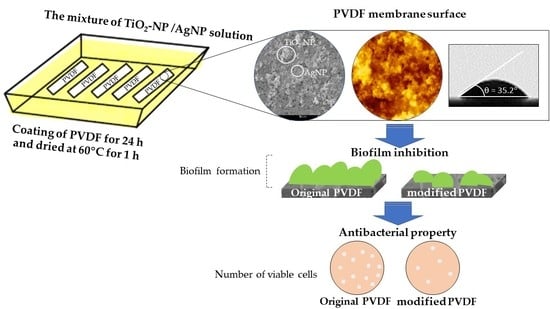
Enhancing the Antibacterial Properties of PVDF Membrane by Hydrophilic Surface Modification Using Titanium Dioxide and Silver Nanoparticles
Abstract
1. Introduction
2. Methodology
2.1. Chemicals and Materials
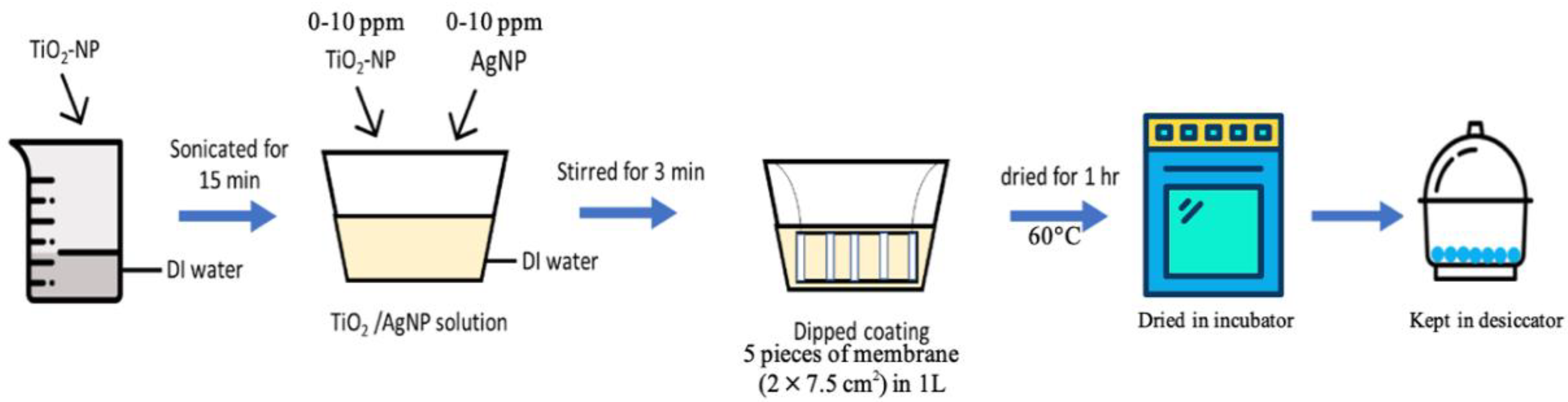
2.2. Experimental Procedures
Membrane Modification via Titanium Dioxide and Silver Nanoparticles (TiO2-NP and AgNP)
2.3. Membrane Characterization
2.3.1. Contact Angle Measurement
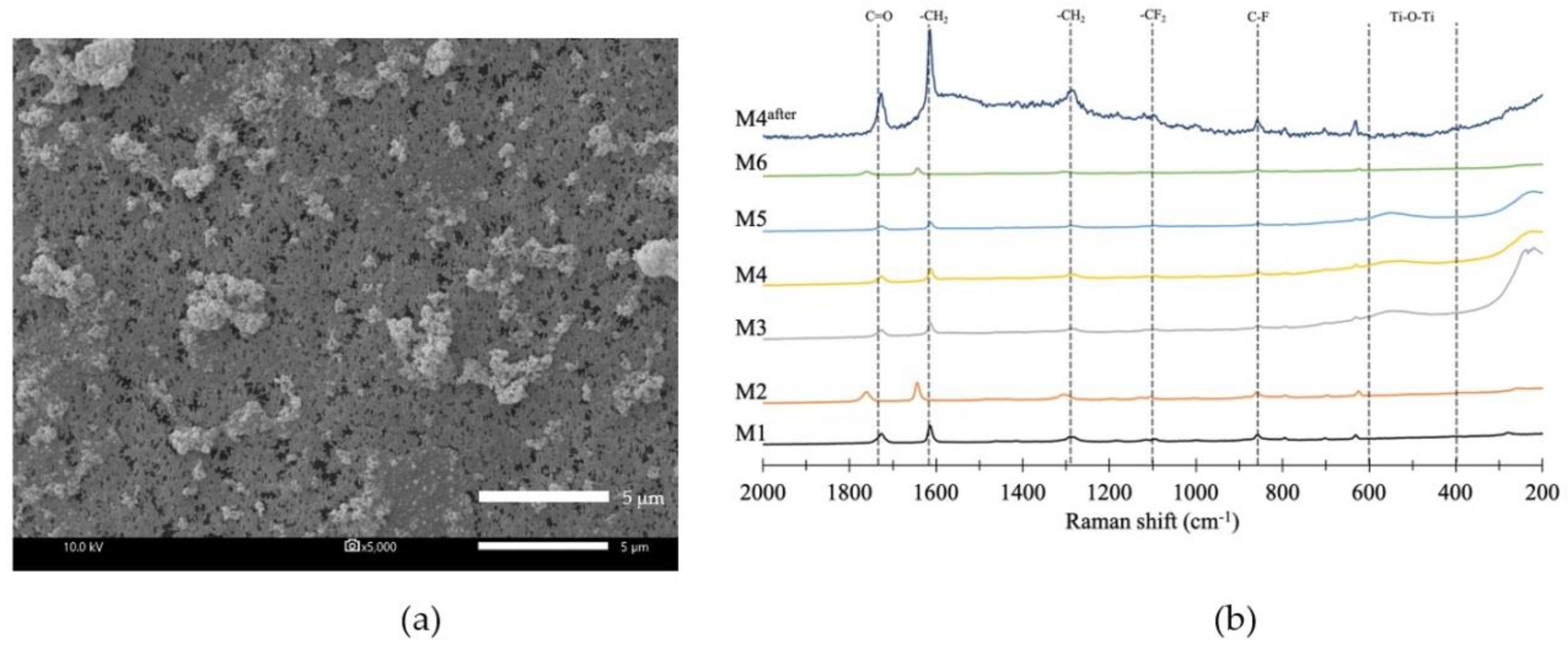
2.3.2. Morphology and Chemical Composition
2.3.3. Porosity Analysis
2.3.4. Permeation Performances
2.3.5. Functional Group Analysis
2.4. Antibacterial Test
2.4.1. E. Coli Strains and Growth Condition
2.4.2. Antibacterial Test
2.4.3. Biofilm Inhibition Test
3. Results and Discussion
3.1. Hydrophilic Membrane Modification
3.1.1. Water Contact Angle (WCA)
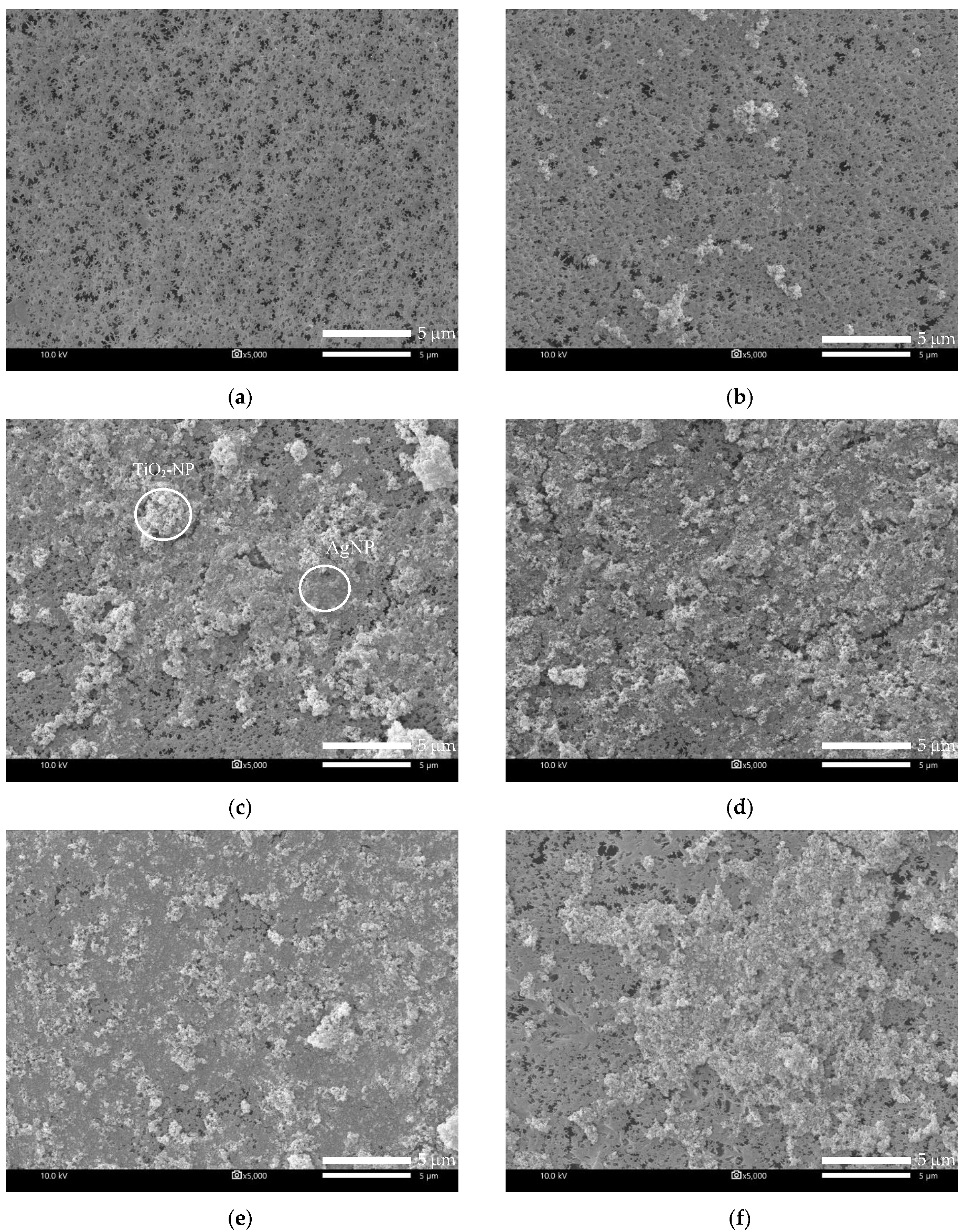
3.1.2. Membrane Morphology
3.1.3. Surface Roughness
3.1.4. Porosity
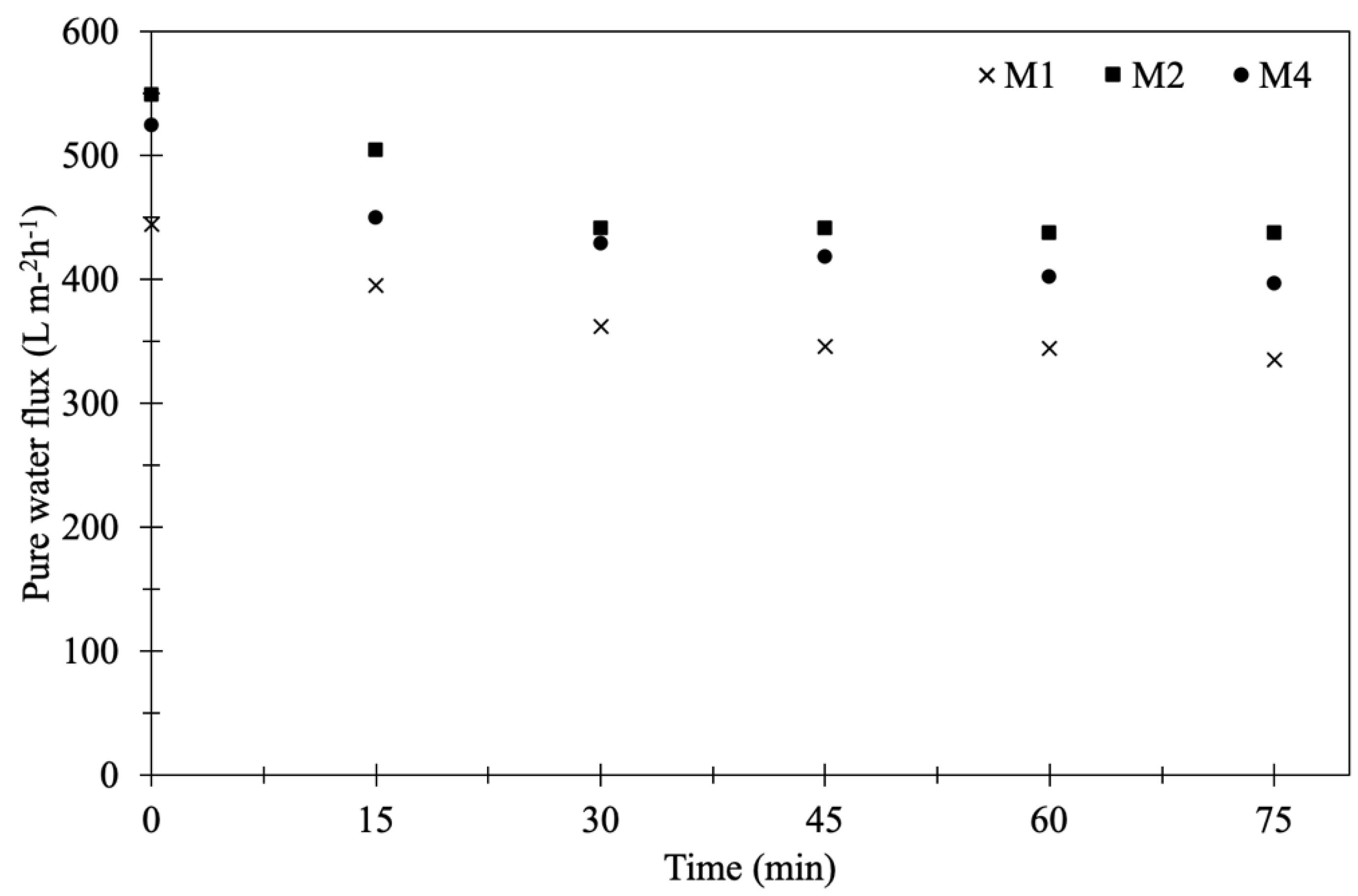
3.1.5. Pure Water Flux of Membranes
3.2. Antibacterial Properties of Modified Membrane
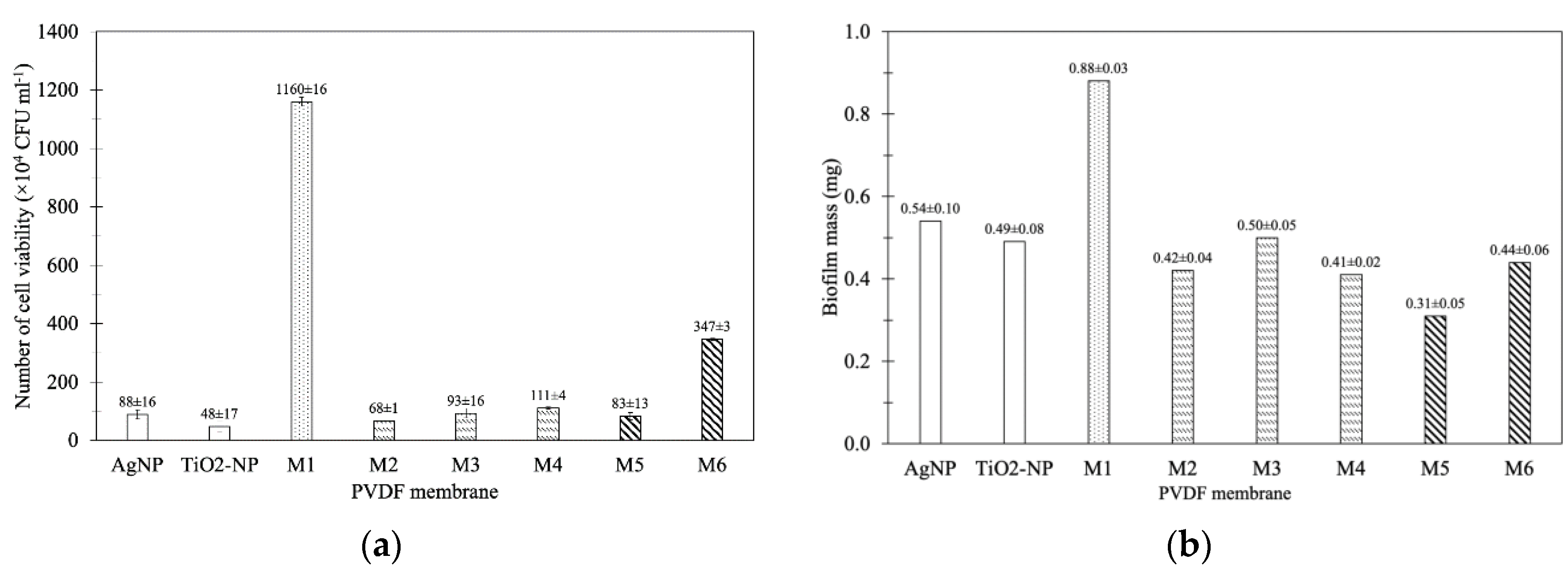
3.2.1. Antibacterial Test
3.2.2. Biofilm Inhibition Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Yao, T.T.; Ren, L.X.; Zhao, Y.H.; Yuan, X.Y. Antibacterial PCL electrospun membranes containing synthetic polypeptides for biomedical purposes. Colloids Surf. B 2018, 172, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.H.; Wang, X.K.; Guo, L.; Zhang, Q.Y.; Guo, X.M.; Li, L.S. Preparation of PU modified PVDF antifouling membrane and its hydrophilic performance. J. Membr. Sci. 2016, 520, 933–940. [Google Scholar] [CrossRef]
- Chew, N.G.P.; Zhao, S.S.; Loh, C.H.; Permogorov, N.; Wang, R. Surfactant effects on water recovery from produced water via direct-contact membrane distillation. J. Membr. Sci. 2017, 528, 126–134. [Google Scholar] [CrossRef]
