Qualitative Assessment of Early Adverse Effects of Pfizer–BioNTech and Sinopharm COVID-19 Vaccines by Telephone Interviews
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Ethical Approval
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Sample
3.2. Frequency of Adverse Reactions Based on the Vaccine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haidere, M.F.; Ratan, Z.A.; Nowroz, S.; Zaman, S.B.; Jung, Y.J.; Hosseinzadeh, H.; Cho, J.Y. COVID-19 Vaccine: Critical Questions with Complicated Answers. Biomol. Ther. 2021, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ratan, Z.A.; Hosseinzadeh, H.; Runa, N.J.; Mahtab Uddin, B.M.; Haidere, M.F.; Sarker, S.K.; Bin Zaman, S. Novel Coronavirus: A new challenge for medical scientist? Bangladesh J. Infect. Dis. 2020, 7, S58–S60. [Google Scholar] [CrossRef]
- Mullard, A. COVID-19 vaccine development pipeline gears up. Lancet 2020, 395, 1751–1752. [Google Scholar] [CrossRef]
- Gopinathan, U.; Peacocke, E.; Gouglas, D.; Ottersen, T.; Røttingen, J.-A. R&D for Emerging Infectious Diseases of Epidemic Potential: Sharing Risks and Benefits Through a New Coalition. In Infectious Diseases in the New Millennium; Springer: London, UK, 2020; pp. 137–165. [Google Scholar]
- Le, T.T.; Cramer, J.P.; Chen, R.; Mayhew, S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Van Riel, D.; de Wit, E. Next-generation vaccine platforms for COVID-19. Nat. Mater. 2020, 19, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Laboratorio Elea Phoenix S.A. Clinical Trial to Evaluate the Efficacy, Immunogenicity and Safety of the Inactivated SARS-CoV-2 Vaccine (COVID-19): ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04560881 (accessed on 20 June 2021).
- BioNTech SE. Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals: ClinicalTrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04368728 (accessed on 20 June 2021).
- Spencer, J.P.; Trondsen Pawlowski, R.H.; Thomas, S. Vaccine Adverse Events: Separating Myth from Reality. Am. Fam. Physician 2017, 95, 786–794. [Google Scholar] [PubMed]
- University of Oxford. A Study of a Candidate COVID-19 Vaccine (COV001): ClinicalTrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04324606 (accessed on 20 June 2021).
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Jordanian Ministry of Health. COVID-19 Vaccination Platform: MOH. 2021. Available online: https://vaccine.jo/cvms/ (accessed on 20 June 2021).
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine-United States, December 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1922–1924. [Google Scholar] [CrossRef] [PubMed]
- Centers for Diseases Control and Prevention (CDC). Reactions and Adverse Events of the Pfizer-BioNTech COVID-19 Vaccine. 2021. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html (accessed on 20 June 2021).
