Development of RALA-Based Mannosylated Nanocarriers for Targeted Delivery of Minicircle DNA Vaccines Encoding HPV-16 Oncogenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Purification of mcDNA Vectors
2.2. Agarose Gel Electrophoresis
2.3. Formulation and Functionalization of RALA-Based Nanoparticles
2.4. Characterization of Nanoparticles
2.4.1. Characterization of Hydrodynamic Diameter, Polydispersity Index and Surface Charge
2.4.2. Evaluation of Complexation Efficiency
2.4.3. Nanoparticle Morphology Analysis
2.4.4. Identification of Functional Groups on Nanoparticles
2.4.5. Conditions of Stability and Decomplexation Assays
2.5. In Vitro Studies
2.5.1. Cell Culture and Growth Conditions
2.5.2. Cell Seeding and Transfections
2.5.3. Cell Viability Study
2.5.4. Reverse Transcription Polymerase Chain Reaction
2.5.5. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
2.5.6. E7 Protein Analysis and Mannose-Receptor Competitive Inhibition Assay
2.6. Data Processing and Statistical Analysis
3. Results
3.1. Optimization of RALA Concentration for mcDNA Complexation
3.2. Physicochemical Characterization of Optimized RALA-Based Nanoparticles
3.2.1. Hydrodynamic Diameter, Polydispersity Index, Zeta Potential, and Complexation Efficiency
3.2.2. Morphological Evaluation by Transmission Electron Microscopy
3.2.3. FTIR Analysis
3.2.4. Stability and Decomplexation Assays
3.3. Cytotoxicity Evaluation in Dendritic Cells
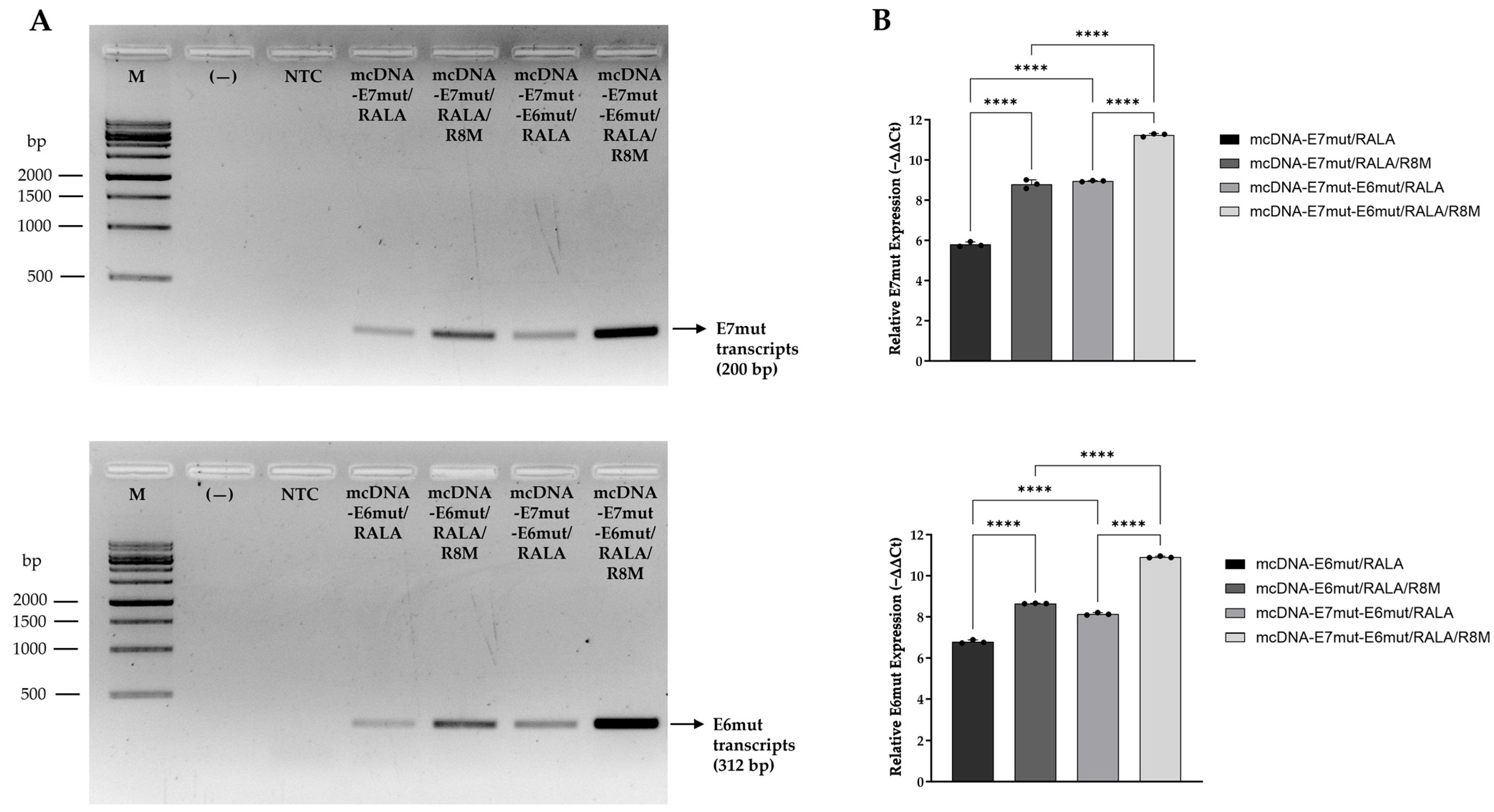
3.4. Evaluation of Relative Gene Expression by RT-PCR and RT-qPCR
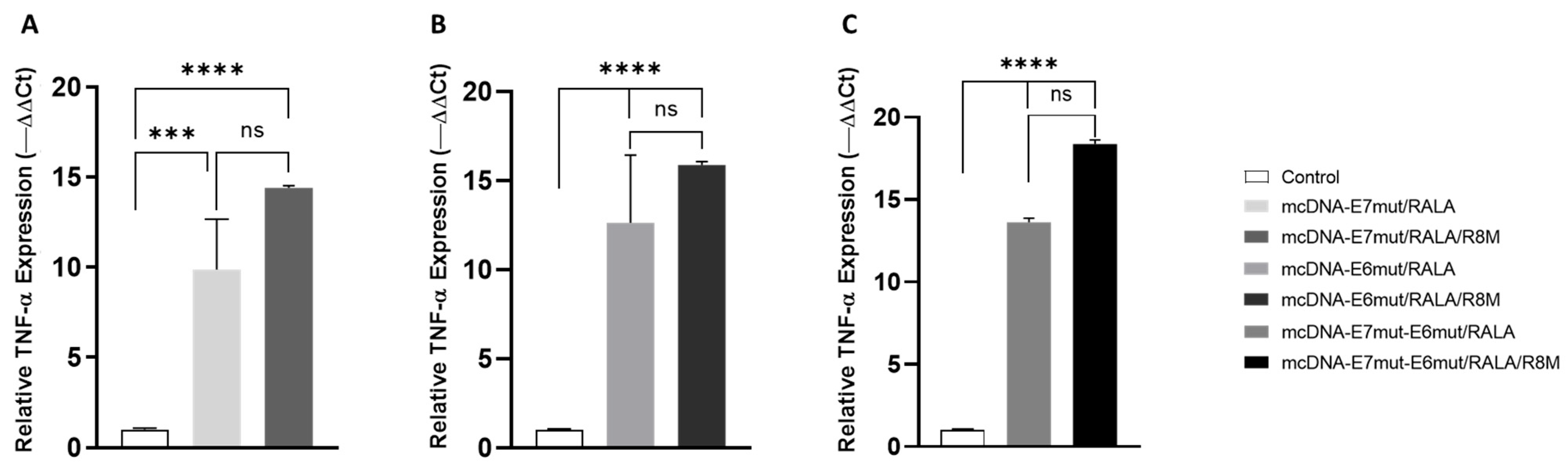
3.5. Evaluation of Pro-Inflammatory Cytokines Gene Expression by RT-qPCR
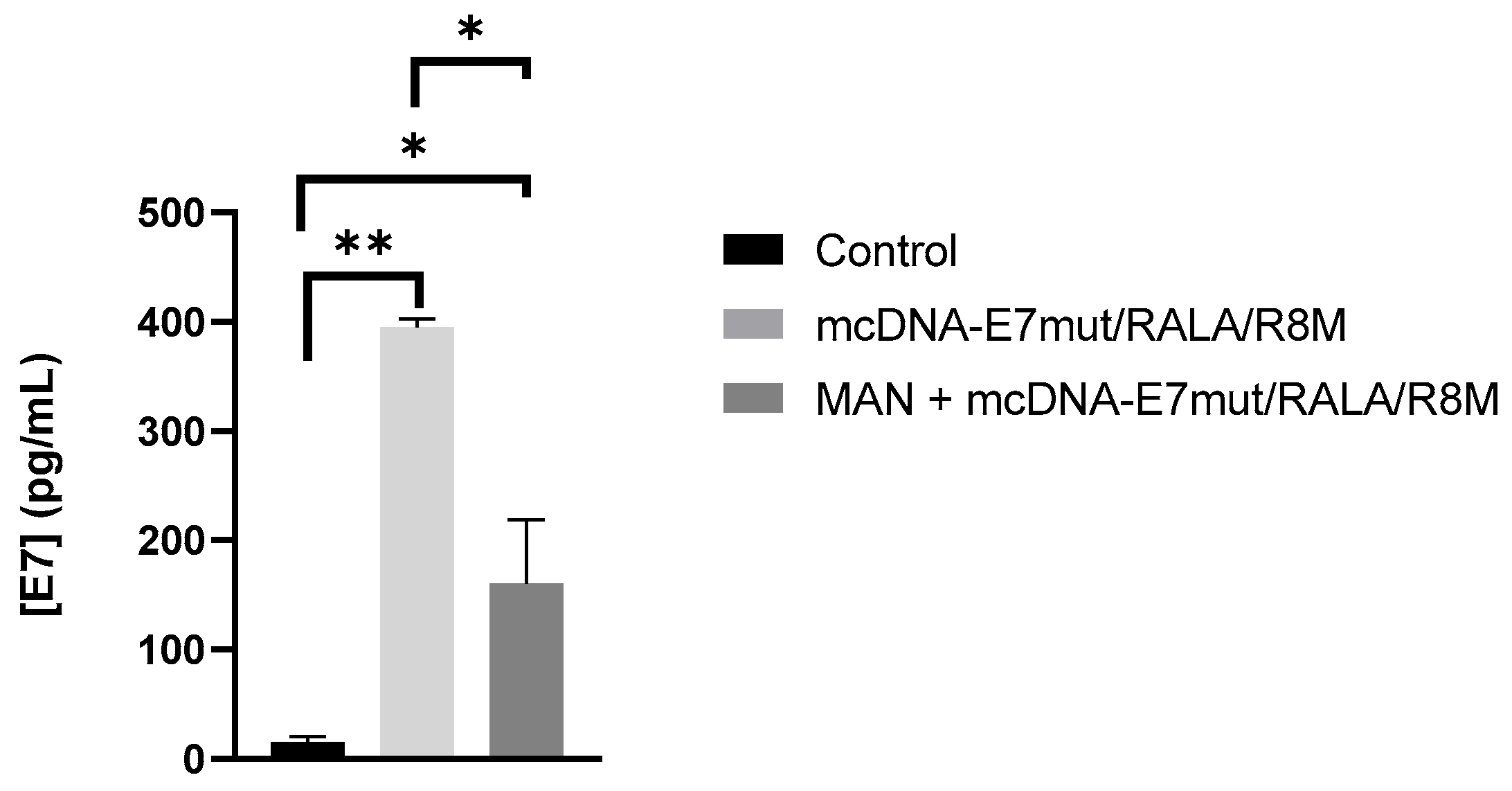
3.6. Evaluation of E7 Protein Levels and Mannose-Receptor Mediated Uptake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| APCs | Antigen Presenting Cells |
| ATCC | American Type Culture Collection |
| cDNA | Complementary DNA |
| CE | Complexation Efficiency |
| CMV | Cytomegalovirus |
| CPPs | Cell-Penetrating Peptides |
| DCs | Dendritic Cells |
| DLS | Dynamic Light Scattering |
| DNA | Deoxyribonucleic Acid |
| dsDNA | Double-Stranded DNA |
| FBS | Fetal Bovine Serum |
| FTIR | Fourier Transform Infrared Spectroscopy |