- Miao, R.; Wang, L.; Feng, L.; Liu, Z.W.; Lv, Y.T. Understanding PVDF ultrafiltration membrane fouling behaviour through model solutions and secondary wastewater effluent. Desalin. Water Treat. 2014, 52, 5061–5067. [Google Scholar] [CrossRef]
- Zeng, K.L.; Zhou, J.; Cui, Z.L.; Zhou, Y.; Shi, C.; Wang, X.Z.; Zhou, L.Y.; Ding, X.B.; Wang, Z.H.; Drioli, E. Insight into fouling behavior of poly(vinylidene fluoride) (PVDF) hollow fiber membranes caused by dextran with different pore size distributions. Chin. J. Chem. Eng. 2018, 26, 268–277. [Google Scholar] [CrossRef]
- Chew, N.G.P.; Zhao, S.S.; Wang, R. Recent advances in membrane development for treating surfactant- and oil-containing feed streams via membrane distillation. Adv. Colloid Interface Sci. 2019, 273, 102022. [Google Scholar] [CrossRef]
- Zuo, G.Z.; Wang, R. Novel membrane surface modification to enhance anti-oil fouling property for membrane distillation application. J. Membr. Sci. 2013, 447, 26–35. [Google Scholar] [CrossRef]
- Aslam, M.; Ahmad, R.; Kim, J. Recent developments in biofouling control in membrane bioreactors for domestic wastewater treatment. Sep. Purif. Technol. 2018, 206, 297–315. [Google Scholar] [CrossRef]
- Wu, L.G.; Zhang, X.Y.; Wang, T.; Du, C.H.; Yang, C.H. Enhanced performance of polyvinylidene fluoride ultrafiltration membranes by incorporating TiO2/graphene oxide. Chem. Eng. Res. Des. 2019, 141, 492–501. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.H.; Williams, C.J.; Derby, B.; Szekely, G. Oil-in-water separation with graphene-based nanocomposite membranes for produced water treatment. J. Membr. Sci. 2020, 603, 118007. [Google Scholar] [CrossRef]
- Wang, F.; Dai, J.W.; Huang, L.Q.; Si, Y.; Yu, J.Y.; Ding, B. Biomimetic and Superelastic Silica Nanofibrous Aerogels with Rechargeable Bactericidal Function for Antifouling Water Disinfection. ACS Nano 2020, 14, 8975–8984. [Google Scholar] [CrossRef] [PubMed]