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [PubMed]
- Kaur, R.J.; Dutta, S.; Bhardwaj, P.; Charan, J.; Dhingra, S.; Mitra, P.; Singh, K.; Yadav, D.; Sharma, P.; Misra, S. Adverse Events Reported From COVID-19 Vaccine Trials: A Systematic Review. Indian J. Clin. Biochem. 2021, 1–13. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, H.C.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Harakeh, S.; Badr-Eldin, S.M.; Bagher, A.M.; Eid, B.; Almukadi, H.; Alghamdi, B.S.; Alahmadi, A.A.; Hassan, N.A.; Sindi, N.; et al. Minor to Moderate Side Effects of Pfizer-BioNTech COVID-19 Vaccine Among Saudi Residents: A Retrospective Cross-Sectional Study. Int. J. Gen. Med. 2021, 14, 1389–1401. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Milken Institute. COVID-19 Treatment and Vaccine Tracker: Milken Institute. 2020. Available online: https://airtable.com/shrSAi6t5WFwqo3GM/tblEzPQS5fnc0FHYR/viweyymxOAtNvo7yH?blocks=bipZFzhJ7wHPv7x9z (accessed on 20 June 2021).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Aloweidi, A.; Bsisu, I.; Suleiman, A.; Abu-Halaweh, S.; Almustafa, M.; Aqel, M.; Amro, A.; Radwan, N.; Assaf, D.; Abdullah, M.Z.; et al. Hesitancy towards COVID-19 Vaccines: An Analytical Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 5111. [Google Scholar] [CrossRef]