| FW | Forward |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| HICs | High-Income Countries |
| HPV | Human Papillomavirus |
| hrHPV | High-Risk Human Papillomavirus |
| LB | Luria–Bertani |
| LMICs | Low And Middle-Income Countries |
| mcDNA | Minicircle DNA |
| MEM-α | Minimum Essential Medium Alpha |
| MHC | Major Histocompatibility Complex |
| mRNA | Messenger Ribonucleic Acid |
| NPs | Nanoparticles |
| N/P | Amine to Phosphate |
| NTC | No-Template Control |
| PCR | Polymerase Chain Reaction |
| PDI | Polydispersity Index |
| pDNA | Plasmid Deoxyribonucleic Acid |
| PEI | Poly(Ethyleneimine) |
| pH | Potential of Hydrogen |
| pRB | Retinoblastoma Protein |
| R8 | Octa-Arginine |
| R8M | Octa-Arginine Mannose |
| RNA | Ribonucleic Acid |
| RTase | Reverse Transcriptase |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| RT-qPCR | Quantitative Reverse Transcription Polymerase Chain Reaction |
| RV | Reverse |
| SDS | Sodium Dodecyl Sulphate |
| TB | Terrific Broth |
| TEM | Transmission Electron Microscopy |
References
- Ci, A.; Ne, M. Cervical Cancer: A Health Limiting Condition. Gynecol. Obs. 2016, 6, 378. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Harden, M.E.; Munger, K. Human papillomavirus molecular biology. Mut. Res. Rev. Mutat. Res. 2017, 772, 3–12. [Google Scholar] [CrossRef]
- Burmeister, C.A.; Khan, S.F.; Schafer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef]
- Almeida, A.M.; Queiroz, J.A.; Sousa, F.; Sousa, Â. Cervical cancer and HPV infection: Ongoing therapeutic research to counteract the action of E6 and E7 oncoproteins. Drug Discov. Today 2019, 24, 2044–2057. [Google Scholar] [CrossRef]
- Movahed, F.; Darzi, S.; Mahdavi, P.; Mahdi, M.S.; Qutaiba, B.; Allela, O.; Sameer, H.N.; Adil, M.; Zarkhah, H.; Yasamineh, S.; et al. The potential use of therapeutics and prophylactic mRNA vaccines in human papillomavirus (HPV). Virol. J. 2024, 21, 124. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2021 update. Int. J. Gynaecol. Obs. 2021, 155, 28–44. [Google Scholar] [CrossRef]
- Burd, E.M.; Dean, C.L. Human Papillomavirus. In Diagnostic Microbiology of the Immunocompromised Host; Wiley: Hoboken, NJ, USA, 2016; pp. 177–195. [Google Scholar]
