Variations in Routine Childhood Vaccination Gaps: A Decomposition Analysis Across 80 Low- and Middle-Income Countries
Abstract
1. Introduction
2. Methods
2.1. Conceptual Model
2.2. Data and Variable Definitions
2.3. Analysis
3. Results
3.1. Data
3.2. Descriptive Analysis
3.3. Decomposition Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALR | Additive log-ratio |
| BCG | Bacille Calmette–Guérin vaccine |
| COVID-19 | Coronavirus disease 2019 |
| DHS | Demographic and Health Surveys |
| DO | Drop-out (received at least DTP1 but not DTP3) |
| DTP | Diphtheria–Tetanus–Pertussis–containing vaccine |
| DTP0/DTP1/DTP3 | 0/1/3 doses of DTP-containing vaccine |
| GVAP | Global Vaccine Action Plan |
| IA2030 | Immunization Agenda 2030 |
| ILR | Isometric log-ratio |
| MCV | Measles-containing vaccine |
| MD | Missed DTP (received ≥1 non-DTP vaccine, such as BCG, polio, or MCV, but no DTP) |
| SEM | Structural Equation Modeling |
| UNICEF | United Nations Children’s Fund |
| WHO | World Health Organization |
| ZD | Zero dose (no recorded doses of BCG, polio, MCV, or DTP) |
Appendix A
| Model code: |
| model = ‘ |
| # define direct effects |
| dpt3_cvg ~ b1 * dropout_prevalence + b2 * misseddtp3_prevalence + b3 * zd_prevalence |
| # define mediator effects |
| dropout_prevalence ~ a1 * zd_prevalence |
| dropout_prevalence ~ c1 * misseddtp3_prevalence |
| misseddtp3_prevalence ~ a2 * zd_prevalence |
| # define indirect and total effects of ZD |
| zd_indirect_DO := a1 * b1 |
| zd_indirect_MD := a2 * b2 |
| zd_total_indirect := zd_indirect_DO + zd_indirect_MD |
| zd_total_effect := b3 + zd_total_indirect |
| # define indirect and total effects of MD |
| md_total_indirect := c1 * b1 |
| md_total_effect := b2 + md_total_indirect |
| ’ |
| Model | Akaike Information Criterion | Bayesian Information Criterion |
|---|---|---|
| Isometric Log-Ratio Transformation | −109.132 | −76.072 |
| Additive Log-Ratio Transformation | −80.086 | −46.903 |
| Parameter | Outcome (Y) | Explanatory (X) | Estimate | Standard Error | p-Value |
|---|---|---|---|---|---|
| Regressions | |||||
| DTP3 | DO | −0.131 | 0.005 | 0.000 | |
| DTP3 | MD | −0.051 | 0.004 | 0.000 | |
| DTP3 | ZD | −0.036 | 0.002 | 0.000 | |
| DO | ZD | 0.104 | 0.021 | 0.000 | |
| DO | MD | 0.522 | 0.033 | 0.000 | |
| MD | ZD | 0.320 | 0.032 | 0.000 | |
| Variances | |||||
| DTP3 | 0.001 | 0.000 | 0.000 | ||
| DO | 0.174 | 0.014 | 0.000 | ||
| MD | 0.557 | 0.046 | 0.000 | ||
| Indirect Effects | |||||
| DTP3 (indirect effect via DO) | ZD | −0.014 | 0.003 | 0.000 | |
| DTP3 (indirect effect via MD) | ZD | −0.016 | 0.002 | 0.000 | |
| DTP3 (total indirect effect) | ZD | −0.030 | 0.003 | 0.000 | |
| DTP3 (total indirect effect) | MD | −0.068 | 0.005 | 0.000 | |
| Total Effects | |||||
| DTP3 (total effect) | ZD | −0.066 | 0.004 | 0.000 | |
| DTP3 (total effect) | MD | −0.119 | 0.004 | 0.000 | |
| DTP3 | DO | −0.131 | 0.008 | 0.000 | |
References
- Shattock, A.J.; Johnson, H.C.; Sim, S.Y.; Carter, A.; Lambach, P.; Hutubessy, R.C.W.; Thompson, K.M.; Badizadegan, K.; Lambert, B.; Ferrari, M.J.; et al. Contribution of vaccination to improved survival and health: Modelling 50 years of the Expanded Programme on Immunization. Lancet 2024, 403, 2307–2316. [Google Scholar] [CrossRef]
- GBD 2020, Release 1, Vaccine Coverage Collaborators Measuring routine childhood vaccination coverage in 204 countries and territories, 1980-2019: A systematic analysis for the Global Burden of Disease Study 2020, Release 1. Lancet 2021, 398, 503–521. [CrossRef] [PubMed]
- Global Vaccine Action Plan 2011–2020. Available online: https://www.who.int/publications/i/item/global-vaccine-action-plan-2011-2020 (accessed on 20 June 2025).