- Gee, J.; Marquez, P.; Su, J.; Calvert, G.M.; Liu, R.; Myers, T.; Nair, N.; Martin, S.; Clark, T.; Markowitz, L.; et al. First Month of COVID-19 Vaccine Safety Monitoring-United States, 14 December 2020–13 January 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hammad, O.; Alduraidi, H.; Abu-Hammad, S.; Alnazzawi, A.; Babkair, H.; Abu-Hammad, A.; Nourwali, I.; Qasem, F.; Dar-Odeh, N. Side Effects Reported by Jordanian Healthcare Workers Who Received COVID-19 Vaccines. Vaccines 2021, 9, 577. [Google Scholar] [CrossRef]

| Variable | Categories | Total | Pfizer | Sinopharm | p-Value |
|---|---|---|---|---|---|
| Total | 1004 (100) | 491 (48.9) | 513 (51.1) | ||
| Gender | 0.7 | ||||
| Male | 673 (67) | 332 (49.3) | 341 (50.7) | ||
| Female | 331 (33) | 159 (48) | 172 (52) | ||
| Age | <0.01 | ||||
| 18–30 | 13 (1.3) | 5 (38.5) | 8 (61.5) | ||
| 31–40 | 36 (3.6) | 22 (61.1) | 14 (38.9) | ||
| 41–50 | 262 (26.1) | 160 (61) | 102 (39) | ||
| 51–60 | 118 (11.7) | 30 (25.4) | 88 (74.6) | ||
| 61–70 | 50 (5) | 31 (62) | 19 (38) | ||
| above 70 | 525 (52.3) | 243 (46.3) | 282 (53.7) | ||
| BMI | 0.08 | ||||
| Underweight | 10 (1) | 8 (80) | 2 (20) | ||
| Normal | 259 (25.8) | 116 (44.8) | 143 (55.2) | ||
| Overweight | 488 (48.7) | 249 (51) | 239 (49) | ||
| Obese | 246 (24.5) | 117 (47.6) | 129 (52.4) | ||
| Chronic diseases | |||||
| Hypertension | 428 (42.6) | 186 (43.5) | 242 (56.5) | <0.01 | |
| Coronary artery disease | 76 (7.6) | 26 (34.2) | 50 (65.8) | <0.01 | |
| Diabetes | 301 (30) | 149 (49.5) | 152 (50.5) | 0.8 | |
| Thyroid disorders | 36 (3.6) | 16 (44.4) | 20 (55.6) | 0.6 | |