- Estêvão, D.; Costa, N.R.; da Costa, R.M.G.; Medeiros, R. Hallmarks of HPV carcinogenesis: The role of E6, E7 and E5 oncoproteins in cellular malignancy. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 153–162. [Google Scholar] [CrossRef]
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 890–907. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef]
- Williamson, A.-L. Recent Developments in Human Papillomavirus (HPV) Vaccinology. Viruses 2023, 15, 1440. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Ho, W.; Gao, M.; Li, F.; Li, Z.; Zhang, X.Q.; Xu, X.A.-O. Next-Generation Vaccines: Nanoparticle-Mediated DNA and mRNA Delivery. Adv. Healthc. Mater. 2021, 10, 2001812. [Google Scholar] [CrossRef]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Primers 2022, 2, 24. [Google Scholar] [CrossRef]
- Eusébio, D.; Neves, A.R.; Costa, D.; Biswas, S.; Alves, G.; Cui, Z.; Sousa, Â. Methods to improve the immunogenicity of plasmid DNA vaccines. Drug Discov. Today 2021, 26, 2575–2592. [Google Scholar] [CrossRef]
- Yang, B.; Jeang, J.; Yang, A.; Wu, T.C.; Hung, C.-F. DNA vaccine for cancer immunotherapy. Hum. Vaccin. Immunother. 2014, 10, 3153–3164. [Google Scholar] [CrossRef]
- Ledesma-Feliciano, C.; Chapman, R.; Hooper, J.W.; Elma, K.; Zehrung, D.; Brennan, M.B.; Spiegel, E.K. Improved DNA Vaccine Delivery with Needle-Free Injection Systems. Vaccines 2023, 11, 280. [Google Scholar] [CrossRef]
- Li, L.; Saade, F.; Petrovsky, N. The future of human DNA vaccines. J. Biotechnol. 2012, 162, 171–182. [Google Scholar] [CrossRef]
- Eusébio, D.; Almeida, A.M.; Alves, J.M.; Maia, C.J.; Queiroz, J.A.; Sousa, F.; Sousa, Â. The Performance of Minicircle DNA Versus Parental Plasmid in p53 Gene Delivery Into HPV-18-Infected Cervical Cancer Cells. Nucleic Acid Ther. 2021, 31, 82–91. [Google Scholar] [CrossRef]
- Gaspar, V.; de Melo-Diogo, D.; Costa, E.; Moreira, A.; Queiroz, J.; Pichon, C.; Correia, I.; Sousa, F. Minicircle DNA vectors for gene therapy: Advances and applications. Expert. Opin. Biol. Ther. 2015, 15, 353–379. [Google Scholar] [CrossRef]
- Gupta, A.; Andresen, J.L.; Manan, R.S.; Langer, R. Nucleic acid delivery for therapeutic applications. Adv. Drug Deliv. Rev. 2021, 178, 113834. [Google Scholar] [CrossRef]