- Penboon, L.; Khrueakham, A.; Sairiam, S. TiO2 coated on PVDF membrane for dye wastewater treatment by a photocatalytic membrane. Water Sci. Technol. 2019, 79, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.K.; Yang, H.C.; Wan, L.S.; Wu, J.; Xu, Z.K. Polypropylene microfiltration membranes modified with TiO2 nanoparticles for surface wettability and antifouling property. J. Membr. Sci. 2016, 500, 8–15. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Ghaemi, N. Characterization of self-cleaning RO membranes coated with TiO2 particles under UV irradiation. J. Membr. Sci. 2007, 303, 221–233. [Google Scholar] [CrossRef]
- Moriyama, A.; Yamada, I.; Takahashi, J.; Iwahashi, H. Oxidative stress caused by TiO2 nanoparticles under UV irradiation is due to UV irradiation not through nanoparticles. Chem. Biol. Interact. 2018, 294, 144–150. [Google Scholar] [CrossRef]
- Chew, N.G.P.; Zhang, Y.J.; Goh, K.; Ho, J.S.; Xu, R.; Wang, R. Hierarchically Structured Janus Membrane Surfaces for Enhanced Membrane Distillation Performance. ACS Appl. Mater. Interfaces 2019, 11, 25524–25534. [Google Scholar] [CrossRef]
- Huang, L.C.; Zhao, S.; Wang, Z.; Wu, J.H.; Wang, J.X.; Wang, S.C. In situ immobilization of silver nanoparticles for improving permeability, antifouling and anti-bacterial properties of ultrafiltration membrane. J. Membr. Sci. 2016, 499, 269–281. [Google Scholar] [CrossRef]
- Qi, L.B.; Liu, Z.Y.; Wang, N.; Hu, Y.X. Facile and efficient in situ synthesis of silver nanoparticles on diverse filtration membrane surfaces for antimicrobial performance. Appl. Surf. Sci. 2018, 456, 95–103. [Google Scholar] [CrossRef]