| Dyslipidemia | 113 (11.3) | 49 (43.4) | 64 (56.6) | 0.2 | |
| Respiratory disorder | 21 (2) | 9 (42.9) | 12 (57.1) | 0.6 | |
| Currently smoking | 332 (33.1) | 176 (53) | 156 (47) | 0.07 | |
| History of COVID-19 infection | 41 (4) | 17 (41.5) | 24 (58.5) | 0.3 |
| First Dose of the Vaccine | Second Dose of the Vaccine | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adverse Reaction | Total | Pfizer | Sinopharm | p-Value | Adverse Reaction | Total | Pfizer | Sinopharm | p-Value |
| Having an adverse reaction | 465 (46.3) | 307 (62.5) | 158 (30.8) | <0.01 | Having an adverse reaction | 488 (48.6) | 320 (65.2) | 168 (32.8) | <0.01 |
| Local side effects | 338 (33.7) | 247 (50.3) | 91 (17.7) | <0.01 | Local side effects | 293 (30) | 217 (44.2) | 76 (14.8) | <0.01 |
| Systemic side effects | 238 (23.7) | 148 (30.2) | 90 (17.5) | <0.01 | Systemic side effects | 313 (31.2) | 200 (40.7) | 113 (22) | <0.01 |
| Frequency | Onset of Symptoms (Hours) | Duration of Symptoms (Hours) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse Reaction | Total | Pfizer | Sinopharm | p-Value | Total | Pfizer | Sinopharm | p-Value | Total | Pfizer | Sinopharm | p-Value |
| Local adverse reactions | ||||||||||||
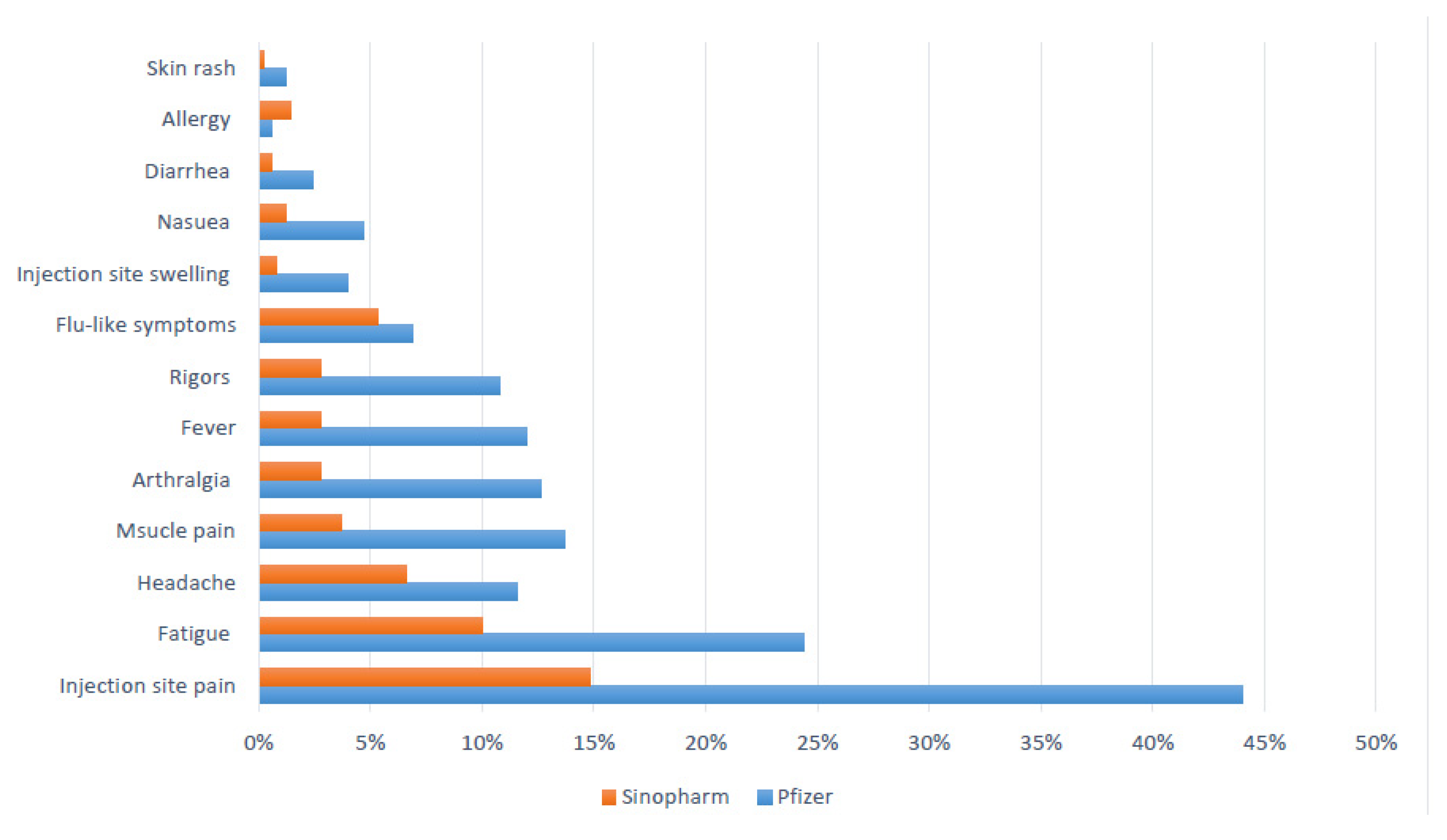
| Pain at the site of the injection | 333 (33.2) | 245 (49.9) | 88 (17.2) | <0.01 | 8.9 ± 10.4 | 9.4 ± 9.9 | 7.8 ± 11.4 | 0.2 | 39.4 ± 34.5 | 40.4 ± 35.3 | 36.5 ± 32.1 | 0.4 |