- Bai, H.; Lester, G.M.S.; Petishnok Laura, C.; Dean, D.A. Cytoplasmic transport and nuclear import of plasmid DNA. Biosci. Rep. 2017, 37, BSR20160616. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Rong, X.; Tang, C.; Liu, H.; Yue, L.; Su, R.; Wang, Y.; Qi, W. Alkylated RALA-Derived Peptides for Efficient Gene Delivery. Biomacromolecules 2024, 25, 8046–8057. [Google Scholar] [CrossRef]
- Massey, A.S.; Pentlavalli, S.; Cunningham, R.; McCrudden, C.M.; McErlean, E.M.; Redpath, P.; Ali, A.A.; Annett, S.; McBride, J.W.; McCaffrey, J.; et al. Potentiating the Anticancer Properties of Bisphosphonates by Nanocomplexation with the Cationic Amphipathic Peptide, RALA. Mol. Pharm. 2016, 13, 1217–1228. [Google Scholar] [CrossRef]
- O’Doherty, M.; Mulholland, E.J.; Chambers, P.; Pentlavalli, S.; Ziminska, M.; Chalanqui, M.J.; Pauly, H.M.; Sathy, B.N.; Donahue, T.H.; Kelly, D.J.; et al. Improving the Intercellular Uptake and Osteogenic Potency of Calcium Phosphate via Nanocomplexation with the RALA Peptide. Nanomaterials 2020, 10, 2442. [Google Scholar] [CrossRef]
- Xing, L.; Fan, Y.-T.; Zhou, T.-J.; Gong, J.-H.; Cui, L.-H.; Cho, K.-H.; Choi, Y.-J.; Jiang, H.-L.; Cho, C.-S. Chemical Modification of Chitosan for Efficient Vaccine Delivery. Molecules 2018, 23, 229. [Google Scholar] [CrossRef]
- Nahar, U.J.; Toth, I.; Skwarczynski, M. Mannose in vaccine delivery. J. Control. Release 2022, 351, 284–300. [Google Scholar] [CrossRef]
- Paurević, M.; Šrajer Gajdošik, M.; Ribić, R. Mannose Ligands for Mannose Receptor Targeting. Int. J. Mol. Sci. 2024, 25, 1370. [Google Scholar] [CrossRef]
- Serra, A.S.; Eusébio, D.; Neves, A.R.; Albuquerque, T.; Bhatt, H.; Biswas, S.; Costa, D.; Sousa, Â. Synthesis and Characterization of Mannosylated Formulations to Deliver a Minicircle DNA Vaccine. Pharmaceutics 2021, 13, 673. [Google Scholar] [CrossRef]
- Eusébio, D.; Paul, M.; Biswas, S.; Cui, Z.; Costa, D.; Sousa, Â. Mannosylated polyethylenimine-cholesterol-based nanoparticles for targeted delivery of minicircle DNA vaccine against COVID-19 to antigen-presenting cells. Int. J. Pharm. 2024, 654, 123959. [Google Scholar] [CrossRef]
