Retrospective Cohort Analysis of Survival After SARS-CoV-2 Infection by Vaccination Status in Jamaica, April–December 2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.3. Procedure
2.4. Inclusion and Exclusion Criteria
2.4.1. Inclusion Criteria
2.4.2. Exclusion Criteria
2.5. Variable Definitions
2.6. Statistical Analysis
2.7. Ethical Approval
3. Results
3.1. Vaccines Administered and Vaccine Coverage
3.2. Cohort Selection
3.3. Demographic Characteristics
3.4. COVID-19 Mortality
3.5. Vaccine Effectiveness
3.6. Sensitivity Analyses
4. Discussion
4.1. Strengths
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://data.who.int/dashboards/covid19/deaths?n=o (accessed on 27 June 2025).
- Webster-Kerr, K.; Grant, A.; Harris, A.; Thorpe, R.; Rowe, D.; Henningham, D.; Mullings, T.; Wellington, I.; Wiggan, J.; Gordon-Johnson, K.A.; et al. Risk factors associated with severe COVID-19 outcomes in Jamaica: A cross-sectional study of national surveillance data. Rev. Panam. De Salud Pública 2024, 48, e36. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, M.; Yin, L.; Wang, K.; Zhou, Y.; Zhou, M.; Lu, Y. COVID-19 treatment: Close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2). Int. J. Antimicrob. Agents 2020, 56, 106080. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Weekly Epidemiological Update on COVID-19–28 December 2021. Issue 72. 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---28-december-2021# (accessed on 2 October 2025).
- Our World in Data. Cumulative Confirmed COVID-19 Cases per Million People. Available online: https://ourworldindata.org/covid-cases (accessed on 2 October 2025).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2022, 114, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Dael, N.; Verweij, S.; Balafas, S.; Mubarik, S.; Rengerink, K.O.; Pasmooij, A.M.G.; van Baarle, D.; Mol, P.G.; de Bock, G.H.; et al. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection and severe outcomes in adults: A systematic review and meta-analysis of European studies published up to 22 January 2024. Eur. Respir. Rev. 2025, 34, 240222. [Google Scholar] [CrossRef]
- Liu, B.; Stepien, S.; Dobbins, T.; Gidding, H.; Henry, D.; Korda, R.; Mills, L.; Pearson, S.-A.; Pratt, N.; Vajdic, C.M.; et al. Effectiveness of COVID-19 vaccination against COVID-19 specific and all-cause mortality in older Australians: A population based study. Lancet Reg. Health–West. Pac. 2023, 40, 100928. [Google Scholar] [CrossRef]
- Rahmani, K.; Shavaleh, R.; Forouhi, M.; Disfani, H.F.; Kamandi, M.; Oskooi, R.K.; Foogerdi, M.; Soltani, M.; Rahchamani, M.; Mohaddespour, M.; et al. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis. Front. Public Health 2022, 10, 873596. [Google Scholar] [CrossRef]
- Soheili, M.; Khateri, S.; Moradpour, F.; Mohammadzedeh, P.; Zareie, M.; Mortazavi, S.M.M.; Manifar, S.; Kohan, H.G.; Moradi, Y. The efficacy and effectiveness of COVID-19 vaccines around the world: A mini-review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 42. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Bloom, D.E.; Cadarette, D.; Ferranna, M. The societal value of vaccination in the age of COVID-19. Am. J. Public Health 2021, 111, 1049–1054. [Google Scholar] [CrossRef]
- Utami, A.M.; Rendrayani, F.; Khoiry, Q.A.; Noviyanti, D.; A Suwantika, A.; Postma, M.J.; Zakiyah, N. Economic evaluation of COVID-19 vaccination: A systematic review. J. Glob. Health 2023, 13, 06001. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhao, J.; Han, P.; Zhang, J.; Wang, Q.; Wang, Q.; Wei, X.; Yang, L.; Ren, T.; Zhan, S.; et al. Cost-effectiveness of COVID-19 vaccination: A systematic review. J. Evid.-Based Med. 2023, 16, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q. Hoarding vaccines or hedging vaccine R&D risks?—Motivation for overbooking COVID-19 vaccines in high-income countries. Int. J. Health Policy Manag. 2024, 13, 8350. [Google Scholar] [PubMed]
- Fox, A.M.; Choi, Y.; Lin, L. Substantial disparities in COVID-19 vaccine uptake and unmet immunization demand in low-and middle-income countries: Study examines COVID-19 vaccine uptake and unmet immunization demand in low-and middle-income countries. Health Aff. 2023, 42, 1697–1705. [Google Scholar] [CrossRef]
- World Health Organization. COVAX: Equitable Access to COVID-19 Vaccines. Available online: https://www.who.int/initiatives/act-accelerator/covax (accessed on 11 June 2025).
- Pan American Health Organization. Jamaica Becomes First Country in the Caribbean to Receive COVID 19 Vaccines Through the COVAX Facility. PAHO. Available online: https://www.paho.org/en/news/15-3-2021-jamaica-becomes-first-country-caribbean-receive-covid-19-vaccines-through-covax (accessed on 25 June 2025).