| Swelling at site of injection | 28 (2.8) | 23 (4.7) | 5 (1) | <0.01 | 20.5 ± 20.4 | 18.5 ± 18 | 29 ± 29.8 | 0.3 | 51.4 ± 37.7 | 47.9 ± 30.8 | 67.2 ± 62.1 | 0.3 |
| Systemic adverse reaction | ||||||||||||
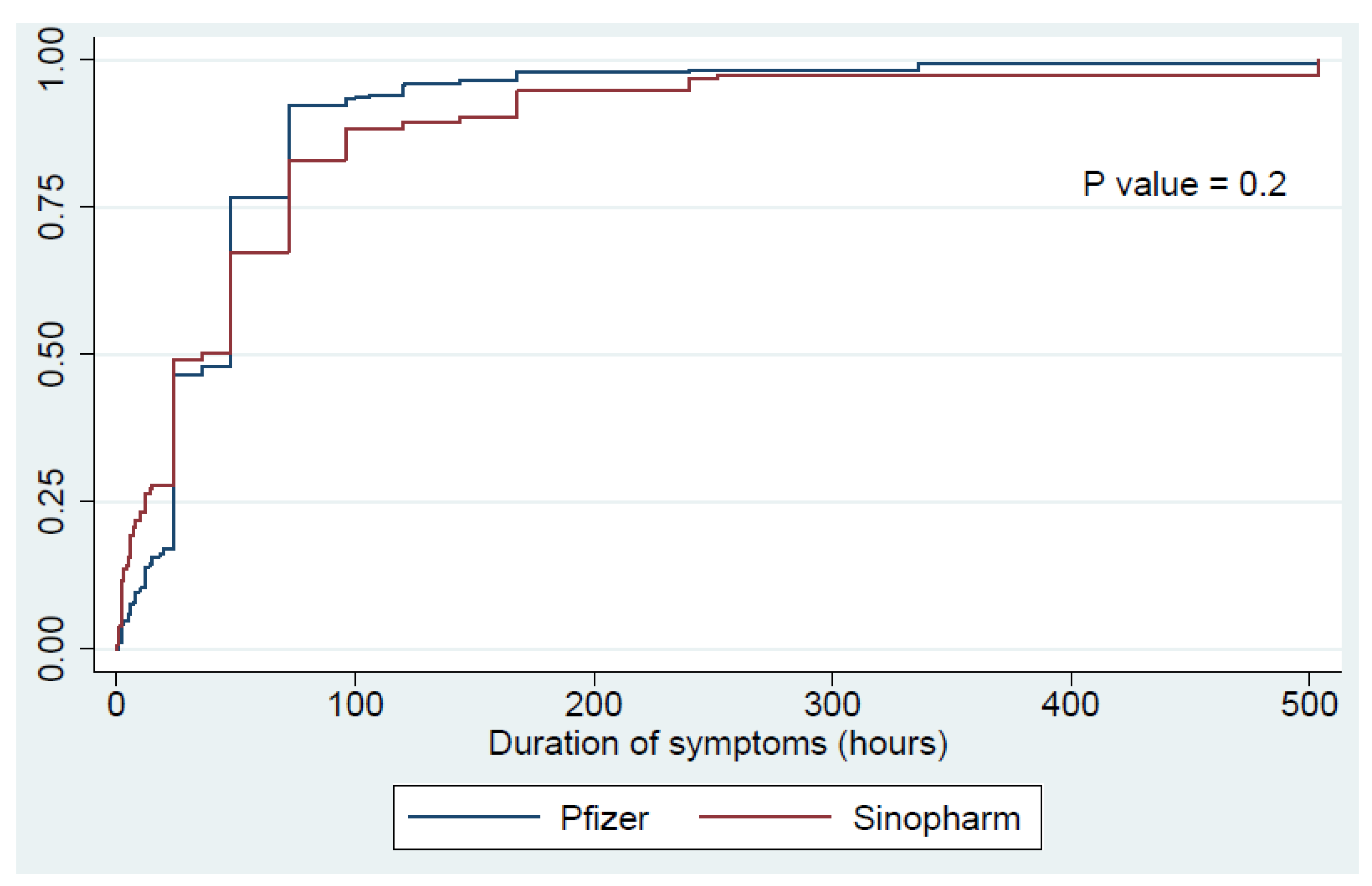
| General weakness | 124 (12.4) | 81 (16.5) | 43 (8.4) | <0.01 | 18.3 ± 21 | 17.4 ± 17 | 20.1 ± 21 | 0.4 | 58 ± 84 | 43.3 ± 43.9 | 83.9 ± 124.6 | 0.01 |
| Headache | 80 (8) | 51 (10.4) | 29 (5.7) | <0.01 | 20 ± 34.3 | 11.4 ± 11 | 35.4 ± 52.5 | <0.01 | 38.1 ± 34.3 | 34.4 ± 28.7 | 45.5 ± 43.1 | 0.2 |
| Muscle pain | 58 (5.8) | 47 (9.6) | 11 (2.1) | <0.01 | 23.2 ± 32.8 | 21.3 ± 27.9 | 30.5 ± 48.6 | 0.4 | 55 ± 81 | 45.4 ± 52.4 | 91.9 ± 144 | 0.09 |
| Rigors | 42 (4.2) | 33 (6.7) | 9 (1.8) | <0.01 | 22.3 ± 30.7 | 20.4 ± 31.5 | 28.9 ± 28.3 | 0.5 | 42.3 ± 81.8 | 48.5 ± 91 | 21.2 ± 21.3 | 0.4 |
| Arthralgia | 48 (4.8) | 38 (7.7) | 10 (2) | <0.01 | 36.3 ± 70 | 33.4 ± 21.3 | 46.1 ± 70 | 0.4 | 44.7 ± 83.6 | 27 ± 28 | 103 ± 157.6 | 0.01 |
| Fever | 47 (4.7) | 31 (6.3) | 16 (3.1) | 0.02 | 19.8 ± 26 | 17.8 ± 26.6 | 23.3 ± 25.4 | 0.5 | 34.5 ± 37.1 | 32 ± 32.8 | 30.3 ± 45.3 | 0.5 |
| Abdominal pain/diarrhea | 14 (1.4) | 7 (1.4) | 7 (1.4) | 0.9 | 40.7 ± 45.5 | 28.3 ± 24.7 | 28.8 ± 64.4 | 0.3 | 31.9 ± 30.9 | 40.8 ± 28.9 | 25.5 ± 32.8 | 0.4 |
| Nausea/vomiting | 24 (2.4) | 13 (2.7) | 11 (2.2) | 0.6 | 35.2 ± 54.4 | 27.3 ± 42.7 | 43.1 ± 65.1 | 0.5 | 34.4 ± 28.5 | 31.9 ± 19.8 | 37.2 ± 36.8 | 0.7 |