- MacDonald, N.; Mohsni, E.; Al-Mazrou, Y.; Kim Andrus, J.; Arora, N.; Elden, S.; Madrid, M.-Y.; Martin, R.; Mahmoud Mustafa, A.; Rees, H.; et al. Global vaccine action plan lessons learned I: Recommendations for the next decade. Vaccine 2020, 38, 5364–5371. [Google Scholar] [CrossRef]
- Immunization Agenda 2030: A Global Strategy to Leave No One Behind. Available online: https://www.who.int/publications/m/item/immunization-agenda-2030-a-global-strategy-to-leave-no-one-behind (accessed on 20 June 2025).
- Global Childhood Immunization Levels Stalled in 2023 Leaving Many Without Life-Saving Protection—PAHO/WHO | Pan American Health Organization. Available online: https://www.paho.org/en/news/15-7-2024-global-childhood-immunization-levels-stalled-2023-leaving-many-without-life-saving (accessed on 20 June 2025).
- Jensen, S.B. Immunization Agenda 2030. Available online: https://www.immunizationagenda2030.org/ia2030-annual-reports (accessed on 20 June 2025).
- Vaccination and Immunization Statistics—UNICEF DATA. Available online: https://data.unicef.org/topic/child-health/immunization/ (accessed on 20 June 2025).
- Immunization Coverage. Available online: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage (accessed on 20 June 2025).
- Highlights from the Meeting of the Strategic Advisory Group of Experts (SAGE) on Immunization. Available online: https://cdn.who.int/media/docs/default-source/immunization/sage/2025/march/sage_march_2025_highlights_final.pdf?sfvrsn=6ad38df_3 (accessed on 20 June 2025).
- Habersaat, K.; MacDonald, N.E.; Dubé, È. Designing tailored interventions to address barriers to vaccination. Can. Commun. Dis. Rep. 2021, 47, 165–168. [Google Scholar] [CrossRef]
- Vaccine Delivery. Available online: https://www.exemplars.health/topics/vaccine-delivery (accessed on 20 June 2025).
- Bednarczyk, R.A.; Hester, K.A.; Dixit, S.M.; Ellis, A.S.; Escoffery, C.; Kilembe, W.; Micek, K.; Sakas, Z.M.; Sarr, M.; Freeman, M.C. Exemplars in vaccine delivery protocol: A case-study-based identification and evaluation of critical factors in achieving high and sustained childhood immunisation coverage in selected low-income and lower-middle-income countries. BMJ Open 2022, 12, e058321. [Google Scholar] [CrossRef]
- Cata-Preta, B.O.; Santos, T.M.; Mengistu, T.; Hogan, D.R.; Barros, A.J.D.; Victora, C.G. Zero-dose children and the immunisation cascade: Understanding immunisation pathways in low and middle-income countries. Vaccine 2021, 39, 4564–4570. [Google Scholar] [CrossRef]
- Phillips, D.E.; Dieleman, J.L.; Lim, S.S.; Shearer, J. Determinants of effective vaccine coverage in low and middle-income countries: A systematic review and interpretive synthesis. BMC Health Serv. Res. 2017, 17, 681. [Google Scholar] [CrossRef]
- Rosseel, Y.; Jorgensen, T.D.; De Wilde, L. lavaan: Latent Variable Analysis 2012, Version 0.6-20. Available online: https://CRAN.R-project.org/package=lavaan (accessed on 22 October 2025).
