–250. [Google Scholar] [CrossRef]
- Balyan, P.; Saini, D.; Das, S.; Kumar, D.; Agarwal, A. Flow induced particle separation and collection through linear array pillar microfluidics device. Biomicrofluidics 2020, 14, 024103. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.K. Designer emulsions using microfluidics and their applications. In Proceedings of the 2008 AIChE Annual Meeting, New York, NY, USA, 16–21 November 2008. [Google Scholar]
- Liu, Y.; Li, Y.; Hensel, A.; Brandner, J.J.; Zhang, K.; Du, X.; Yang, Y. A review on emulsification via microfluidic processes. Front. Chem. Sci. Eng. 2020, 14, 350–364. [Google Scholar] [CrossRef]
- Schroën, K.; Bliznyuk, O.; Muijlwijk, K.; Sahin, S.; Berton-Carabin, C.C. Microfluidic emulsification devices: From micrometer insights to large-scale food emulsion production. Curr. Opin. Food Sci. 2015, 3, 33–40. [Google Scholar] [CrossRef]
- Sahin, S.; Bliznyuk, O.; Cordova, A.R.; Schroën, K. Microfluidic EDGE emulsification: The importance of interface interactions on droplet formation and pressure stability. Sci. Rep. 2016, 6, 26407. [Google Scholar] [CrossRef]
- Warriner, K.; Reddy, S.M.; Namvar, A.; Neethirajan, S. Developments in nanoparticles for use in biosensors to assess food safety and quality. Trends Food Sci. Technol. 2014, 40, 183–199. [Google Scholar] [CrossRef]
- Garcia-Cordero, J.L.; Maerkl, S.J. Microfluidic systems for cancer diagnostics. Curr. Opin. Biotechnol. 2020, 65, 37–44. [Google Scholar] [CrossRef]
- Malankowska, M.; Julian, I.; Pellejero, I.; Rho, H.S.; Schlautmann, S.; Tiggelaar, R.M.; Pina, M.P.; Gardeniers, H.; Mallada, R. Understanding blood oxygenation in a microfluidic meander double side membrane contactor. Sens. Actuators B Chem. 2019, 288, 414–424. [Google Scholar] [CrossRef]
- Morsink, M.A.J.; Willemen, N.G.A.; Leijten, J.; Bansal, R.; Shin, S.R. Immune Organs and Immune Cells on a Chip: An Overview of Biomedical Applications. Micromachines 2020, 11, 849. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouhid de Aguiar, I.; Schroën, K. Microfluidics Used as a Tool to Understand and Optimize Membrane Filtration Processes. Membranes 2020, 10, 316. https://doi.org/10.3390/membranes10110316
Bouhid de Aguiar I, Schroën K. Microfluidics Used as a Tool to Understand and Optimize Membrane Filtration Processes. Membranes. 2020; 10(11):316. https://doi.org/10.3390/membranes10110316
Chicago/Turabian StyleBouhid de Aguiar, Izabella, and Karin Schroën. 2020. "Microfluidics Used as a Tool to Understand and Optimize Membrane Filtration Processes" Membranes 10, no. 11: 316. https://doi.org/10.3390/membranes10110316
APA StyleBouhid de Aguiar, I., & Schroën, K. (2020). Microfluidics Used as a Tool to Understand and Optimize Membrane Filtration Processes. Membranes, 10(11), 316. https://doi.org/10.3390/membranes10110316