| Skin rash | 4 (0.4) | 2 (0.4) | 2 (0.4) | 0.9 | 92.3 ± 162.8 | 180 ± 220.6 | 4.5 ± 2.1 | 0.4 | 13 ± 15.6 | 13 ± 15.6 | 13 ± 15.6 | 1 |
| Allergic reaction | 8 (0.8) | 4 (0.8) | 4 (0.8) | 1 | 7 ± 16.6 | 40 ± 13.9 | 16 ± 9.8 | 0.04 | 145.5 ± 158 | 75 ± 62.3 | 216 ± 203.7 | 0.2 |
| Flu-like symptoms | 30 (3) | 18 (3.7) | 12 (2.3) | 0.2 | 53.3 ± 75.2 | 55.6 ± 82.9 | 40.1 ± 62.4 | 0.8 | 92.8 ± 102.1 | 72.6 ± 109 | 127.7 ± 82 | 0.2 |
| Frequency | Onset of Symptoms (Hours) | Duration of Symptoms (Hours) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adverse Reaction | Total | Pfizer | Sinopharm | Adverse Reaction | Total | Pfizer | Sinopharm | Adverse Reaction | Total | Pfizer | Sinopharm | Adverse Reaction |
| Local Adverse reactions | ||||||||||||
| Pain at the site of the injection | 292 (29) | 216 (44) | 76 (14.8) | <0.01 | 9.9 ± 14.8 | 10.3 ± 12.1 | 8.7 ± 20.3 | 0.4 | 46.5 ± 60.8 | 44 ± 46 | 46.5 ± 60.8 | 0.7 |
| Swelling at site of injection | 24 (2.4) | 20 (4) | 4 (0.8) | <0.01 | 11.4 ± 12.2 | 10.7 ± 12.5 | 14.1 ± 11.8 | 0.6 | 50.5 ± 30.6 | 49.2 ± 31.3 | 57 ± 9 | 0.7 |
| Systemic Adverse reactions | ||||||||||||
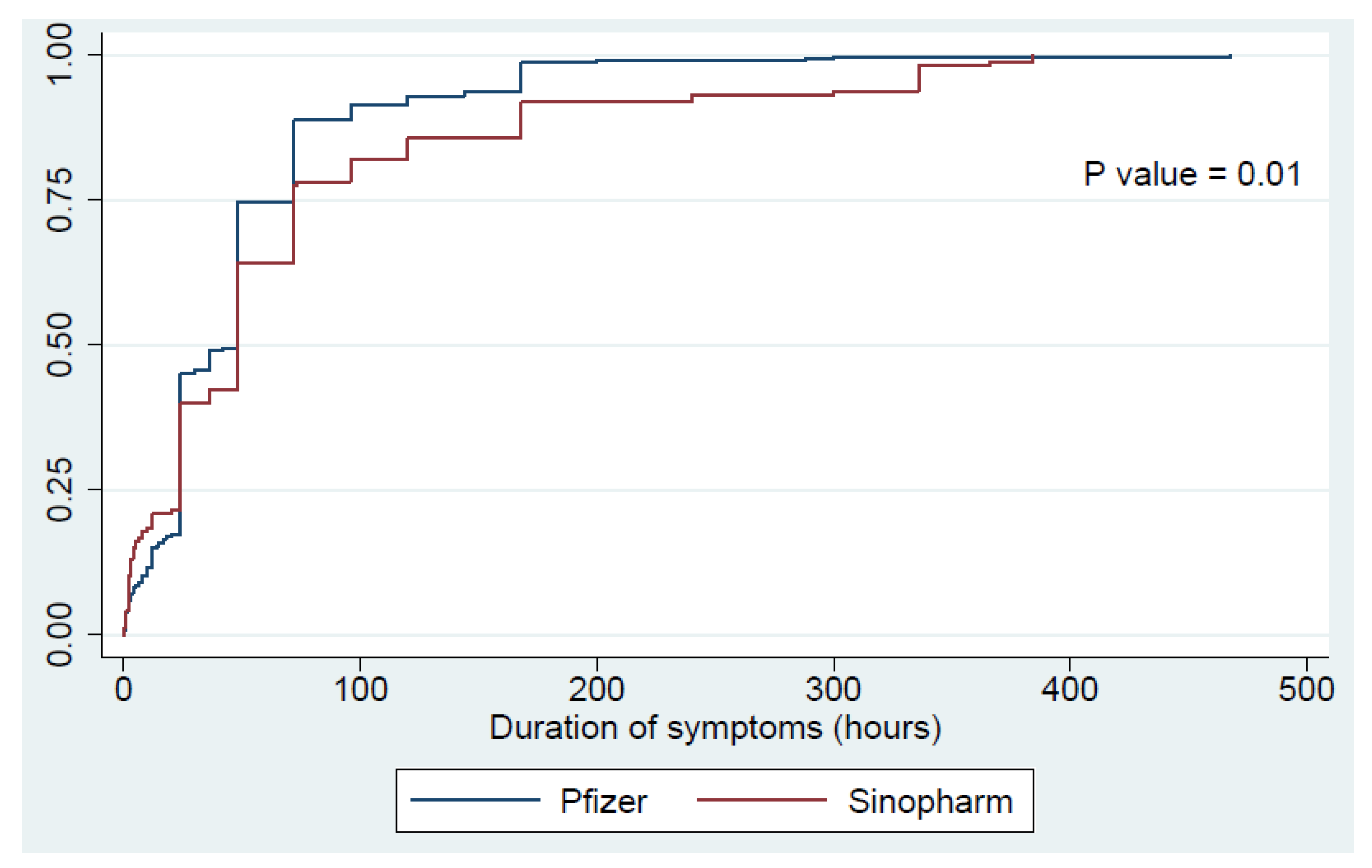
| General weakness | 171 (17) | 120 (24.4) | 51 (10) | <0.01 | 19.5 ± 26.5 | 17.1 ± 15.6 | 25.5 ± 42.3 | 0.06 | 54.1 ± 61.8 | 45.5 ± 36.4 | 75.1 ± 97.2 | <0.01 |
| Headache | 91 (9.1) | 57 (11.6) | 34 (6.6) | <0.01 | 21.2 ± 21.1 | 18.5 ± 29.3 | 25.6 ± 21.9 | 0.1 | 51.3 ± 46.1 | 45.7 ± 40.2 | 61 ± 54 | 0.1 |