- Hodges, P.G. Health Ministry Assures of Safety and Efficacy of Vaccines. Jamaica Information Service. Available online: https://jis.gov.jm/features/health-ministry-assures-of-safety-and-efficacy-of-vaccines/ (accessed on 11 June 2025).
- Ministry of Health & Wellness (Jamaica). Vaccination Continues This Weekend—Astrazeneca, Sinopharm, Johnson & Johnson, Pfizer Available to the Public. Available online: https://www.moh.gov.jm/vaccination-continues-this-weekend-astrazeneca-sinopharm-johnson-johnson-pfizer-available-to-the-public/ (accessed on 11 June 2025).
- Ministry of Health & Wellness (Jamaica). Vitals (COVID-19)—Quarterly Report. Available online: https://www.moh.gov.jm/covidvitals/ (accessed on 25 June 2025).
- Laurencin, C.T.; Wu, Z.H.; Grady, J.J.; Wu, R.; Walker, J.M. Changes in COVID-19-Associated deaths during a year among blacks and Hispanics compared to whites in the state of Connecticut. J. Racial Ethn. Health Disparities 2022, 9, 2049–2055. [Google Scholar] [CrossRef]
- Robinson, E.; Jones, A.; Daly, M. International estimates of intended uptake and refusal of COVID-19 vaccines: A rapid systematic review and meta-analysis of large nationally representative samples. Vaccine 2021, 39, 2024–2034. [Google Scholar] [CrossRef]
- Restrepo, N.; Krouse, H.J. COVID-19 disparities and vaccine hesitancy in Black Americans: What ethical lessons can Be learned? Otolaryngol.–Head Neck Surg. 2022, 166, 1147–1160. [Google Scholar] [CrossRef]
- Katz, M.A.; Cohuet, S.; Bino, S.; Tarkhan-Mouravi, O.; Kryeziu, B.; Otorbaeva, D.; Stavridis, K.; Stosic, M.; Sulo, J.; Machablishvili, A.; et al. COVID-19 vaccine effectiveness against SARS-CoV-2-confirmed hospitalisation in the eastern part of the WHO European Region (2022–2023): A test-negative case-control study from the EuroSAVE network. Lancet Reg. Health–Eur. 2024, 47, 101095. [Google Scholar] [CrossRef] [PubMed]
- International Vaccine Access Center (IVAC). “VIEW-Hub.” Johns Hopkins Bloomberg School of Public Health. Available online: https://view-hub.org/vaccine/covid/ (accessed on 16 June 2025).
- Raji, T.; Fallah, M.P.; Dereje, N.; Kakooza, F.; Ndembi, N.; Abdulaziz, M.; Aragaw, M.; Kaseya, J.; Ngongo, A.N. Efficacy and effectiveness of COVID-19 vaccines in Africa: A systematic review. PLoS ONE 2024, 19, e0306309. [Google Scholar] [CrossRef] [PubMed]
- Arregocés-Castillo, L.; Fernández-Niño, J.; Rojas-Botero, M.; Palacios-Clavijo, A.; Galvis-Pedraza, M.; Rincón-Medrano, L.; Pinto-Álvarez, M.; Ruiz-Gómez, F.; Trejo-Valdivia, B. Effectiveness of COVID-19 vaccines in older adults in Colombia: A retrospective, population-based study of the ESPERANZA cohort. Lancet Healthy Longev. 2022, 3, e242–e252. [Google Scholar] [CrossRef]
- Arregocés-Castillo, L.; Fernández-Niño, J.; Rojas-Botero, M.; Palacios-Clavijo, A.; Galvis-Pedraza, M.; Rincón-Medrano, L.; Pinto-Álvarez, M.; Ruiz-Gómez, F.; Trejo-Valdivia, B. Efficacy of COVID-19 vaccines by race and ethnicity. Public Health 2022, 208, 14–17. [Google Scholar] [CrossRef]
- Cai, S.; Chang, C.; Zhang, X.; Qiao, W. Comparative analysis of the effectiveness difference of SARS-COV-2 mRNA vaccine in different populations in the real world: A review. Medicine 2023, 102, e34805. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Pilot Protocol for A COVID-19 Vaccine Effectiveness Study Using Health Data Registries; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2022; Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-pilot-protocol-vaccine-effectiveness-study-using-health-data-registries (accessed on 2 October 2025).
- Renoux, C. COVID-19 Real-World Evidence Primer, Version 8; ch. Immortal Time Bias [Chapter 3]; Cavet, C.C.A., Galaznik, A., Patrick-Lake, B., Talwai, A., Eds.; Reagan-Udall Foundation for the FDA: Washington, DC, USA, 2023; pp. 49–64. [Google Scholar]
- World Health Organization. Global Surveillance for COVID-19 Caused by Human Infection with COVID-19 Virus: Interim Guidance, 20 March 2020. Available online: https://apps.who.int/iris/handle/10665/331506 (accessed on 11 October 2021).
- World Health Organization. International Guidelines for Certification and Classification (Coding) of COVID-19 as Cause of Death. Available online: https://www.who.int/publications/m/item/international-guidelines-for-certification-and-classification-(coding)-of-covid-19-as-cause-of-death (accessed on 11 October 2021).
