A Review of the Effects of Ipsilateral or Contralateral Vaccine Boosting on the Adaptive Immune Response
Abstract
1. Introduction
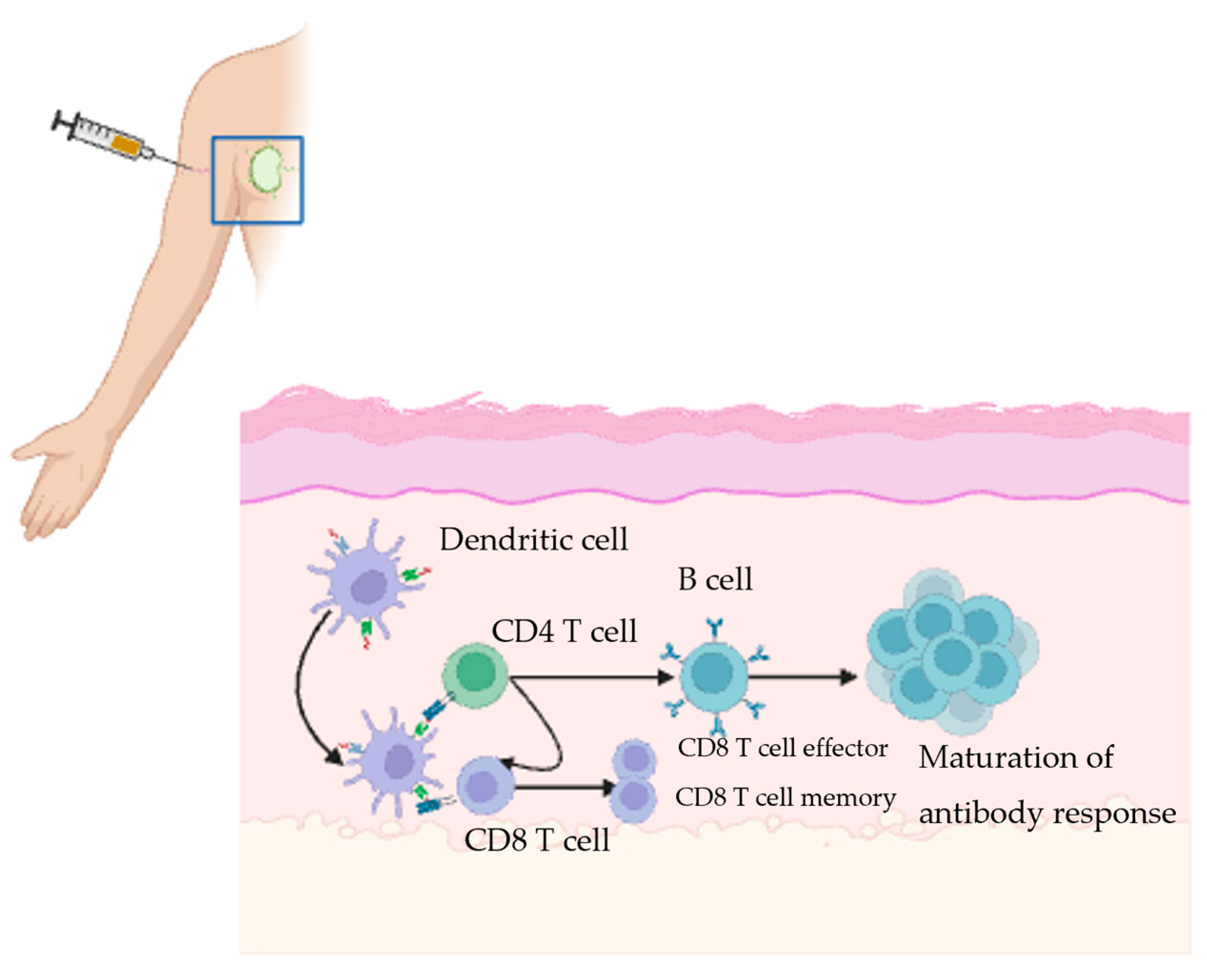
2. Immune Response to Vaccine in Draining Lymph Node
3. Effect of Administering the Vaccine Boosters in the Ipsilateral Versus Contralateral Arm on Germinal Center Response
4. Impact of Administering Vaccine Boosters in the Ipsilateral Versus Contralateral Arm on Antibody Response
| Study | Model | Primary End Point | Time Evaluated Post-Vaccination | Outcomes |
|---|---|---|---|---|
| Animal Studies | ||||
| Jacobson et al., 1969 [31] | Rabbit | Antibody response To DT and BCG vaccines | 7 days; 2–3 weeks; 4 weeks, 3–4 months | -Ipsilateral lymph nodes showed an antibody response at day 7 -The contralateral lymph nodes showed a response at day 9 -The antibody response in the contralateral lymph nodes was significantly lower over time |
| Kuraoka et al., 2022 [34] | Mice | Levels of serum IgG, secondary GCBC response to influenza vaccine | 4–5 weeks; 8–10 weeks; 12–14 weeks | -Similar IgG and plasmacyte responses between groups -Higher antigen-specific clonal IgG in ipsilateral group -More highly mutated B cells in ipsilateral boost group -More efficient recruitment of primary GC B cells in ipsilateral group |
| Human Studies | ||||
| Peck et al., 1964 [32] | Adults | Antibody response to rabies vaccine | 52 days | -Higher antibody response in the ipsilateral group compared to the contralateral group |
| Iro et al., 2015 [33] | 6–12 weeks old infants | Geometric mean concentration of anti-tetanus toxoid, anti-pneumococcal and anti-H influenzae type B antibodies | 1 months, 5 months, 12 months, 24 months | -Anti-Hib GMTs lower in consistent limb than alternating legs at 5 months and 12 months -Anti-tetanus toxoid IgG were lower in consistent than alternating legs at 13 months and 24 months -Anti-pneumococcal IgG means were similar between both groups at all time points |
5. Effect of Site of Administration on the T Cell Response
6. Summary and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting cell |
| BGG | Bovin gamma globulin |
| DT | Diphtheria toxoid |
| DTaP | Diphtheria—tetanus- acellular pertussis |
| GC | Germinal center |
| GCBC | Germinal center B cell |
| GMC | geometric mean concentration |
| Hib | Haemophilus influenzae type b |
| Hib-MenC-TT | Haemophilus influenzae type b-capsular group C Neisseria meningitidis-tetanus toxoid conjugate |
| IP | Inactivated polio |
| PCV13 | 13-valent pneumococcal conjugate |
| RBD | Receptor binding domain |
| TCR | T cell receptor |
| Tfh | T follicular helper |
References
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef]
- Bärnighausen, T.; Bloom, D.E.; Cafiero-Fonseca, E.T.; O’Brien, J.C. Valuing vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12313–12319. [Google Scholar] [CrossRef]
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- den Boon, S.; Ahmed, S.; Sarker, A.R. Economic evaluations of immunization programs as an indispensable tool for policymakers. BMC Health Serv. Res. 2023, 23, 1284. [Google Scholar] [CrossRef] [PubMed]