| Muscle pain | 86 (8.6) | 67 (13.7) | 19 (3.7) | <0.01 | 24.8 ± 25 | 20.7 ± 21.1 | 39 ± 32.3 | <0.01 | 58 ± 60.8 | 45 ± 32.1 | 103.6 ± 103.6 | <0.01 |
| Rigors | 67 (6.7) | 53 (10.8) | 14 (2.8) | <0.01 | 27.1 ± 41.8 | 22.7 ± 30.5 | 43.4 ± 68.7 | 0.1 | 43.2 ± 52.6 | 38.9 ± 35.7 | 59.1 ± 92 | 0.2 |
| Arthralgia | 84 (8.4) | 62 (12.6) | 22 (4.3) | <0.01 | 64.5 ± 110.5 | 38.4 ± 32.4 | 136.3 ± 192.9 | <0.01 | 52.4 ± 87.8 | 45.9 ± 82.7 | 69.7 ± 100 | 0.3 |
| Fever | 73 (7.3) | 59 (12) | 14 (2.8) | <0.01 | 18 ± 17.5 | 16.9 ± 14.8 | 22.4 ± 26.2 | 0.3 | 50.7 ± 61 | 44.8 ± 38.8 | 75.3 ± 113.6 | 0.09 |
| Abdominal pain/diarrhea | 15 (1.5) | 12 (2.4) | 3 (0.6) | 0.02 | 35.9 ± 38.5 | 28.9 ± 27.3 | 64 ± 69.3 | 0.2 | 42.7 ± 41.7 | 42.4 ± 45.8 | 44 ± 25 | 0.96 |
| Nausea/vomiting | 29 (2.9) | 23 (4.7) | 6 (1.2) | <0.01 | 29.9 ± 45 | 30.9 ± 45.5 | 26 ± 46.8 | 0.8 | 29 ± 42.4 | 46 ± 44.1 | 18.1 ± 27.9 | 0.15 |
| Skin rash | 7 (0.7) | 6 (1.2) | 1 (0.2) | 0.05 | 17.6 ± 15.1 | 21.3 ± 20.2 | 3 | N/A | 34.5 ± 68.9 | 2 | 63 ± 62 | N/A |
| Allergic reaction | 10 (1) | 3 (0.6) | 7 (1.4) | 0.2 | 32.6 ± 20 | 34.7 ± 33.3 | 18 ± 9.3 | 0.3 | 109.3 ± 93.8 | 56 ± 13.8 | 136 ± 107 | 0.3 |
| Flu-like symptoms | 61 (6.1) | 34 (6.9) | 27 (5.3) | 0.3 | 50.9 ± 59.6 | 30.1 ± 43.3 | 77 ± 67.3 | <0.01 | 79.3 ± 70.6 | 12 ± 70.3 | 80.8 ± 72.2 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Halaweh, S.; Alqassieh, R.; Suleiman, A.; Al-Sabbagh, M.Q.; AbuHalaweh, M.; AlKhader, D.; Abu-Nejem, R.; Nabulsi, R.; Al-Tamimi, M.; Alwreikat, M.; et