- Costa, A.; Sarmento, B.; Seabra, V. Mannose-functionalized solid lipid nanoparticles are effective in targeting alveolar macrophages. Eur. J. Pharm. Sci. 2018, 114, 103–113. [Google Scholar] [CrossRef]
- Ventura, C.; Eusébio, D.; Gonçalves, A.M.; Barroca-Ferreira, J.; Costa, D.; Cui, Z.; Passarinha, L.A.; Sousa, Â. Maximization of the Minicircle DNA Vaccine Production Expressing SARS-CoV-2 RBD. Biomedicines 2022, 10, 990. [Google Scholar] [CrossRef]
- Diogo, M.M.; Queiroz, J.A.; Monteiro, G.A.; Martins, S.A.M.; Ferreira, G.N.M.; Prazeres, D.M.F.; Prazeres, D.M. Purification of a cystic fibrosis plasmid vector for gene therapy using hydrophobic interaction chromatography. Biotechnol. Bioeng. 2000, 68, 576–583. [Google Scholar] [CrossRef]
- Almeida, A.M.; Eusébio, D.; Queiroz, J.A.; Sousa, F.; Sousa, A. The use of size-exclusion chromatography in the isolation of supercoiled minicircle DNA from Escherichia coli lysate. J. Chromatogr. A 2020, 1609, 460444. [Google Scholar] [CrossRef]
- Chen, K.; Singh, V.K.; Tang, P.; Bao, Z.; He, T.; Xiang, Y.; Gong, W.; Yoshimura, T.; Le, Y.; Tessarollo, L.; et al. Deficiency in Fpr2 results in reduced numbers of Lin−cKit+Sca1+ myeloid progenitor cells. J. Biol. Chem. 2018, 293, 13452–13463. [Google Scholar] [CrossRef]
- Mady, M.M.; Mohammed, W.A.; El-Guendy, N.M.; Elsayed, A.A. Interaction of DNA and polyethylenimine: Fourier-transform infrared (FTIR) and differential scanning calorimetry (DSC) studies. Int. J. Phys. Sci. 2011, 6, 7328–7334. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef]
- Petit, T.; Puskar, L. FTIR spectroscopy of nanodiamonds: Methods and interpretation. Diam. Relat. Mater. 2018, 89, 52–66. [Google Scholar] [CrossRef]
- Imani, R.; Emami, S.H.; Faghihi, S. Synthesis and characterization of an octaarginine functionalized graphene oxide nano-carrier for gene delivery applications. Phys. Chem. Chem. Phys. 2015, 17, 6328–6339. [Google Scholar] [CrossRef]
- Horák, D.; Babic, M.; Jendelová, P.; Herynek, V.; Trchová, M.; Pientka, Z.; Pollert, E.; Hájek, M.; Syková, E. D-mannose-modified iron oxide nanoparticles for stem cell labeling. Bioconjug. Chem. 2007, 18, 635–644. [Google Scholar] [CrossRef]