- Wang, L.; Ali, J.; Zhang, C.B.; Mailhot, G.; Pan, G. Simultaneously enhanced photocatalytic and antibacterial activities of TiO2/Ag composite nanofibers for wastewater purification. J. Environ. Chem. Eng. 2020, 8, 102104. [Google Scholar] [CrossRef]
- Zou, Y.J.; Liang, J.; She, Z.; Kraatz, H.B. Gold nanoparticles-based multifunctional nanoconjugates for highly sensitive and enzyme-free detection of E.coli K12. Talanta 2019, 193, 15–22. [Google Scholar] [CrossRef]
- Behboudi, A.; Jafarzadeh, Y.; Yegani, R. Enhancement of antifouling and antibacterial properties of PVC hollow fiber ultrafiltration membranes using pristine and modified silver nanoparticles. J. Environ. Chem. Eng. 2018, 6, 1764–1773. [Google Scholar] [CrossRef]
- Rompre, A.; Servais, P.; Baudart, J.; de Roubin, M.R.; Laurent, P. Detection and enumeration of coliforms in drinking water: Current methods and emerging approaches. J. Microbiol. Methods 2002, 49, 31–54. [Google Scholar] [CrossRef]
- Huang, J.; Wang, H.T.; Zhang, K.S. Modification of PES membrane with Ag-SiO2: Reduction of biofouling and improvement of filtration performance. Desalination 2014, 336, 8–17. [Google Scholar] [CrossRef]
- Ghaemi, N. Novel antifouling nano-enhanced thin-film composite membrane containing cross-linkable acrylate-alumoxane nanoparticles for water softening. J. Colloid Interface Sci. 2017, 485, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, P.; Prasannadevi, D.; Mohan, D. Preparation and performance of silver nanoparticle incorporated polyetherethersulfone nanofiltration membranes. High Perform. Polym. 2013, 25, 174–187. [Google Scholar] [CrossRef]
- Gebru, K.A.; Das, C. Removal of bovine serum albumin from wastewater using fouling resistant ultrafiltration membranes based on the blends of cellulose acetate, and PVP-TiO2 nanoparticles. J. Environ. Manag. 2017, 200, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Peng, H.D.; Wang, W.Z.; Liu, T.X. Crystallization behavior of poly(epsilon-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar]