- Katikireddi, S.V.; Cerqueira-Silva, T.; Vasileiou, E.; Robertson, C.; Amele, S.; Pan, J.; Taylor, B.; Boaventura, V.; Werneck, G.L.; Flores-Ortiz, R.; et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: A retrospective, population-based cohort study in Scotland and Brazil. Lancet 2022, 399, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Ikeokwu, A.E.; Lawrence, R.; Osieme, E.D.; Gidado, K.M.; Guy, C.; Dolapo, O. Unveiling the impact of COVID-19 vaccines: A meta-analysis of survival rates among patients in the United States based on vaccination status. Cureus 2023, 15, e43282. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.L.G.; Oprescu, A.M.; Lezcano, S.M.; Ramos, J.C.; Cabrera, J.L.R.; de la Hoz, M.Á.A.; Estella, Á. Assessing the impact of vaccines on COVID-19 efficacy in survival rates: A survival analysis approach for clinical decision support. Front. Public Health 2024, 12, 1437388. [Google Scholar]
- Escobar-Agreda, S.; Silva-Valencia, J.; Rojas-Mezarina, L.; Vargas-Herrera, J. Survival of health workers infected by SARS-CoV-2 in the context of vaccination against COVID-19 in Peru. medRxiv 2021, 82, 106–112. [Google Scholar]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Shan, R.; Zhao, L.; Bai, Y.; Feng, L. Paxlovid for the treatment of COVID-19: A systematic review and meta-analysis. J. Infect. Dev. Ctries. 2024, 18, 1169–1178. [Google Scholar] [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Iacobucci, G. COVID-19: “Grotesque Inequity” That Only a Quarter of Paxlovid Courses Go to Poorer Countries; British Medical Journal Publishing Group: London, UK, 2022. [Google Scholar]
- Ahmad, S.J.S.; Degiannis, J.R.; Borucki, J.; Pouwels, S.; Rawaf, D.L.; Lala, A.; Whiteley, G.S.; Head, M.; Simpson, A.; Archid, R.; et al. Fatality rates after infection with the Omicron variant (B. 1.1. 529): How deadly has it been? A systematic review and meta-analysis. J. Acute Med. 2024, 14, 51. [Google Scholar]
- Guo, K.; Ni, P.; Chang, S.; Jin, Y.; Duan, G.; Zhang, R. Effectiveness of mRNA vaccine against Omicron-related infections in the real world: A systematic review and meta-analysis. Am. J. Infect. Control 2023, 51, 1049–1055. [Google Scholar] [CrossRef]
- Wu, N.; Joyal-Desmarais, K.; Ribeiro, P.A.B.; Vieira, A.M.; Stojanovic, J.; Sanuade, C.; Yip, D.; Bacon, S.L. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: Findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 2023, 11, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Wratil, P.R.; Stern, M.; Priller, A.; Willmann, A.; Almanzar, G.; Vogel, E.; Feuerherd, M.; Cheng, C.-C.; Yazici, S.; Christa, C.; et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat. Med. 2022, 28, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Hussey, H.; Davies, M.-A.; Heekes, A.; Williamson, C.; Valley-Omar, Z.; Hardie, D.; Korsman, S.; Doolabh, D.; Preiser, W.; Maponga, T.; et al. Assessing the clinical severity of the Omicron variant in the Western Cape Province, South Africa, using the diagnostic PCR proxy marker of RdRp target delay to distinguish between Omicron and Delta infections—A survival analysis. Int. J. Infect. Dis. 2022, 118, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Flacco, M.E.; Soldato, G.; Acuti Martellucci, C.; Carota, R.; Di Luzio, R.; Caponetti, A.; Manzoli, L. Interim estimates of COVID-19 vaccine effectiveness in a mass vaccination setting: Data from an Italian Province. Vaccines 2021, 9, 628. [Google Scholar] [CrossRef]
- Mirahmadizadeh, A.; Heiran, A.; Lankarani, K.B.; Serati, M.; Habibi, M.; Eilami, O.; Heiran, F.; Moghadami, M. Effectiveness of coronavirus disease 2019 vaccines in preventing infection, hospital admission, and death: A historical cohort study using Iranian registration data during vaccination program. In Open Forum Infectious Diseases; Oxford University Press: New York, NY, USA, 2022; Volume 9, p. ofac177. [Google Scholar]
- World Health Organisation. Achieving 70% COVID-19 Immunization Coverage by Mid-2022. Available online: https://www.who.int/news/item/23-12-2021-achieving-70-covid-19-immunization-coverage-by-mid-2022#_ftn7 (accessed on 3 January 2023).
- Centers for Disease Control and Prevention. Principles of Epidemiology in Public Health Practice an Introduction to Applied Epidemiology and Biostatistics, 3rd ed.; Centers for Disease Control and Prevention: Altlanta, GA, USA, 2006. Available online: https://stacks.cdc.gov/view/cdc/6914 (accessed on 27 June 2025).