- Egozcue, J.J.; Pawlowsky-Glahn, V.; Mateu-Figueras, G.; Barceló-Vidal, C. Isometric Logratio Transformations for Compositional Data Analysis. Math. Geol. 2003, 35, 279–300. [Google Scholar] [CrossRef]
- van den Boogaart, K.G.; Tolosana-Delgado, R.; Bren, M. Compositions: Compositional Data Analysis 2005, Version 2.0-8. Available online: https://CRAN.R-project.org/package=compositions (accessed on 20 June 2025).
- Ozawa, S.; Yemeke, T.T.; Evans, D.R.; Pallas, S.E.; Wallace, A.S.; Lee, B.Y. Defining hard-to-reach populations for vaccination. Vaccine 2019, 37, 5525–5534. [Google Scholar] [CrossRef]
- Singh, S.; Sahu, D.; Agrawal, A.; Vashi, M.D. Barriers and opportunities for improving childhood immunization coverage in slums: A qualitative study. Prev. Med. Rep. 2019, 14, 100858. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.; Barros, A. Within-Country Inequalities in Zero-Dose Prevalence. Available online: https://www.countdown2030.org/wp-content/uploads/2023/06/SOWC-background-paper-on-zero-dose-inequalities_for-design_edited.pdf (accessed on 20 June 2025).
- Bergen, N.; Cata-Preta, B.O.; Schlotheuber, A.; Santos, T.M.; Danovaro-Holliday, M.C.; Mengistu, T.; Sodha, S.V.; Hogan, D.R.; Barros, A.J.D.; Hosseinpoor, A.R. Economic-Related Inequalities in Zero-Dose Children: A Study of Non-Receipt of Diphtheria–Tetanus–Pertussis Immunization Using Household Health Survey Data from 89 Low- and Middle-Income Countries. Vaccines 2022, 10, 633. [Google Scholar] [CrossRef]
- Wigley, A.; Lorin, J.; Hogan, D.; Utazi, C.E.; Hagedorn, B.; Dansereau, E.; Tatem, A.J.; Tejedor-Garavito, N. Estimates of the number and distribution of zero-dose and under-immunised children across remote-rural, urban, and conflict-affected settings in low and middle-income countries. PLOS Glob. Public Health 2022, 2, e0001126. [Google Scholar] [CrossRef]
- Wonodi, C.; Farrenkopf, B.A. Defining the Zero Dose Child: A Comparative Analysis of Two Approaches and Their Impact on Assessing the Zero Dose Burden and Vulnerability Profiles across 82 Low- and Middle-Income Countries. Vaccines 2023, 11, 1543. [Google Scholar] [CrossRef]
- Oromidayo Olakunmi, O.; Shola, A.I. Assessment of Zero Dose, Under-Immunized, and Dropout Children in Ifelodun Local Government Area, Kwara State Nigeria. Texila Int. J. Acad. Res. 2025, 12. [Google Scholar] [CrossRef]
- Karlsson, O.; Rajpal, S.; Johri, M.; Kim, R.; Subramanian, S.V. Prevalence and Trends of Not Receiving a Dose of DPT-Containing Vaccine Among Children 12-35 Months: An Analysis of 81 Low- And Middle-Income Countries. J. Epidemiol. Glob. Health 2024, 14, 1490–1503. [Google Scholar] [CrossRef] [PubMed]
- Avila-Aguero, M.L.; Brenes-Chacon, H.; Melgar, M.; Becerra-Posada, F.; Chacon-Cruz, E.; Gentile, A.; Ospina, M.; Sandoval, N.; Sanwogou, J.; Urena, A.; et al. Zero-dose children in Latin America: Analysis of the problem and possible solutions. F1000Res 2024, 13, 1060. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.; Gupta, A. Why Reaching Zero-Dose Children Holds the Key to Achieving the Sustainable Development Goals. Vaccines 2023, 11, 781. [Google Scholar] [CrossRef]
- Jain, M.; Shisler, S.; Lane, C.; Bagai, A.; Brown, E.; Engelbert, M. Use of community engagement interventions to improve child immunisation in low-income and middle-income countries: A systematic review and meta-analysis. BMJ Open 2022, 12, e061568. [Google Scholar] [CrossRef]
- Picillo, B.; Ewald, L.; Chee, G. Leveraging Immunization Campaigns to Strengthen Routine Immunization and Health Systems. Available online: https://www.acceleratehss.org/wp-content/uploads/2021/09/Accelerator_Campaigns-and-RI-Lit-Review_R2_LR.pdf (accessed on 20 June 2025).