- Radaic, A.; Pugliese, G.O.; Campese, G.C.; Pessine, F.B.T.; Jesus, M.B.D. Studying the Interactions Between Nanoparticles and Biological Systems. Quím Nova 2016, 39, 1236–1244. [Google Scholar]
- Ferreira, L.A.B.; Radaic, A.; Pugliese, G.O.; Valentini, M.B.; Oliveira, M.R.; de Jesus, M.B. Endocytosis and intracellular trafficking of nanomaterials. Acta Farm. Port. 2014, 3, 143–154. [Google Scholar]
- Marcucci, F.; Lefoulon, F. Active targeting with particulate drug carriers in tumor therapy: Fundamentals and recent progress. Drug Discov. Today 2004, 9, 219–228. [Google Scholar] [CrossRef]
- McCrudden, C.M.; McBride, J.W.; McCaffrey, J.; McErlean, E.M.; Dunne, N.J.; Kett, V.L.; Coulter, J.A.; Robson, T.; McCarthy, H.A.-O. Gene therapy with RALA/iNOS composite nanoparticles significantly enhances survival in a model of metastatic prostate cancer. Cancer Nanotechnol. 2018, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.; Ali, A.A.; McErlean, E.; Mulholland, E.J.; Short, A.; McCrudden, C.M.; McCaffrey, J.; Robson, T.; Kett, V.L.; Coulter, J.A.; et al. DNA vaccination via RALA nanoparticles in a microneedle delivery system induces a potent immune response against the endogenous prostate cancer stem cell antigen. Acta Biomater. 2019, 96, 480–490. [Google Scholar] [CrossRef]
- McCarthy, H.O.; McCaffrey, J.; McCrudden, C.M.; Zholobenko, A.; Ali, A.A.; McBride, J.W.; Massey, A.S.; Pentlavalli, S.; Chen, K.-H.; Cole, G.; et al. Development and characterization of self-assembling nanoparticles using a bio-inspired amphipathic peptide for gene delivery. J. Control. Release 2014, 189, 141–149. [Google Scholar] [CrossRef]
- Bombin, A.D.J.; Dunne, N.; McCarthy, H.O. Delivery of a peptide/microRNA blend via electrospun antimicrobial nanofibres for wound repair. Acta Biomater. 2023, 155, 304–322. [Google Scholar] [CrossRef]
- Silva, J.M.; Vandermeulen, G.; Oliveira, V.G.; Pinto, S.N.; Rodrigues, C.; Salgado, A.; Afonso, C.A.; Viana, A.S.; Jérôme, C.; Silva, L.C.; et al. Development of Functionalized Nanoparticles for Vaccine Delivery to Dendritic Cells: A Mechanistic Approach. Nanomedicine 2014, 9, 2639–2656. [Google Scholar] [CrossRef]