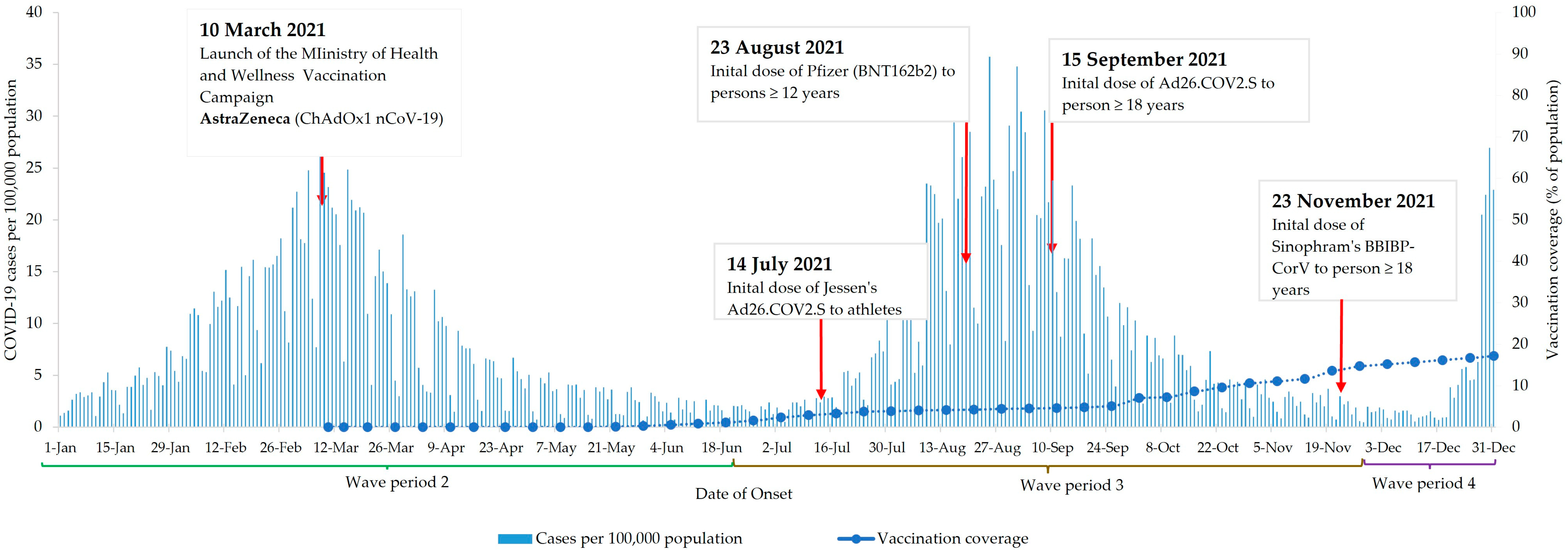
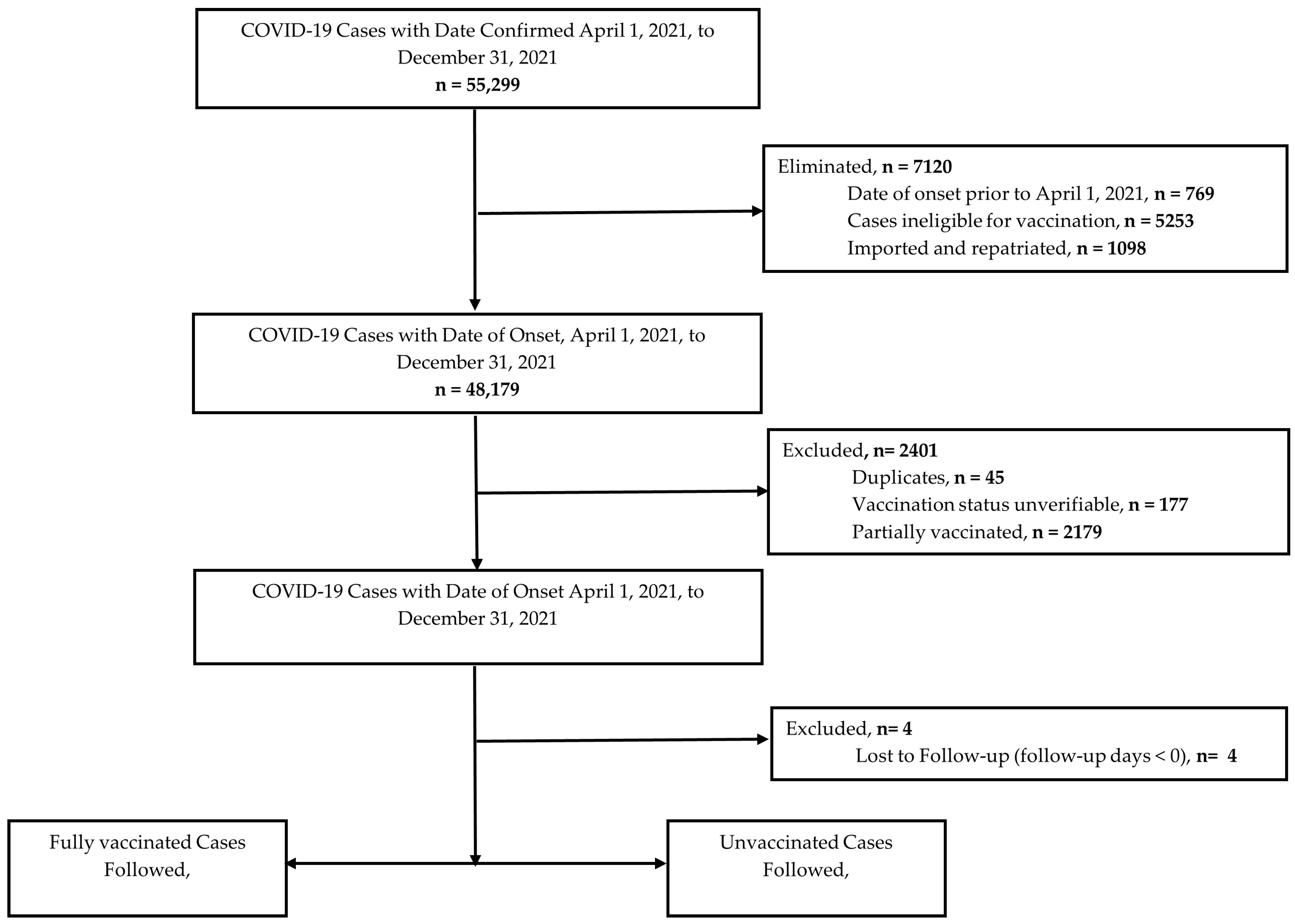
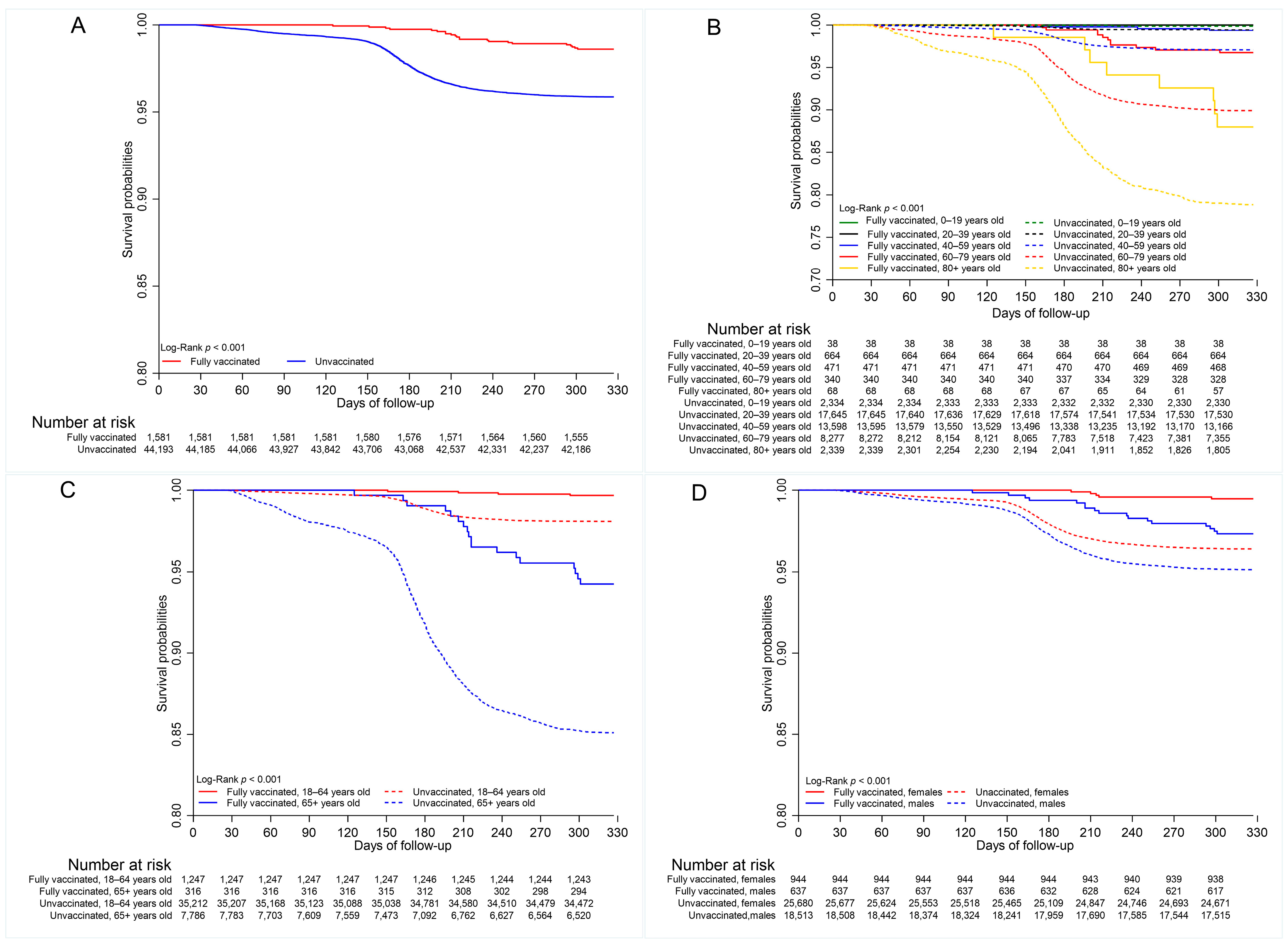
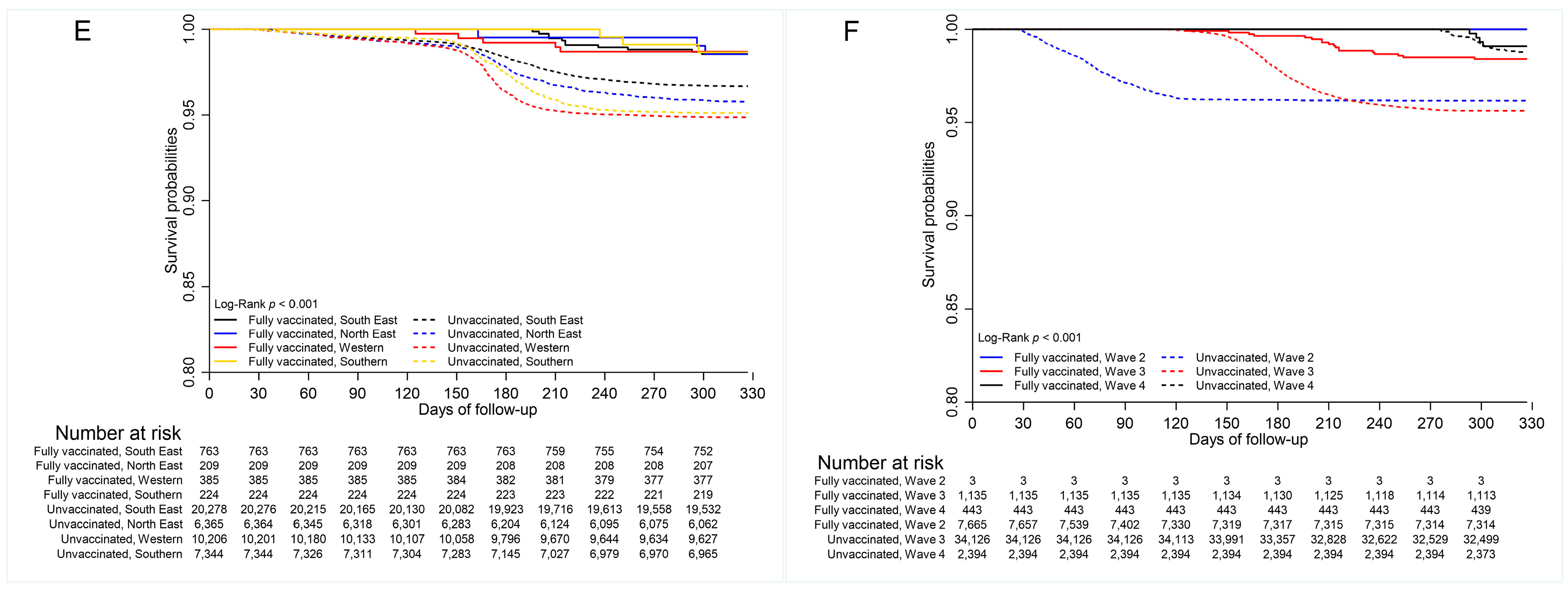
| Characteristics | Total Cases n = 45,774 | COVID-19 Death | |||
|---|---|---|---|---|---|
| Yes n = 1843 | No n = 43,931 | p-Value | Case Fatality Rate | ||
| n (%) | n (%) | n (%) | % | ||
| Vaccination status | |||||
| Vaccinated | 1581 (3.5) | 22 (1.2) | 1559 (3.5) | <0.001 | 1.5 |
| Unvaccinated | 44,193 (96.5) | 1821 (98.8) | 42,372 (96.5) | 4.1 | |
| Median age (IQR) 1, years | 43 (30) | 70 (22) | 41 (28) | <0.001 | |
| Age group, years | |||||
| 12–19 | 2372 (5.2) | 3 (0.2) | 2369 (5.4) | <0.001 | 0.1 |
| 20–39 | 18,309 (40.0) | 102 (5.6) | 18,207 (41.4) | 0.6 | |
| 40–59 | 14,069 (30.7) | 402 (21.8) | 13,667 (31.1) | 2.9 | |
| 60–79 | 8617 (18.8) | 840 (45.6) | 7777 (17.7) | 9.8 | |
| ≥80 | 2407 (5.3) | 496 (26.9) | 1911 (4.4) | 20.6 | |
| Age group, years | |||||
| 18–64 | 36,459 (81.8) | 675 (36.7) | 35,784 (83.8) | <0.05 | 1.8 |
| ≥65 | 8102 (18.2) | 1167 (63.3) | 6935 (16.2) | 13.5 | |
| Sex | |||||
| Female | 26,624 (58.2) | 929 (50.4) | 25,697 (58.5) | 0.216 | 3.5 |
| Male | 19,150 (41.8) | 914 (49.6) | 18,237 (41.5) | 4.8 | |
| Geographic region | |||||
| South East | 21,041 (46.0) | 684 (37.2) | 20,357 (46.3) | <0.050 | 3.3 |
| North East | 6574 (14.4) | 271 (14.7) | 6303 (14.3) | 4.1 | |
| Western | 10,591 (23.1) | 527 (28.6) | 10,064 (22.9) | 5.0 | |
| Southern | 7568 (16.5) | 361 (19.6) | 7207 (16.4) | 4.8 | |
| Comorbidity | |||||
| Yes | 3591 (7.8) | 1562 (84.8) | 2029 (4.6) | <0.001 | |