- Shah, M.P.; Morgan, C.J.; Beeson, J.G.; Peach, E.; Davis, J.; McPake, B.; Wallace, A.S. Integrated Approaches for the Delivery of Maternal and Child Health Services with Childhood Immunization Programs in Low- and Middle-Income Countries: Systematic Review Update 2011–2020. Vaccines 2024, 12, 1313. [Google Scholar] [CrossRef]
- Phillips, D.E.; Dieleman, J.L.; Shearer, J.C.; Lim, S.S. Childhood vaccines in Uganda and Zambia: Determinants and barriers to vaccine coverage. Vaccine 2018, 36, 4236–4244. [Google Scholar] [CrossRef]
- Jain, A.K. Measuring the Effect of Fertility Decline on the Maternal Mortality Ratio. Stud. Fam. Plan. 2011, 42, 247–260. [Google Scholar] [CrossRef]
- Leite, M.L.C. Applying compositional data methodology to nutritional epidemiology. Stat. Methods Med. Res. 2016, 25, 3057–3065. [Google Scholar] [CrossRef]
- Migueles, J.H.; Delisle Nyström, C.; Dumuid, D.; Leppänen, M.H.; Henriksson, P.; Löf, M. Longitudinal associations of movement behaviours with body composition and physical fitness from 4 to 9 years of age: Structural equation and mediation analysis with compositional data. