Role of NKT Cells during Viral Infection and the Development of NKT Cell-Based Nanovaccines
Abstract
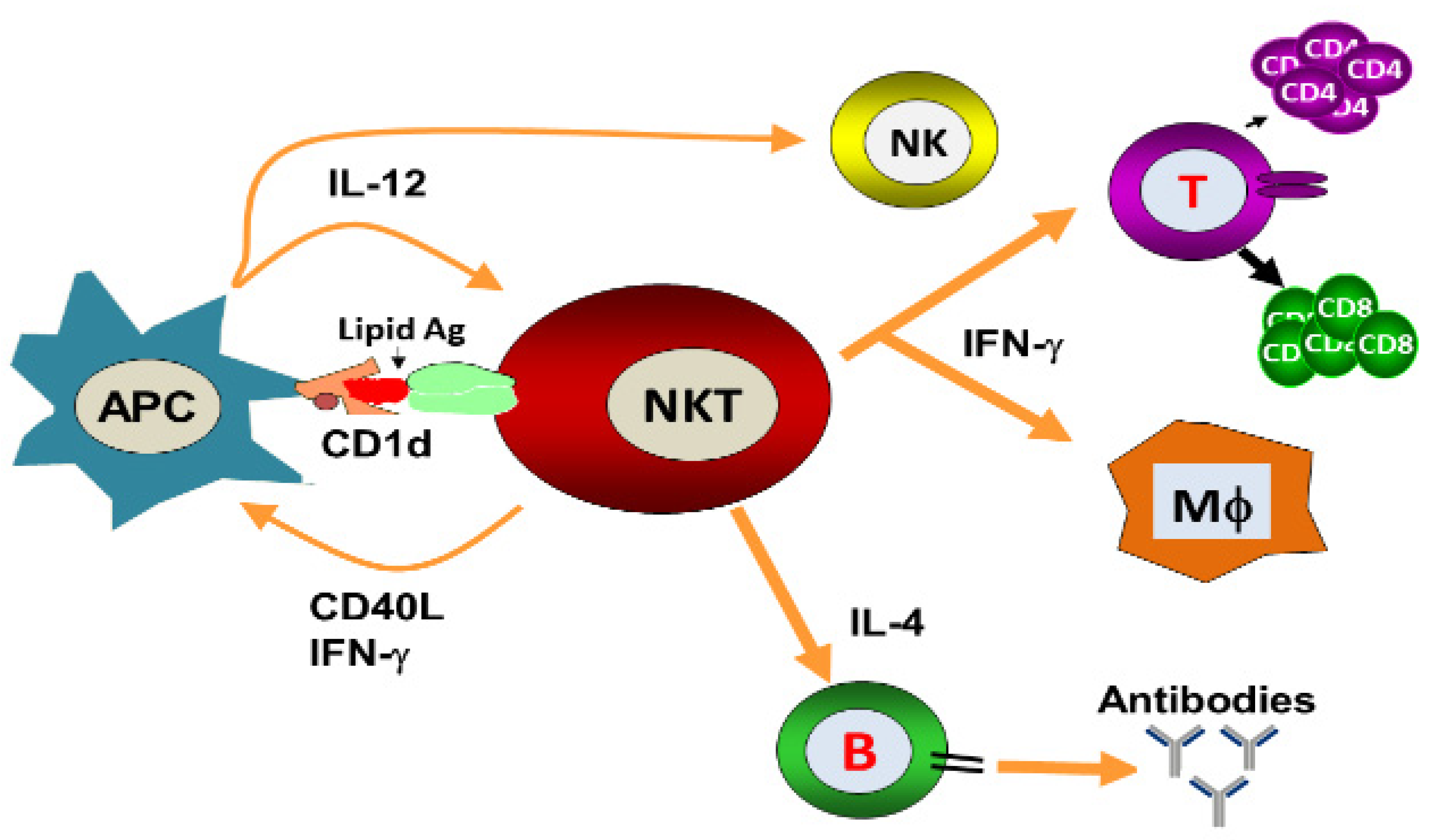
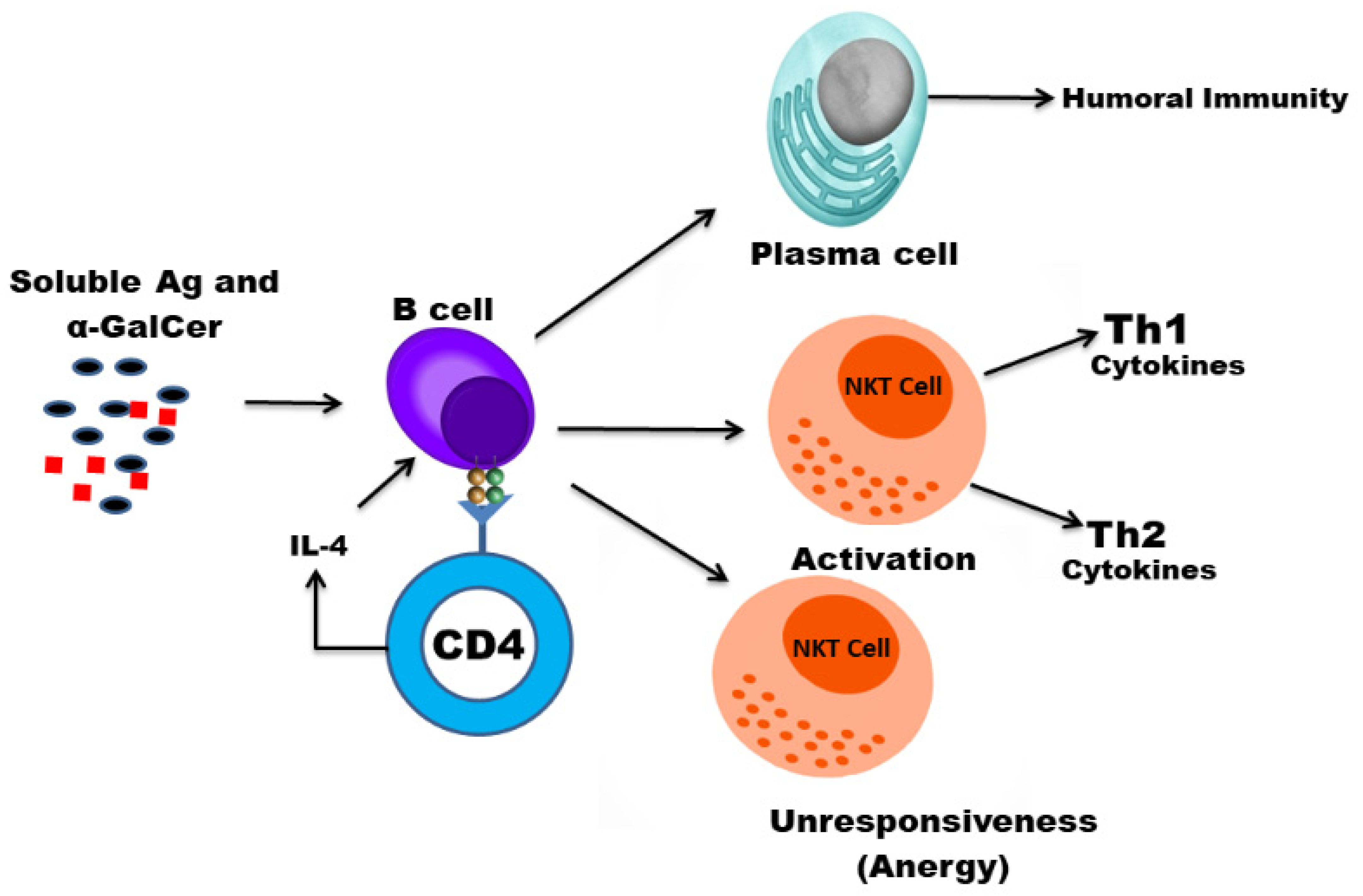
1. Introduction
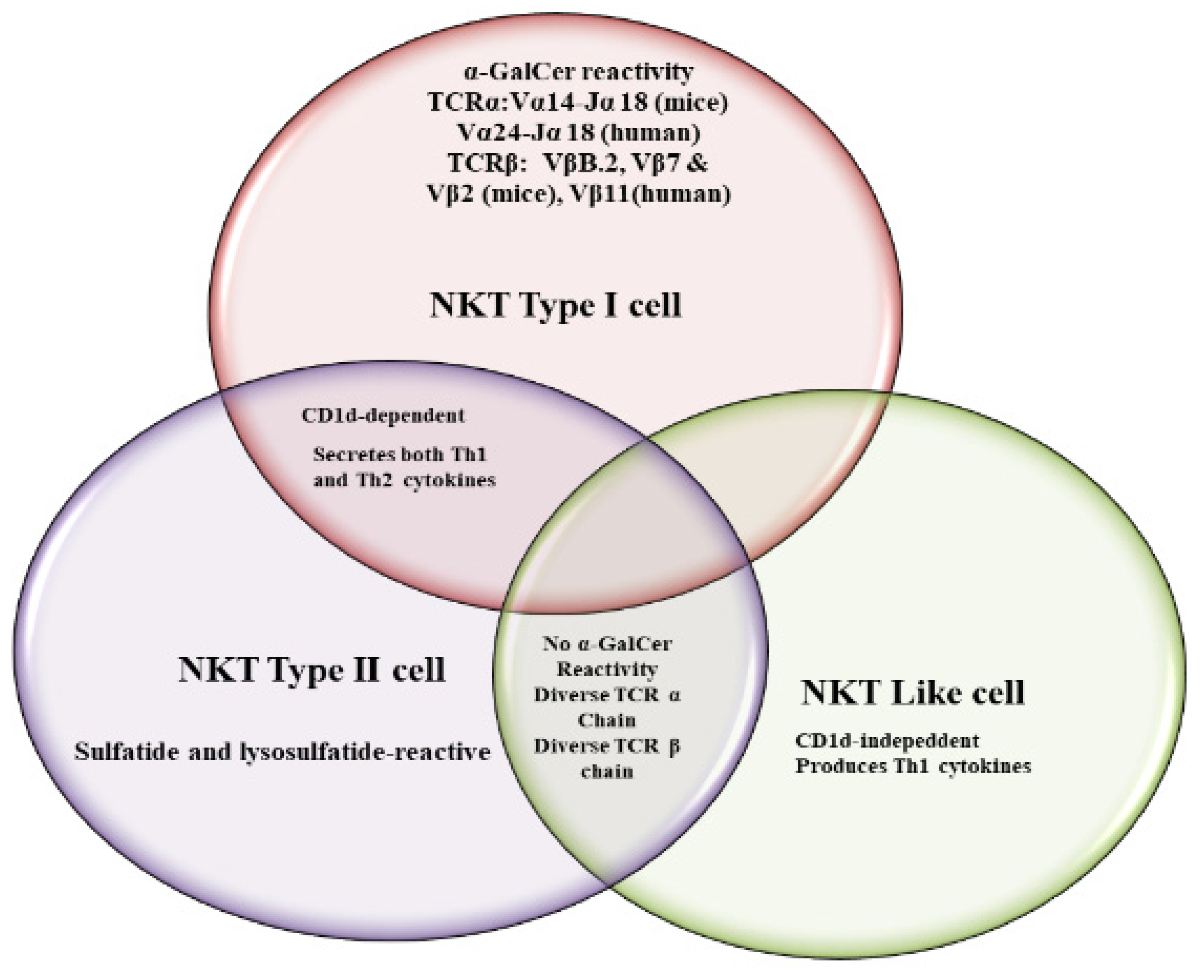
2. NKT Cell Ligands
3. Role of iNKT Cells against Viral Infections
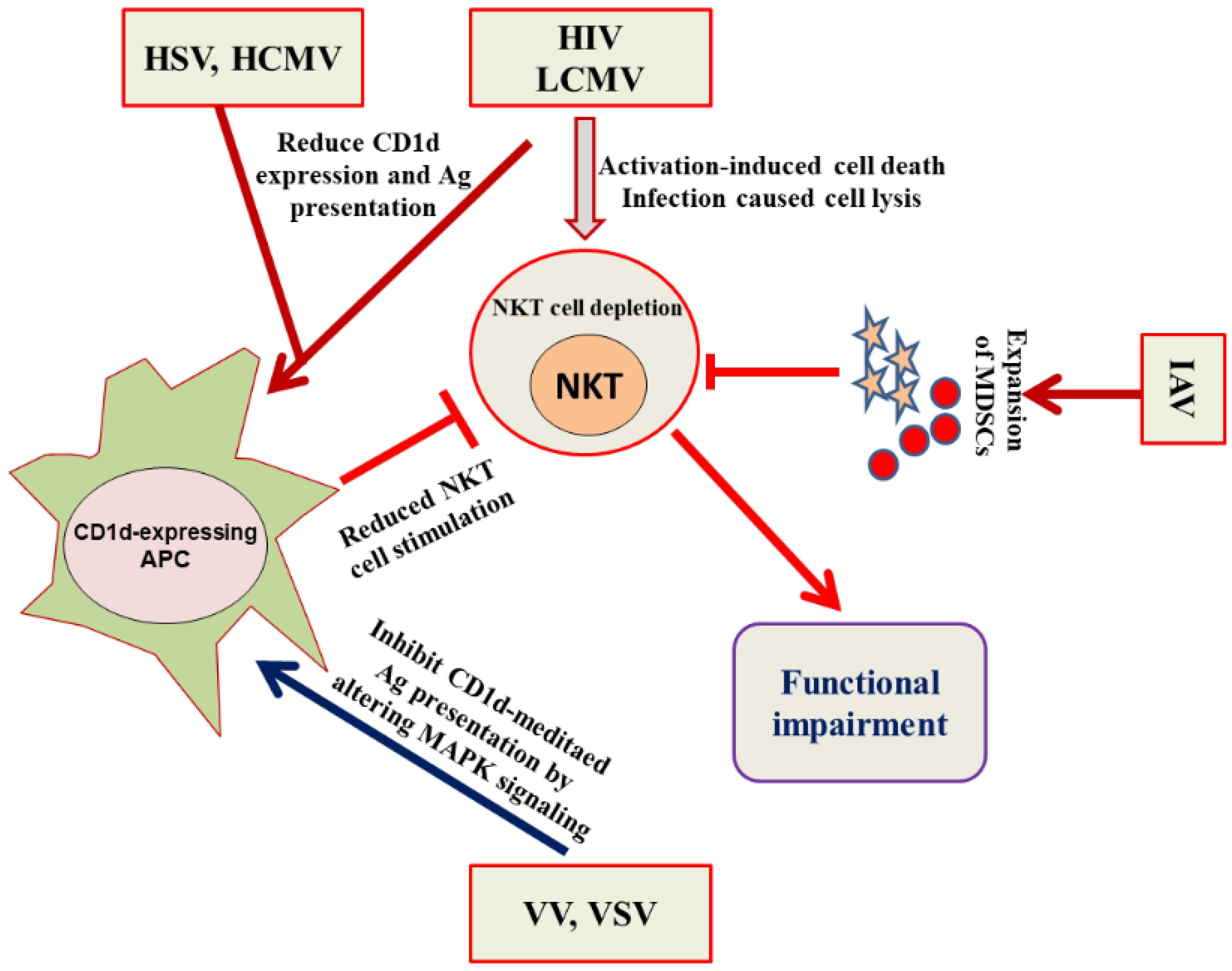
4. Evasion of the NKT Cell Functioning by Viruses
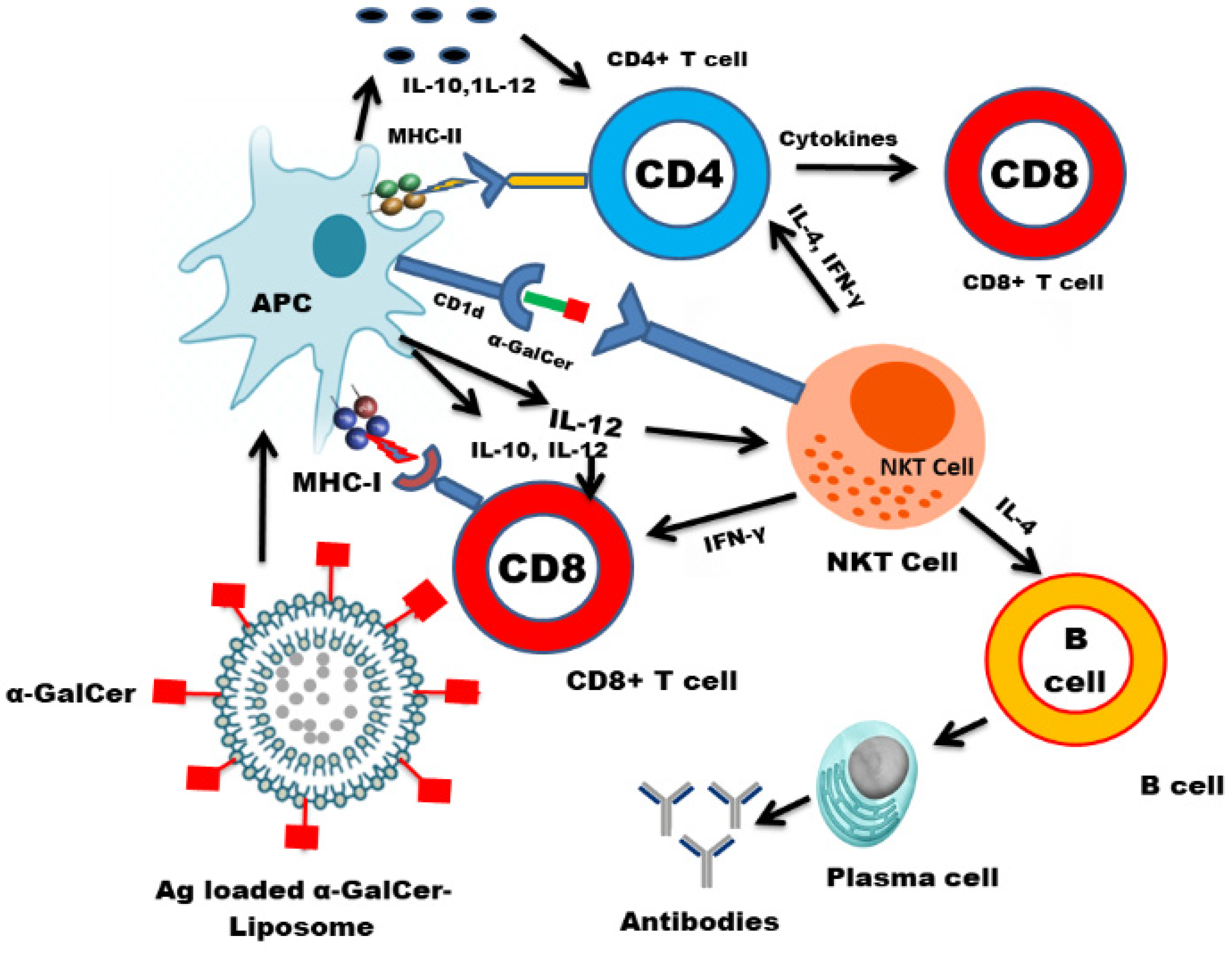