| No | 297 (0.6) | 276 (15.0) | 21 (0) | ||
| Missing | 41,886 (91.5) | 5 (0.3) | 41,881 (95.3) | ||
| Wave period 1 | |||||
| Wave 2: 1 Apr–22 Jun 2021 | 7668 (16.8) | 293 (14.9) | 7375 (16.8) | <0.001 | 3.6 |
| Wave 3: 23 Jun–3 Dec 2021 | 35,261 (77.1) | 1509 (82.7) | 33,752 (76.9) | 4.3 | |
| Wave 4: 4 Dec–31 Dec 2021 | 2837 (6.2) | 33 (2.5) | 2804 (6.5) | 1.6 | |
| Time Since Vaccination (among vaccinated, n = 1581) | |||||
| 0–3 months | 111 (7.0) | 10 (45.5) | 101 (6.5) | <0.001 | |
| 3–6 months | 346 (21.9) | 12 (54.5) | 334 (21.4) | ||
| >6 months | 1124 (71.1) | 0 (0) | 1124 (72.1) | ||
| Vaccine type (among vaccinated, n = 1581) | |||||
| ChAdOx1 nCoV-19 | 1405 (88.8) | 16 (69.6) | 1389 (89.0) | <0.001 | 1.1 |
| BNT162b2 | 101 (6.4) | 4 (17.4) | 97 (6.3) | 4.0 | |
| Ad26.COV2.S | 75 (4.7) | 2 (8.7) | 73 (4.7) | 2.7 | |
| BBIBP-CorV | 1 (0.1) | 1 (4.3) | 0 (0) | - | |
| Characteristics | All n = 45,774 | Vaccination Status | ||
|---|---|---|---|---|
| Fully Vaccinated n = 1581 | Unvaccinated n = 44,193 | p-Value | ||
| n (%) | n (%) | n (%) | ||
| COVID-19 death | ||||
| Yes | 1843 (4.0) | 22 (1.4) | 1821 (4.1) | <0.001 |
| No | 43,931 (96.0) | 1559 (98.6) | 42,372 (95.9) | |
| Age group, years | ||||
| 12–19 | 2372 (5.2) | 38 (2.4) | 2334 (5.1) | <0.001 |
| 20–39 | 18,309 (40.0) | 664 (42.0) | 17,645 (38.5) | |
| 40–59 | 14,069 (30.7) | 471 (29.8) | 13,598 (29.7) | |
| 60–79 | 8617 (18.8) | 340 (21.5) | 8277 (18.1) | |
| ≥80 | 2407 (5.3) | 68 (4.3) | 2339 (5.1) | |
| Age group, years | ||||
| 18–64 | 36,459 (81.8) | 1247 (79.8) | 35,212 (81.9) | <0.050 |
| ≥65 | 8102 (18.2) | 316 (20.2) | 7786 (18.1) | |