- Chew, N.G.P.; Zhao, S.S.; Malde, C.; Wang, R. Superoleophobic surface modification for robust membrane distillation performance. J. Membr. Sci. 2017, 541, 162–173. [Google Scholar] [CrossRef]
- Chew, N.G.P.; Zhao, S.S.; Malde, C.; Wang, R. Polyvinylidene fluoride membrane modification via oxidant-induced dopamine polymerization for sustainable direct-contact membrane distillation. J. Membr. Sci. 2018, 563, 31–42. [Google Scholar] [CrossRef]
- Abedini, R.; Mousavi, S.M.; Aminzadeh, R. A novel cellulose acetate (CA) membrane using TiO2 nanoparticles: Preparation, characterization and permeation study. Desalination 2011, 277, 40–45. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Rajaeian, B.; Rahimnejad, M. TiO2 entrapped nano-composite PVDF/SPES membranes: Preparation, characterization, antifouling and antibacterial properties. Desalination 2011, 278, 343–353. [Google Scholar] [CrossRef]
- Ayyaru, S.; Ahn, Y.H. Fabrication and separation performance of polyethersulfone/sulfonated TiO2 (PES-STiO2) ultrafiltration membranes for fouling mitigation. J. Ind. Eng. Chem. 2018, 67, 199–209. [Google Scholar] [CrossRef]
- Lou, L.H.; Kendall, R.J.; Smith, E.; Ramkumar, S.S. Functional PVDF/rGO/TiO2 nanofiber webs for the removal of oil from water. Polymer 2020, 186, 122028. [Google Scholar] [CrossRef]
- Lihua, L.; Kendall, R.J.; Ramkumar, S. Comparision of hydrophilic PVA/TiO2 and hydrophobic PVDF/TiO2 microfiber webs on the dye pollutant photo-catalyzation. J. Environ. Chem. Eng. 2020, 9, 103914. [Google Scholar]
- Chen, X.J.; Huan, G.; An, C.J.; Feng, R.F.; Wu, Y.H.; Huang, C. Plasma-induced PAA-ZnO coated PVDF membrane for oily wastewater treatment: Preparation, optimization, and characterization through Taguchi OA design and synchrotron-based X-ray analysis. J. Membr. Sci. 2019, 582, 70–82. [Google Scholar] [CrossRef]