- Peng, M. Outbreak of COVID-19: An emerging global pandemic threat. Biomed. Pharmacother. 2020, 129, 110499. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef]
- Lynn, D.J.; Benson, S.C.; Lynn, M.A.; Pulendran, B. Modulation of immune responses to vaccination by the microbiota: Implications and potential mechanisms. Nat. Rev. Immunol. 2022, 22, 33–46. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef]
- Falahi, S.; Kenarkoohi, A. Host factors and vaccine efficacy: Implications for COVID-19 vaccines. J. Med. Virol. 2022, 94, 1330–1335. [Google Scholar] [CrossRef]
- Lau, C.S.; Aw, T.C. Considerations in Understanding Vaccine Effectiveness. Vaccines 2022, 11, 20. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. Factors influencing estimated effectiveness of COVID-19 vaccines in non-randomised studies. BMJ Evid. Based Med. 2022, 27, 324–329. [Google Scholar] [CrossRef]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Omer, S.B. Why and How Vaccines Work. Cell 2020, 183, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Casey, G. Vaccines--How and why they work. Nurs. N. Z. 2016, 22, 20–24. [Google Scholar] [PubMed]
- Reddy, K.S. Boosters appear effective, but are they always needed? Lancet 2021, 398, 2055–2057. [Google Scholar] [CrossRef]
- Ziegler, L.; Klemis, V.; Schmidt, T.; Schneitler, S.; Baum, C.; Neumann, J.; Becker, S.L.; Gärtner, B.C.; Sester, U.; Sester, M. Differences in SARS-CoV-2 specific humoral and cellular immune responses after contralateral and ipsilateral COVID-19 vaccination. eBioMedicine 2023, 95, 104743. [Google Scholar] [CrossRef]
- Pettini, E.; Medaglini, D.; Ciabattini, A. Profiling the B cell immune response elicited by vaccination against the respiratory virus SARS-CoV-2. Front. Immunol. 2022, 13, 1058748. [Google Scholar] [CrossRef]
- Lapuente, D.; Winkler, T.H.; Tenbusch, M. B-cell and antibody responses to SARS-CoV-2: Infection, vaccination, and hybrid immunity. Cell Mol. Immunol. 2024, 21, 144–158. [Google Scholar] [CrossRef]
- Lederer, K.; Bettini, E.; Parvathaneni, K.; Painter, M.M.; Agarwal, D.; Lundgreen, K.A.; Weirick, M.; Muralidharan, K.; Castaño, D.; Goel, R.R.; et al. Germinal center responses to SARS-CoV-2 mRNA vaccines in healthy and immunocompromised individuals. Cell 2022, 185, 1008–1024.e15. [Google Scholar] [CrossRef]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef]
- Chen, M.; Venturi, V.; Munier, C.M.L. Dissecting the Protective Effect of CD8(+) T Cells in Response to SARS-CoV-2 mRNA Vaccination and the Potential Link with Lymph Node CD8(+) T Cells. Biology 2023, 12, 1035. [Google Scholar] [CrossRef]
- Yang, Y.; Miller, H.; Byazrova, M.G.; Cndotti, F.; Benlagha, K.; Camara, N.O.S.; Shi, J.; Forsman, H.; Lee, P.; Yang, L.; et al. The characterization of CD8(+) T-cell responses in COVID-19. Emerg. Microbes Infect. 2024, 13, 2287118. [Google Scholar] [CrossRef] [PubMed]
- Reinscheid, M.; Luxenburger, H.; Karl, V.; Graeser, A.; Giese, S.; Ciminski, K.; Reeg, D.B.; Oberhardt, V.; Roehlen, N.; Lang-Meli, J.; et al. COVID-19 mRNA booster vaccine induces transient CD8+ T effector cell responses while conserving the memory pool for subsequent reactivation. Nat. Commun. 2022, 13, 4631. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal Center B Cell Dynamics. Immunity 2016, 45, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Ritz, N.; Perrett, K.P.; Messina, N.L.; van der Klis, F.R.M.; Curtis, N. Correlation of Vaccine Responses. Front. Immunol. 2021, 12, 646677. [Google Scholar] [CrossRef]