al. Qualitative Assessment of Early Adverse Effects of Pfizer–BioNTech and Sinopharm COVID-19 Vaccines by Telephone Interviews. Vaccines 2021, 9, 950. https://doi.org/10.3390/vaccines9090950
Abu-Halaweh S, Alqassieh R, Suleiman A, Al-Sabbagh MQ, AbuHalaweh M, AlKhader D, Abu-Nejem R, Nabulsi R, Al-Tamimi M, Alwreikat M, et al. Qualitative Assessment of Early Adverse Effects of Pfizer–BioNTech and Sinopharm COVID-19 Vaccines by Telephone Interviews. Vaccines. 2021; 9(9):950. https://doi.org/10.3390/vaccines9090950
Chicago/Turabian StyleAbu-Halaweh, Sami, Rami Alqassieh, Aiman Suleiman, Mohammed Qussay Al-Sabbagh, Maram AbuHalaweh, Duaa AlKhader, Rozan Abu-Nejem, Roa’a Nabulsi, Mohammad Al-Tamimi, Mallak Alwreikat, and et al. 2021. "Qualitative Assessment of Early Adverse Effects of Pfizer–BioNTech and Sinopharm COVID-19 Vaccines by Telephone Interviews" Vaccines 9, no. 9: 950. https://doi.org/10.3390/vaccines9090950
APA StyleAbu-Halaweh, S., Alqassieh, R., Suleiman, A., Al-Sabbagh, M. Q., AbuHalaweh, M., AlKhader, D., Abu-Nejem, R., Nabulsi, R., Al-Tamimi, M., Alwreikat, M., Alnouti, M., Suleiman, B., Yousef, M., Jarbeh, M. E., Al-Shudifat, A.-E., Alqassieh, A., & Bsisu, I. (2021). Qualitative Assessment of Early Adverse Effects of Pfizer–BioNTech and Sinopharm COVID-19 Vaccines by Telephone Interviews. Vaccines, 9(9), 950. https://doi.org/10.3390/vaccines9090950