- Xiang, K.; Li, Y.; Cong, H.; Yu, B.; Shen, Y. Peptide-based non-viral gene delivery: A comprehensive review of the advances and challenges. Int. J. Biol. Macromol. 2024, 266, 131194. [Google Scholar] [CrossRef]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Gene therapy and DNA delivery systems. Int. J. Pharm. 2014, 459, 70–83. [Google Scholar] [CrossRef]
- Neves, A.R.; Sousa, A.; Faria, R.; Albuquerque, T.; Queiroz, J.A.; Costa, D. Cancer gene therapy mediated by RALA/plasmid DNA vectors: Nitrogen to phosphate groups ratio (N/P) as a tool for tunable transfection efficiency and apoptosis. Colloids Surf. B Biointerfaces 2020, 185, 110610. [Google Scholar] [CrossRef]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Xu, R.; Zhang, C.; Wang, X.; Li, C.; Hu, Y. Mannose-functionalized antigen nanoparticles for targeted dendritic cells, accelerated endosomal escape and enhanced MHC-I antigen presentation. Colloids Surf. B Biointerfaces 2021, 197, 111378. [Google Scholar] [CrossRef]
- Blanco, P.; Palucka, A.K.; Pascual, V.; Banchereau, J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008, 19, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-D.; Cheng, M.; Shang, P.-P.; Yang, Y.-Q. Role of IL-6 in dendritic cell functions. J. Leukoc. Biol. 2022, 111, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, F.; Liu, J.; Wang, G. Comparative study of dendritic cells matured by using IL-1β, IL-6, TNF-α and prostaglandins E2 for different time span. Exp. Ther. Med. 2017, 14, 1389–1394. [Google Scholar] [CrossRef]
- Zanna, M.Y.; Yasmin, A.R.; Omar, A.R.; Arshad, S.S.; Mariatulqabtiah, A.R.; Nur-Fazila, S.H.; Mahiza, M.I.N. Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species. Int. J. Mol. Sci. 2021, 22, 8044. [Google Scholar] [CrossRef]
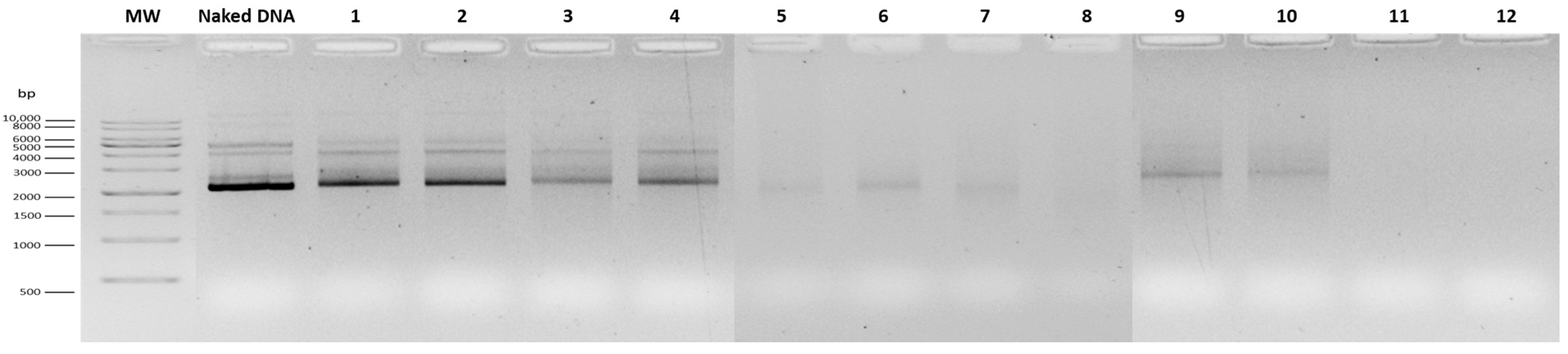
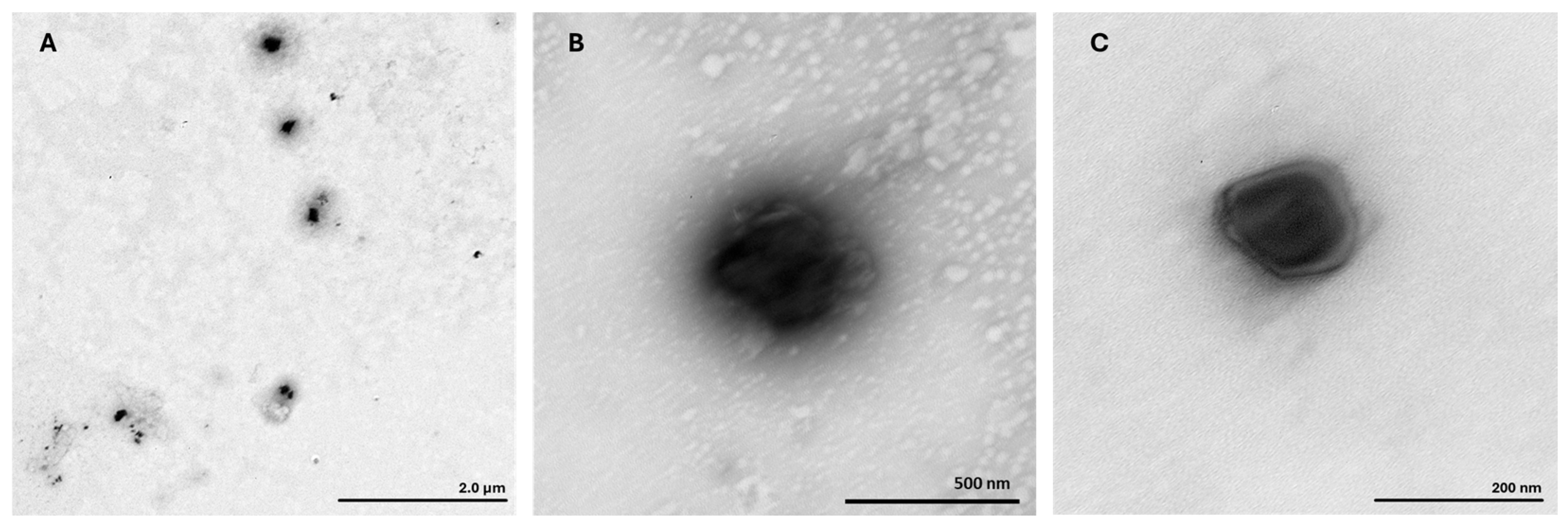
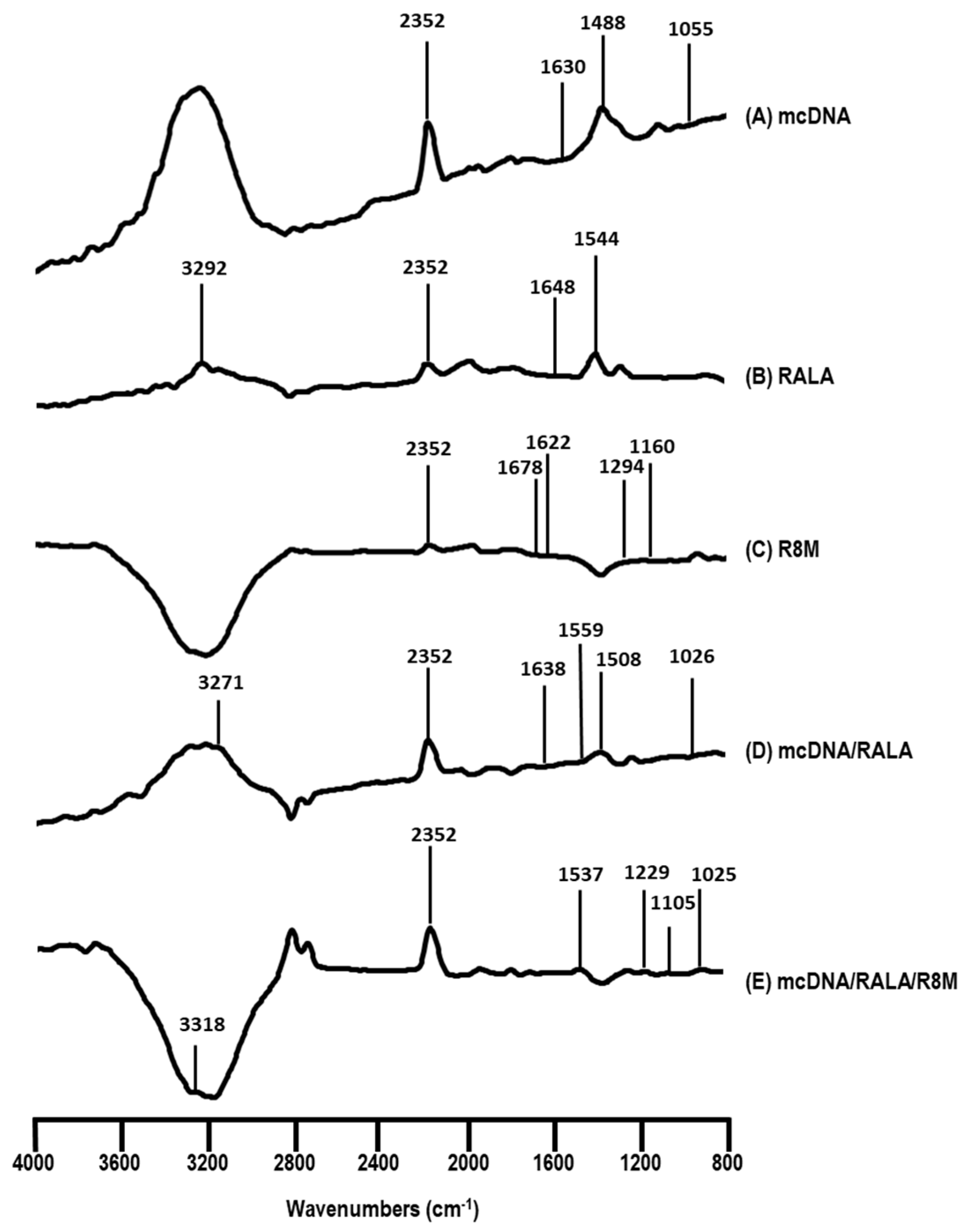
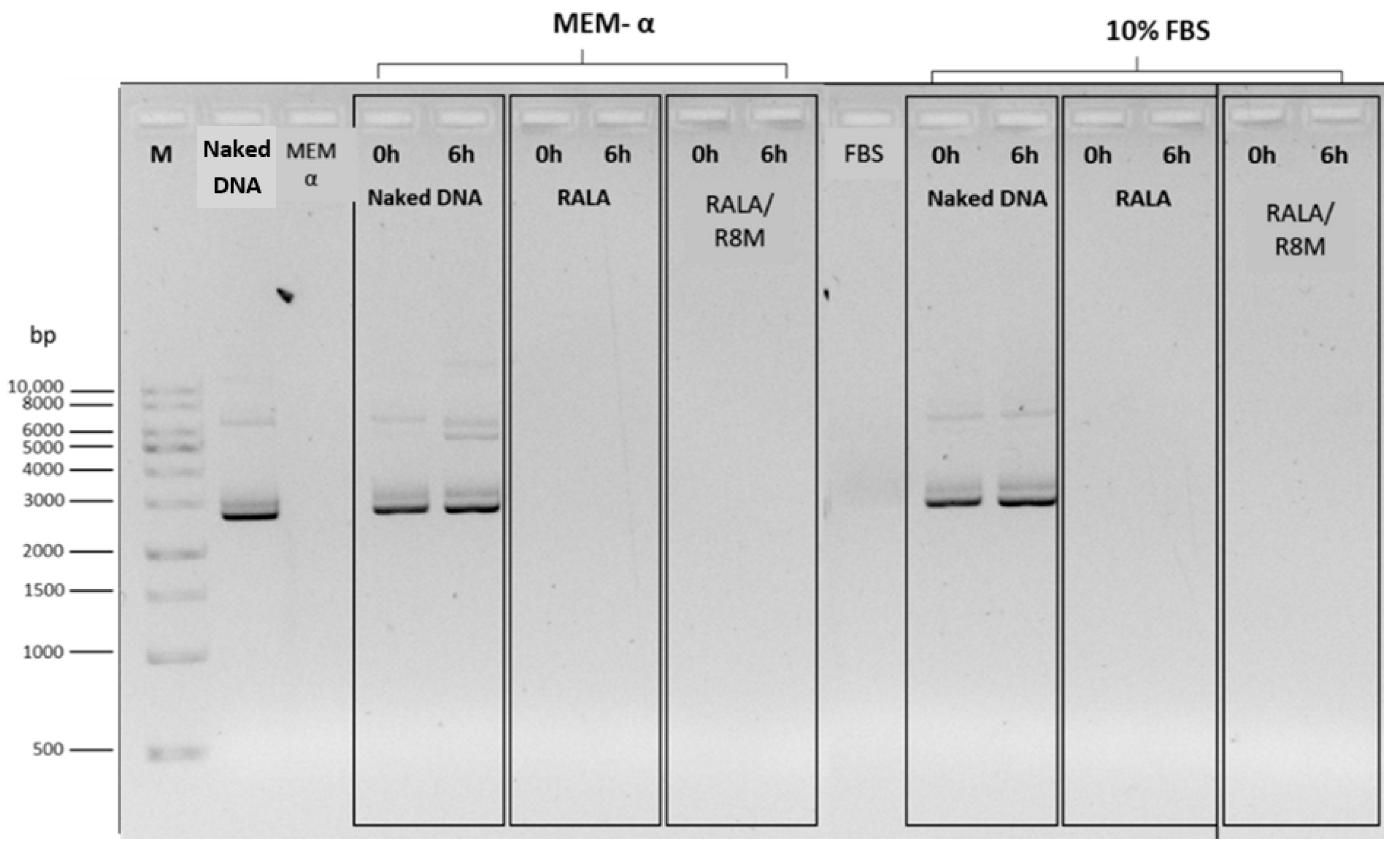



| Formulation | RALA Concentration (µg/mL) | R8M | Hydrodynamic Diameter (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|
| 1 | 29 | No | 164.51 ± 21.01 a | 0.60 ± 0.10 | −5.83 ± 0.18 |
| 2 | 29 | Yes | NV | NV | - |
| 3 | 58 | No | 172.54 ± 15.91 a | 0.44 ± 0.02 | −22.10 ± 0.53 |
| 4 | 58 | Yes | 181.36 ± 9.41 a | 0.46 ± 0.03 | −19.21 ± 2.75 |
| 5 | 72.5 | No | 82.72 ± 1.62 d | 0.20 ± 0.13 | −10.56 ± 3.47 |