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 11. [Google Scholar] [CrossRef]
- Pearl, J. Interpretation and identification of causal mediation. Psychol. Methods 2014, 19, 459–481. [Google Scholar] [CrossRef]
- Binyaruka, P.; Borghi, J. Validity of parental recalls to estimate vaccination coverage: Evidence from Tanzania. BMC Health Serv. Res. 2018, 18, 440. [Google Scholar] [CrossRef]
- Adetifa, I.M.O.; Karia, B.; Mutuku, A.; Bwanaali, T.; Makumi, A.; Wafula, J.; Chome, M.; Mwatsuma, P.; Bauni, E.; Hammitt, L.L.; et al. Coverage and timeliness of vaccination and the validity of routine estimates: Insights from a vaccine registry in Kenya. Vaccine 2018, 36, 7965–7974. [Google Scholar] [CrossRef] [PubMed]
- Dansereau, E.; Brown, D.; Stashko, L.; Danovaro-Holliday, M.C. A systematic review of the agreement of recall, home-based records, facility records, BCG scar, and serology for ascertaining vaccination status in low and middle-income countries. Gates Open Res. 2019, 3, 923. [Google Scholar] [CrossRef] [PubMed]
- Danovaro-Holliday, M.C.; Dansereau, E.; Rhoda, D.A.; Brown, D.W.; Cutts, F.T.; Gacic-Dobo, M. Collecting and using reliable vaccination coverage survey estimates: Summary and recommendations from the “Meeting to share lessons learnt from the roll-out of the updated WHO Vaccination Coverage Cluster Survey Reference Manual and to set an operational research agenda around vaccination coverage surveys”, Geneva, 18–21 April 2017. Vaccine 2018, 36, 5150–5159. [Google Scholar] [CrossRef] [PubMed]
- Modi, R.N.; King, C.; Bar-Zeev, N.; Colbourn, T. Caregiver recall in childhood vaccination surveys: Systematic review of recall quality and use in low- and middle-income settings. Vaccine 2018, 36, 4161–4170. [Google Scholar] [CrossRef]
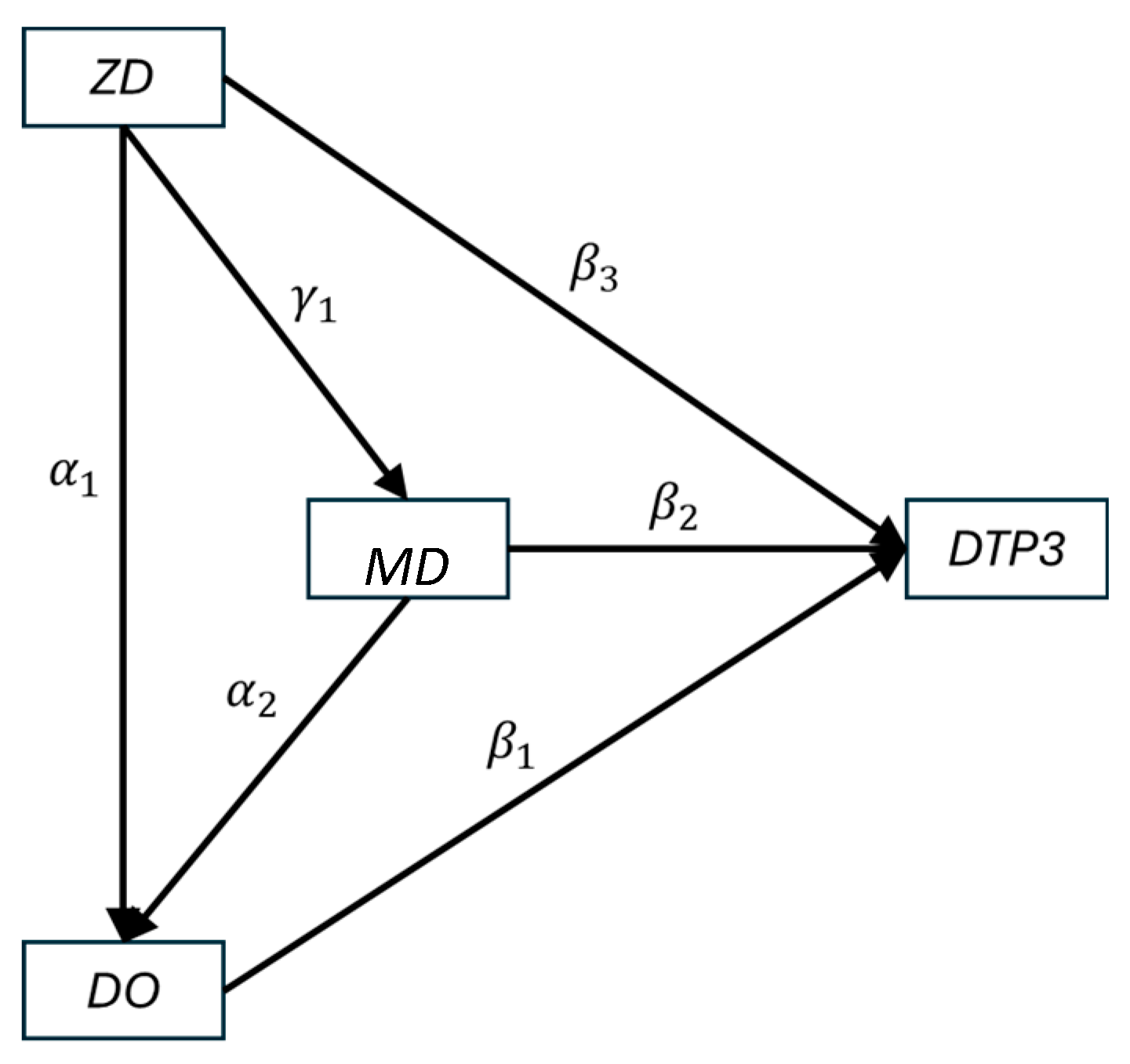
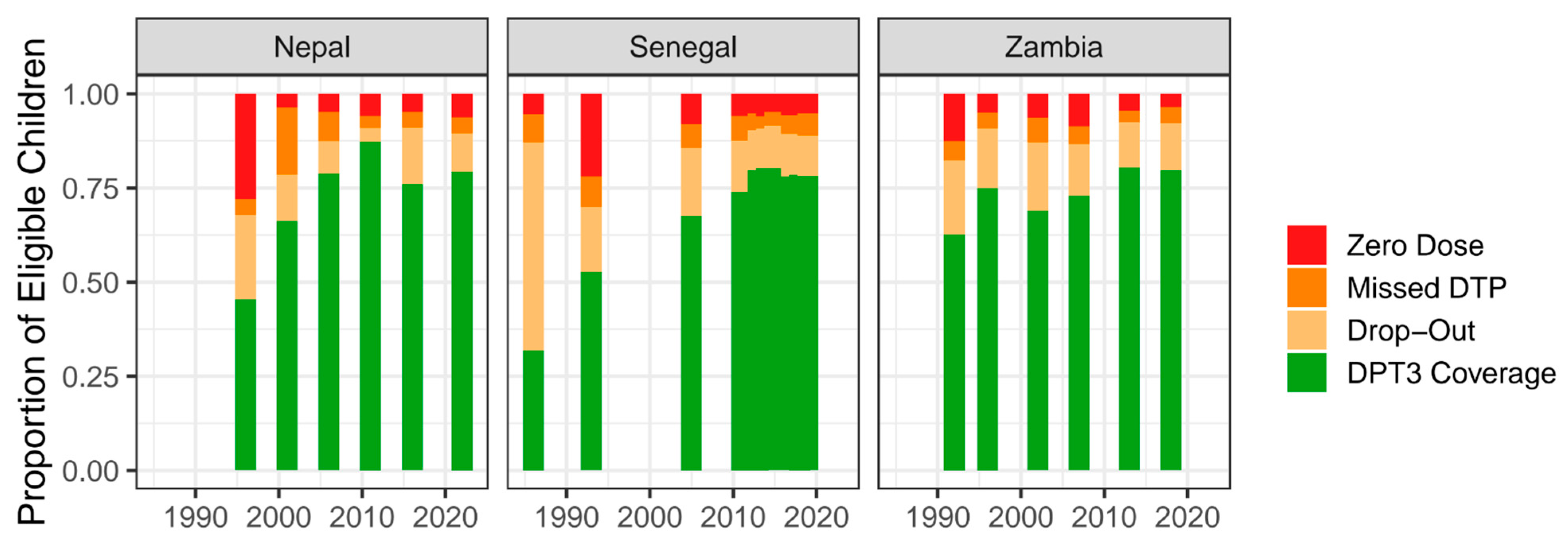
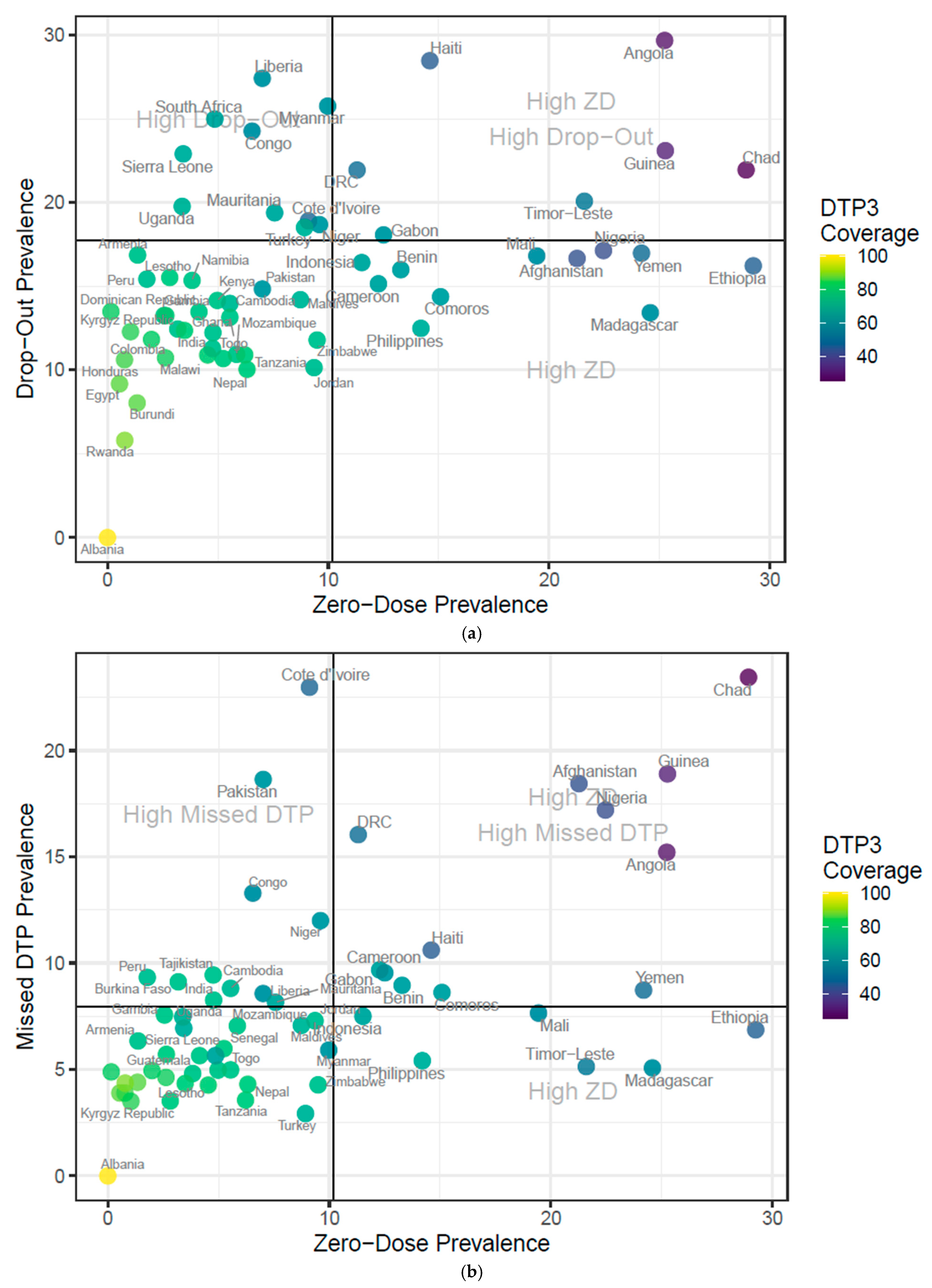