- Bonan, R.F.; Mota, M.F.; Farias, R.M.D.; da Silva, S.D.; Bonan, P.R.F.; Diesel, L.; Menezes, R.R.; Perez, D.E.D. In vitro antimicrobial and anticancer properties of TiO2 blow-spun nanofibers containing silver nanoparticles. J. Ind. Eng. Chem. 2019, 104, 109876. [Google Scholar] [CrossRef]
- Sunada, K.; Kikuchi, Y.; Hashimoto, K.; Fujishima, A. Bactericidal and detoxification effects of TiO2 thin film photocatalysts. Environ. Sci. Technol. 1998, 32, 726–728. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Takeshima, T.; Tada, Y.; Sakaguchi, N.; Watari, F.; Fugetsu, B. DNA/Ag nanoparticles as antibacterial agents against gram-negative bacteria. Nanomaterials 2015, 5, 284–297. [Google Scholar] [CrossRef]
- Soo, J.Z.; Chai, L.C.; Ang, B.C.; Ong, B.H. Enhancing the Antibacterial Performance of Titanium Dioxide Nanofibers by Coating with Silver Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 5743–5751. [Google Scholar] [CrossRef]
- Yang, L.; Ye, F.Y.; Liu, P.; Wang, F.Z. The Visible-Light Photocatalytic Activity and Antibacterial Performance of Ag/AgBr/TiO2 Immobilized on Activated Carbon. Photochem. Photobiol. 2016, 92, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Dalai, S.; Pakrashi, S.; Kumar, R.S.S.; Chandrasekaran, N.; Mukherjee, A. A comparative cytotoxicity study of TiO2 nanoparticles under light and dark conditions at low exposure concentrations. Toxicol. Res. 2012, 1, 116–130. [Google Scholar] [CrossRef]
- Reisner, A.; Haagensen, J.A.J.; Schembri, M.A.; Zechner, E.L.; Molin, S. Development and maturation of Escherichia coli K-12 biofilms. Mol. Microbiol. 2003, 48, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Malamis, S.; Andreadakis, A. Fractionation of proteins and carbohydrates of extracellular polymeric substances in a membrane bioreactor system. Bioresour. Technol. 2009, 100, 3350–3357. [Google Scholar] [CrossRef]