| 6 | 72.5 | Yes | 90.78 ± 10.15 d | 0.27 ± 0.04 | −6.17 ± 0.24 |
| 7 | 81.2 | No | 112.16 ± 21.65 c | 0.25 ± 0.08 | −15.98 ± 7.39 |
| 8 | 81.2 | Yes | 137.24 ± 22.04 b | 0.32 ± 0.05 | −13.54 ± 2.71 |
| 9 | 87 | No | 125.02 ± 6.46 c | 0.38 ± 0.02 | −23.53 ± 1.33 |
| 10 | 87 | Yes | 138.91 ± 9.96 b | 0.36 ± 0.07 | −20.53 ± 1.70 |
| 11 | 116 | No | NV | NV | - |
| 12 | 116 | Yes | NV | NV | - |
| Formulation | Vector | R8M | Hydrodynamic Diameter (nm) | PDI | Zeta Potential (mV) | CE (%) |
|---|---|---|---|---|---|---|
| 1 | mcDNA E7mut | No | 86.70 ± 1.98 | 0.25 ± 0.03 | −9.87 ± 0.51 | 98.5 ± 0.91 |
| 2 | Yes | 122.04 ± 17.39 | 0.30 ± 0.02 | −11.04 ± 3.31 | 97.9 ± 2.23 | |
| 3 | mcDNA E6mut | No | 87.05 ± 8.16 | 0.23 ± 0.03 | −7.49 ± 0.19 | 99.5 ± 0.51 |
| 4 | Yes | 101.79 ± 17.94 | 0.25 ± 0.04 | −12.35 ± 0.42 | 99.5 ± 0.10 | |
| 5 | mcDNA E7mut-E6mut | No | 106.73 ± 12.07 | 0.29 ± 0.03 | −13.25 ± 2.88 | 99.4 ± 0.23 |
| 6 | Yes | 122.77 ± 20.54 | 0.29 ± 0.05 | −12.71 ± 3.09 | 99.2 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Giusti, A.; Eusébio, D.; Costa, M.; Silveira, I.; Biswas, S.; Costa, D.; Sousa, Â. Development of RALA-Based Mannosylated Nanocarriers for Targeted Delivery of Minicircle DNA Vaccines Encoding HPV-16 Oncogenes. Vaccines 2026, 14, 18. https://doi.org/10.3390/vaccines14010018
Giusti A, Eusébio D, Costa M, Silveira I, Biswas S, Costa D, Sousa Â. Development of RALA-Based Mannosylated Nanocarriers for Targeted Delivery of Minicircle DNA Vaccines Encoding HPV-16 Oncogenes. Vaccines. 2026; 14(1):18. https://doi.org/10.3390/vaccines14010018
Chicago/Turabian StyleGiusti, Andressa, Dalinda Eusébio, Matilde Costa, Inês Silveira, Swati Biswas, Diana Costa, and Ângela Sousa. 2026. "Development of RALA-Based Mannosylated Nanocarriers for Targeted Delivery of Minicircle DNA Vaccines Encoding HPV-16 Oncogenes" Vaccines 14, no. 1: 18. https://doi.org/10.3390/vaccines14010018
APA StyleGiusti, A., Eusébio, D., Costa, M., Silveira, I., Biswas, S., Costa, D., & Sousa, Â. (2026). Development of RALA-Based Mannosylated Nanocarriers for Targeted Delivery of Minicircle DNA Vaccines Encoding HPV-16 Oncogenes. Vaccines, 14(1), 18. https://doi.org/10.3390/vaccines14010018