| Sex | ||||
| Female | 26,624 (58.2) | 944 (59.7) | 25,680 (58.1) | 0.205 |
| Male | 19,150 (41.8) | 637 (40.3) | 18,513 (41.9) | |
| Geographic region | ||||
| South East | 21,041 (46.0) | 763 (48.3) | 20,278 (45.9) | <0.05 |
| North East | 6574 (14.4) | 209 (13.2) | 6365 (14.4) | |
| Western | 10,591 (23.1) | 385 (24.4) | 10,206 (23.1) | |
| Southern | 7568 (16.5) | 224 (14.2) | 7344 (16.6) | |
| Comorbidity | ||||
| Yes | 3591 (7.8) | 85 5.4) | 3506 (7.9) | <0.001 |
| No | 297 (0.6) | 2 (0.1) | 295 (0.7) | |
| Missing | 41,886 (91.5) | 1494 (94.5) | 40,392 (91.4) | |
| Wave period 1 | ||||
| Wave 2: 1 Apr–22 Jun 2021 | 1405 (88.8) | 2 (0.1) | 7549 (17.1) | <0.001 |
| Wave 3: 23 Jun–3 Dec 2021 | 101 (6.4) | 1123 (71) | 34,186 (77.4) | |
| Wave 4: 4 Dec–31 Dec 2021 | 75 (4.7) | 457 (28.9) | 2461 (5.6) | |
| Time Since Vaccination (among vaccinated, n = 1581) | ||||
| 0–3 months | 111 (7) | 111 (7) | -- | __ |
| 3–6 months | 346 (21.9) | 346 (21.9) | -- | |
| >6 months | 1124 (71.1) | 1124 (71.1) | -- | |
| Univariable | Multivariable 1 | |||
|---|---|---|---|---|
| HR (95% CI) | VE% (95% CI) | HR (95% CI) | VE% (95% CI) | |
| All vaccines | 0.34 (0.22–0.52) | 66 (48–78) | 0.32 (0.21–0.49) | 68 (51–79) |
| Sub-Group | ||||
| Sex | ||||
| Female | 0.15 (0.06–0.36) | 85 (64–94) | 0.16 (0.07–0.37) | 84 (63–93) |
| Male | 0.56 (0.35–0.9) | 44 (10–65) | 0.47 (0.29–0.76) | 53 (24–71) |
| Age | ||||
| 18–64 | 0.18 (0.07–0.47) | 82 (53–93) | 0.18 (0.07–0.48) | 82 (52–93) |
| ≥65 | 0.37 (0.23–0.59) | 63 (41–77) | 0.37 (0.23–0.59) | 63 (41–77) |
| Regional Health Authority | ||||