- Röltgen, K.; Nielsen, S.C.A.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185, 1025–1040.e14. [Google Scholar] [CrossRef]
- Silva-Cayetano, A.; Foster, W.S.; Innocentin, S.; Belij-Rammerstorfer, S.; Spencer, A.J.; Burton, O.T.; Fra-Bidó, S.; Le Lee, J.; Thakur, N.; Conceicao, C.; et al. A booster dose enhances immunogenicity of the COVID-19 vaccine candidate ChAdOx1 nCoV-19 in aged mice. Med 2021, 2, 243–262.e8. [Google Scholar] [CrossRef]
- Ying, B.; Liang, C.Y.; Desai, P.; Scheaffer, S.M.; Elbashir, S.M.; Edwards, D.K.; Thackray, L.B.; Diamond, M.S. Ipsilateral or contralateral boosting of mice with mRNA vaccines confers equivalent immunity and protection against a SARS-CoV-2 Omicron strain. J. Virol. 2024, 98, e0057424. [Google Scholar] [CrossRef]
- Jiang, W.; Maldeney, A.R.; Yuan, X.; Richer, M.J.; Renshaw, S.E.; Luo, W. Ipsilateral immunization after a prior SARS-CoV-2 mRNA vaccination elicits superior B cell responses compared to contralateral immunization. Cell Rep. 2024, 43, 113665. [Google Scholar] [CrossRef]
- Jacobson, E.B.; Thorbecke, G.J. The proliferative and anamnestic antibody response of rabbit lymphoid cells in vitro. I. Immunological memory in the lymph nodes draining and contralateral to the site of a primary antigen injection. J. Exp. Med. 1969, 130, 287–297. [Google Scholar] [CrossRef]
- Peck, F.B., Jr.; Kohlstaedt, K.C. PRE-EXPOSURE RABIES PROPHYLAXIS PROBLEMS AND PROCEDURES. Ind. Med. Surg. 1964, 33, 17–21. [Google Scholar] [PubMed]
- Iro, M.A.; Khatami, A.; Marshall, A.S.; Pace, D.; Voysey, M.; McKenna, J.; Campbell, D.; Attard-Montalto, S.; Finn, A.; White, C.; et al. Immunological effect of administration of sequential doses of Haemophilus influenzae type b and pneumococcal conjugate vaccines in the same versus alternating limbs in the routine infant immunisation schedule: An open-label randomised controlled trial. Lancet Infect. Dis. 2015, 15, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Kuraoka, M.; Yeh, C.H.; Bajic, G.; Kotaki, R.; Song, S.; Windsor, I.; Harrison, S.C.; Kelsoe, G. Recall of B cell memory depends on relative locations of prime and boost immunization. Sci. Immunol. 2022, 7, eabn5311. [Google Scholar] [CrossRef] [PubMed]
- Fazli, S.; Thomas, A.; Estrada, A.E.; Ross, H.A.; Xthona Lee, D.; Kazmierczak, S.; Slifka, M.K.; Montefiori, D.; Messer, W.B.; Curlin, M.E. Contralateral second dose improves antibody responses to a 2-dose mRNA vaccination regimen. J. Clin. Investig. 2024, 134, e176411. [Google Scholar] [CrossRef]
- Pattinson, D.; Jester, P.; Gu, C.; Guan, L.; Armbrust, T.; Petrie, J.G.; King, J.P.; Nguyen, H.Q.; Belongia, E.A.; Halfmann, P.; et al. Ipsilateral and contralateral coadministration of influenza and COVID-19 vaccines produce similar antibody responses. eBioMedicine 2024, 103, 105103. [Google Scholar] [CrossRef]