- Jimenez-Pardo, I.; van der Ven, L.G.J.; van Benthem, R.A.T.M.; de With, G.; Esteves, A.C.C. Hydrophilic self-replenishing coatings with long-term water stability for anti-fouling applications. Coatings 2018, 8, 184. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Hou, J.W.; Zhang, Y.T.; Tian, M.M.; He, T.; Liu, J.D.; Chen, V. Polymeric antimicrobial membranes enabled by nanomaterials for water treatment. J. Membr. Sci. 2018, 550, 173–197. [Google Scholar] [CrossRef]



| Pore Size (µm) | 0.45 |
| Membrane Porosity (%) | 80 |
| Thickness (µm) | 110 |
| Color | White |
| Properties | TiO2-NP | AgNP |
|---|---|---|
| Appearance | White Powder | Yellow Brown Colloid |
| Crystalline Structure | 80% Anatase, 20% Rutile | - |
| Primary Particle Size (nm) | 21 | - |
| Average Particle Size (nm) | - | 5–20 |
| Particle Shape | - | Nanospheres |
 |  | |
| pH | - | 6 ± 1 |
| Specific gravity (g mL−1) | - | 1.01 |
| Tamped density (g L−1) | 130 | - |
| Specific surface area (m2 g−1) | 50 | - |
| Content (wt%) | ˃99.5 | - |
| Membrane | Concentration of TiO2-NP (ppm) | Concentration of AgNP (ppm) | Immersion Time (h) |
|---|---|---|---|
| Original PVDF | |||
| M1 | 0 | 0 | 0 |
| Control PVDF | |||
| TiO2-NP | 10 | 0 | 24 |
| AgNP | 0 | 10 | 24 |
| Modified PVDF | |||
| M2 | 10 | 10 | 8 |
| M3 | 10 | 10 | 16 |
| M4 | 10 | 10 | 24 |
| M5 | 10 | 20 | 24 |
| M6 | 20 | 10 | 24 |
| Membrane | C (%) | F (%) | O (%) | Ti (%) | Ag (%) |
|---|---|---|---|---|---|
| M1 | 55.11 ± 0.18 | 43.61 ± 0.22 | 1.28 ± 0.08 | - | - |
| M2 | 58.16 ± 0.21 | 35.05 ± 0.28 | 4.36 ± 0.14 | 1.39 ± 0.13 | 1.03 ± 0.07 |
| M3 | 35.89 ± 0.17 | 30.42 ± 0.34 | 20.51 ± 0.27 | 8.33 ± 0.22 | 4.84 ± 0.11 |
| M4 | 45.02 ± 0.17 | 34.42 ± 0.27 | 12.65 ± 0.18 | 4.29 ± 0.16 | 3.62 ± 0.08 |