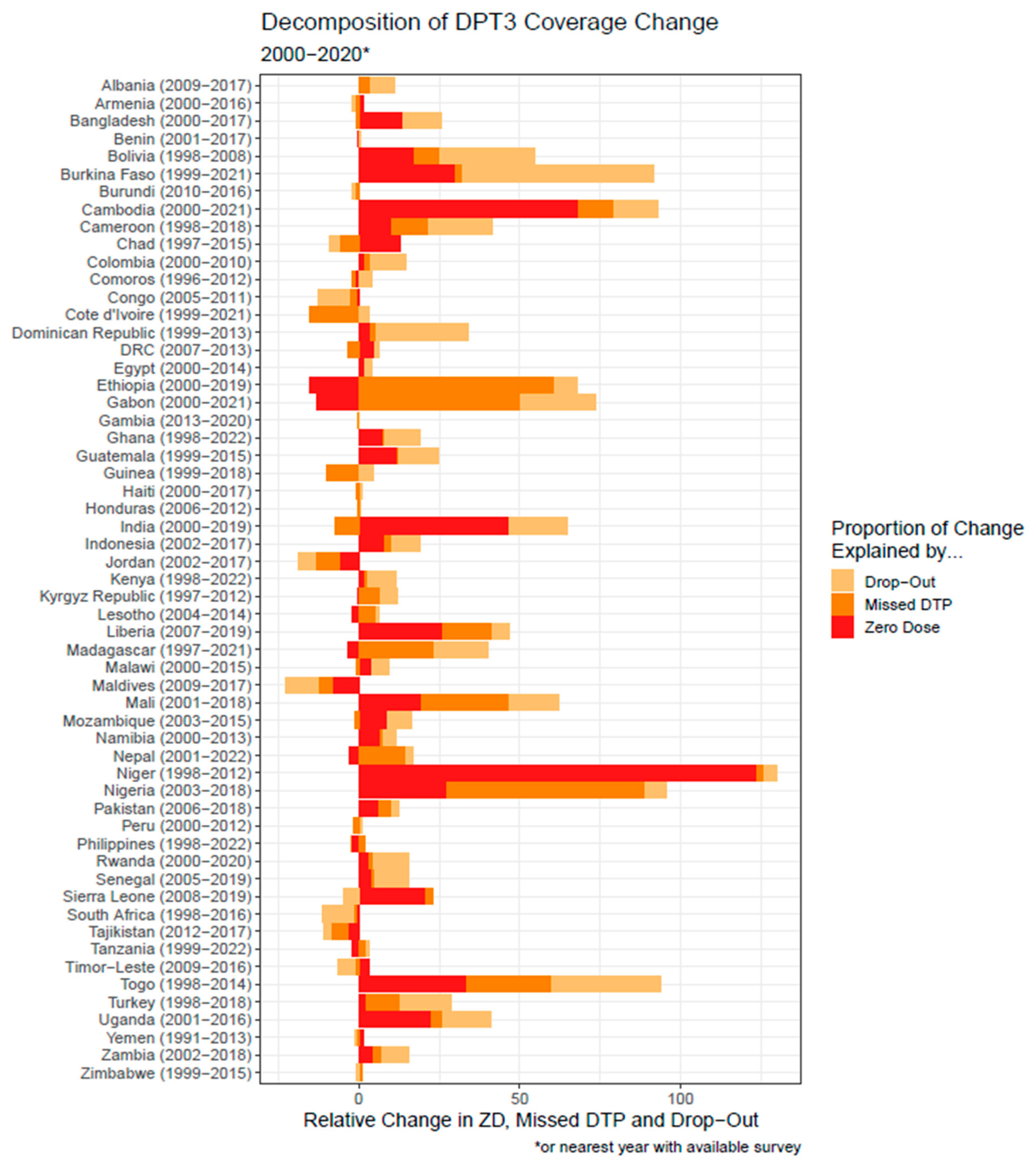
| Parameter | Outcome (Y) | Explanatory (X) | Estimate | Standard Error | p-Value |
|---|---|---|---|---|---|
| Regressions | |||||
| DTP3 | DO | −0.243 | 0.008 | 0.000 | |
| DTP3 | MD | −0.066 | 0.007 | 0.000 | |
| DTP3 | ZD | −0.039 | 0.003 | 0.000 | |
| DO | ZD | 0.092 | 0.023 | 0.000 | |
| DO | MD | 0.443 | 0.045 | 0.000 | |
| MD | ZD | 0.076 | 0.030 | 0.011 | |
| Variances | |||||
| DTP3 | 0.003 | 0.000 | 0.000 | ||
| DO | 0.164 | 0.013 | 0.000 | ||
| MD | 0.274 | 0.023 | 0.000 | ||
| Indirect Effects | |||||
| DTP3 (indirect effect via DO) | ZD | −0.022 | 0.006 | 0.000 | |
| DTP3 (indirect effect via MD) | ZD | −0.005 | 0.002 | 0.014 | |
| DTP3 (total indirect effect) | ZD | −0.027 | 0.006 | 0.000 | |
| DTP3 (total indirect effect) | MD | −0.108 | 0.012 | 0.000 | |
| Total Effects | |||||
| DTP3 (total effect) | ZD | −0.067 | 0.007 | 0.000 | |
| DTP3 (total effect) | MD | −0.174 | 0.012 | 0.000 | |
| DTP3 | DO | −0.243 | 0.008 | 0.000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, D.; Thomas, J.-T.; Ikilezi, G. Variations in Routine Childhood Vaccination Gaps: A Decomposition Analysis Across 80 Low- and Middle-Income Countries. Vaccines 2025, 13, 1136. https://doi.org/10.3390/vaccines13111136
Phillips D, Thomas J-T, Ikilezi G. Variations in Routine Childhood Vaccination Gaps: A Decomposition Analysis Across 80 Low- and Middle-Income Countries. Vaccines. 2025; 13(11):1136. https://doi.org/10.3390/vaccines13111136
Chicago/Turabian StylePhillips, David, Jordan-Tate Thomas, and Gloria Ikilezi. 2025. "Variations in Routine Childhood Vaccination Gaps: A Decomposition Analysis Across 80 Low- and Middle-Income Countries" Vaccines 13, no. 11: 1136. https://doi.org/10.3390/vaccines13111136
APA StylePhillips, D., Thomas, J.-T., & Ikilezi, G. (2025). Variations in Routine Childhood Vaccination Gaps: A Decomposition Analysis Across 80 Low- and Middle-Income Countries. Vaccines, 13(11), 1136. https://doi.org/10.3390/vaccines13111136