- Grupel, D.; Pasternak, Y.; Schonmann, Y. Effect of same-arm versus cross-arm administration of sequential doses of BNT162b2 on short-term vaccine effectiveness-a retrospective cohort study. Clin. Microbiol. Infect. 2023, 29, 540.e1–540.e7. [Google Scholar] [CrossRef]
| Study | Model | Primary End Point | Time Evaluated Post-Vaccination | Outcomes |
|---|---|---|---|---|
| Ying et al., 2024 [29] | Mice | Impact of site of COVID vaccination on adaptive B and T cell immune response | 1 day; 4 weeks | -Both booster locations induce similar IgG titers -Small variation in neutralizing activity of antibodies -Similar GCBC and T cell response in contralateral vs. ipsilateral groups |
| Jiang et al., 2024 [30] | Mice | GCBC and antibody response to COVID vaccines | 2 days; 4 days; 9 days; 19 weeks | -Higher RBD-specific GCBC response in ipsilateral group -Higher high-affinity RBD-specific antibodies in ipsilateral boost group -More plasma cell differentiation of pre-existing GCBC in ipsilateral group |
| Study | Primary End Point | Time Evaluated Post- Vaccination | Outcomes |
|---|---|---|---|
| Ziegler et al., 2023 [17] | Levels of spike-specific IgG, IgG-avidity, neutralizing antibodies in response to COVID vaccine | 15 days | -Spike-specific IgG levels were similar, but neutralizing activity was lower in the contralateral group -CD4 levels similar; higher CTLA-4 expression in contralateral group -Lower spike-specific CD8 T cells in the contralateral group |
| Fazli et al., 2024 [35] | Serological Response to COVID vaccine | 2.5 weeks; 8 months; 1.1 years | -Higher spike-specific antibodies in the contralateral group |
| Pattison et al., 2024 [36] | Raw mean pre- and post-vaccination titers in response to COVID and influenza vaccine | 22–55 days | -No difference in the immune response between the contralateral and ipsilateral group |
| Grupel et al., 2022 [37] | Positive RT-PCR in response to COVID vaccine | 28 days (10 days after second vaccine dose) | -Positive SARS-CoV-2 PCR was more common in the contralateral group than the ipsilateral group -Adjustments for age and sex showed similar effect -COVID-19 related hospitalization and death was more frequently seen in the contralateral group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naji, A.; El Sahly, H.M.; Whitaker, J.A. A Review of the Effects of Ipsilateral or Contralateral Vaccine Boosting on the Adaptive Immune Response. Vaccines 2025, 13, 1225. https://doi.org/10.3390/vaccines13121225
Naji A, El Sahly HM, Whitaker JA. A Review of the Effects of Ipsilateral or Contralateral Vaccine Boosting on the Adaptive Immune Response. Vaccines. 2025; 13(12):1225. https://doi.org/10.3390/vaccines13121225
Chicago/Turabian StyleNaji, Amal, Hana M. El Sahly, and Jennifer A. Whitaker. 2025. "A Review of the Effects of Ipsilateral or Contralateral Vaccine Boosting on the Adaptive Immune Response" Vaccines 13, no. 12: 1225. https://doi.org/10.3390/vaccines13121225
APA StyleNaji, A., El Sahly, H. M., & Whitaker, J. A. (2025). A Review of the Effects of Ipsilateral or Contralateral Vaccine Boosting on the Adaptive Immune Response. Vaccines, 13(12), 1225. https://doi.org/10.3390/vaccines13121225