| South East | 0.44 (0.24–0.8) | 56 (20–76) | 0.46 (0.26–0.83) | 54 (17–74) |
| North East | 0.34 (0.11–1.05) | 66 (-5–89) | 0.3 (0.1–0.9) | 70 (10–90) |
| Western | 0.26 (0.11–0.62) | 74 (38–89) | 0.22 (0.09–0.54) | 78 (46–91) |
| Southern | 0.28 (0.09–0.86) | 72 (14–91) | 0.26 (0.08–0.79) | 74 (21–92) |
| ChAdOx1 nCoV-19 | 0.28 (0.17–0.45) | 72 (55–83) | 0.25 (0.16–0.41) | 75 (59–84) |
| BNT162b2 | 0.66 (0.17–2.64) | 34 (−164–83) | 1.46 (0.38–5.67) | −46 (−467–62) |
| Ad26.COV2.S | 0.98 (0.37–2.62) | 2 (−162–63) | 1.09 (0.43–2.73) | −9 (−173–57) |
| Time Since Vaccination (among fully vaccinated), months | ||||
| 0–3 | 0.29 (0.16–0.54) | 0 (1–71) | 0.29 (0.15–0.53) | 71 (47–85) |
| 3–6 | 0.5 (0.28–0.88) | 0 (1–56) | 0.44 (0.25–0.77) | 56 (23–75) |
| >6 | -- | -- | -- | -- |
| Vaccine type–ChAdOx1 nCoV-19 | 0.27 (0.17–0.44) | 73 (56–83) | 0.25 (0.16–0.41) | 75 (59–84) |
| Subgroup | ||||
| Sex | ||||
| Female | 0.16 (0.07–0.38) | 84 (62–93) | 0.17 (0.07–0.4) | 83 (60–93) |
| Male | 0.41 (0.23–0.74) | 59 (26–77) | 0.34 (0.19–0.61) | 66 (39–81) |
| Age, years | ||||
| 18–64 | 0.1 (0.02–0.39) | 90 (61–98) | 0.1 (0.02–0.39) | 90 (61–98) |
| ≥65 | 0.31 (0.18–0.52) | 69 (48–82) | 0.31 (0.18–0.52) | 69 (48–82) |
| Regional Health Authority | ||||
| South-East | 0.36 (0.18–0.72) | 64 (28–82) | 0.33 (0.17–0.66) | 67 (34–83) |
| North-East | 0.13 (0.02–0.93) | 87 (7–98) | 0.11 (0.01–0.74) | 89 (26–99) |
| Western | 0.22 (0.08–0.59) | 78 (41–92) | 0.2 (0.07–0.52) | 80 (48–93) |
| Southern | 0.29 (0.09–0.89) | 71 (11–91) | 0.24 (0.08–0.75) | 76 (25–92) |
| Time since vaccination, months | ||||