| M4after | 55.62 ± 0.07 | 37. 59 ± 0.11 | 5. 36 ± 0.06 | 0.93 ± 0.02 | 0.51 ± 0.01 |
| M5 | 46.57 ± 0.18 | 33.61 ± 0.30 | 12.19 ± 0.20 | 4.06 ± 0.16 | 3.57 ± 0.09 |
| M6 | 30.87 ± 0.16 | 25.46 ± 0.31 | 24.48 ± 0.30 | 13.80 ± 0.26 | 5.39 ± 0.11 |
| Membrane | Porosity (%) |
|---|---|
| M1 | 61.33 ± 2.83 |
| M2 | 67.19 ± 3.70 |
| M3 | 67.81 ± 3.79 |
| M4 | 66.74 ± 2.45 |
| M5 | 66.45 ± 2.78 |
| M6 | 64.10 ± 4.73 |
| TiO2-NP (control) | 65.99 ± 10.35 |
| AgNP (control) | 66.52 ± 3.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samree, K.; Srithai, P.-u.; Kotchaplai, P.; Thuptimdang, P.; Painmanakul, P.; Hunsom, M.; Sairiam, S. Enhancing the Antibacterial Properties of PVDF Membrane by Hydrophilic Surface Modification Using Titanium Dioxide and Silver Nanoparticles. Membranes 2020, 10, 289. https://doi.org/10.3390/membranes10100289
Samree K, Srithai P-u, Kotchaplai P, Thuptimdang P, Painmanakul P, Hunsom M, Sairiam S. Enhancing the Antibacterial Properties of PVDF Membrane by Hydrophilic Surface Modification Using Titanium Dioxide and Silver Nanoparticles. Membranes. 2020; 10(10):289. https://doi.org/10.3390/membranes10100289
Chicago/Turabian StyleSamree, Kajeephan, Pen-umpai Srithai, Panaya Kotchaplai, Pumis Thuptimdang, Pisut Painmanakul, Mali Hunsom, and Sermpong Sairiam. 2020. "Enhancing the Antibacterial Properties of PVDF Membrane by Hydrophilic Surface Modification Using Titanium Dioxide and Silver Nanoparticles" Membranes 10, no. 10: 289. https://doi.org/10.3390/membranes10100289
APA StyleSamree, K., Srithai, P.-u., Kotchaplai, P., Thuptimdang, P., Painmanakul, P., Hunsom, M., & Sairiam, S. (2020). Enhancing the Antibacterial Properties of PVDF Membrane by Hydrophilic Surface Modification Using Titanium Dioxide and Silver Nanoparticles. Membranes, 10(10), 289. https://doi.org/10.3390/membranes10100289