| 0–3 | 0.13 (0.05–0.36) | 87 (65–95) | 0.13 (0.05–0.35) | 87 (65–95) |
| 3–6 | 0.52 (0.29–0.92) | 55 (21–74) | 0.45 (0.26–0.79) | 55 (21–74) |
| >6 | -- | -- | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Webster-Kerr, K.; Grant, A.; Harris, A.; Campbell, E.; Henningham, D.; Brown, M.; Rowe, D.; Lord, C.; Thorpe, R.; Mullings, T.; et al. Retrospective Cohort Analysis of Survival After SARS-CoV-2 Infection by Vaccination Status in Jamaica, April–December 2021. Vaccines 2025, 13, 1250. https://doi.org/10.3390/vaccines13121250
Webster-Kerr K, Grant A, Harris A, Campbell E, Henningham D, Brown M, Rowe D, Lord C, Thorpe R, Mullings T, et al. Retrospective Cohort Analysis of Survival After SARS-CoV-2 Infection by Vaccination Status in Jamaica, April–December 2021. Vaccines. 2025; 13(12):1250. https://doi.org/10.3390/vaccines13121250
Chicago/Turabian StyleWebster-Kerr, Karen, Andriene Grant, Ardene Harris, Eon Campbell, Deborah Henningham, Marsha Brown, Daidre Rowe, Carol Lord, Romae Thorpe, Tanielle Mullings, and et al. 2025. "Retrospective Cohort Analysis of Survival After SARS-CoV-2 Infection by Vaccination Status in Jamaica, April–December 2021" Vaccines 13, no. 12: 1250. https://doi.org/10.3390/vaccines13121250
APA StyleWebster-Kerr, K., Grant, A., Harris, A., Campbell, E., Henningham, D., Brown, M., Rowe, D., Lord, C., Thorpe, R., Mullings, T., Wiggan, J., Martin-Chen, N., Dawkins-Beharie, T., & Duncan, J. (2025). Retrospective Cohort Analysis of Survival After SARS-CoV-2 Infection by Vaccination Status in Jamaica, April–December 2021. Vaccines, 13(12), 1250. https://doi.org/10.3390/vaccines13121250