5. Nanotechnology-Based Vaccine Delivery Platforms and the Development of an NKT Cell-Based Nanovaccine
5.1. Inorganic Nanoparticles
5.2. Liposomes
5.3. Polymeric Nanoparticles
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bendelac, A.; Savage, P.B.; Teyton, L. The Biology of NKT Cells. Annu. Rev. Immunol. 2007, 25, 297–336. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Stankovic, S.; Baxter, A. Raising the NKT cell family. Nat. Immunol. 2010, 11, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Berzins, S.P.; Smyth, M.; Baxter, A. Presumed guilty: Natural killer T cell defects and human disease. Nat. Rev. Immunol. 2011, 11, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Peralbo, E.; Alonso, C.; Solana, R. Invariant NKT and NKT-like lymphocytes: Two different T cell subsets that are differentially affected by ageing. Exp. Gerontol. 2007, 42, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Terrazzano, G.; Bruzzaniti, S.; Rubino, V.; Santopaolo, M.; Palatucci, A.T.; Giovazzino, A.; La Rocca, C.; de Candia, P.; Puca, A.; Perna, F.; et al. T1D progression is associated with loss of CD3+CD56+ regulatory T cells that control CD8+ T cell effector functions. Nat. Metab. 2020, 2, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Uldrich, A.; Patel, O.; Cameron, G.; Pellicci, D.; Day, E.B.; Sullivan, L.; Kyparissoudis, K.; Kjer-Nielsen, L.; Vivian, J.; Cao, B.; et al. A semi-invariant Vα10+ T cell antigen receptor defines a population of natural killer T cells with distinct glycolipid antigen—Recognition properties. Nat. Immunol. 2011, 12, 616–623. [Google Scholar] [CrossRef]
- Reantragoon, R.; Corbett, A.; Sakala, I.G.; Gherardin, N.; Furness, J.B.; Chen, Z.; Eckle, S.; Uldrich, A.; Birkinshaw, R.; Patel, O.; et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J. Exp. Med. 2013, 210, 2305–2320. [Google Scholar] [CrossRef]
- Tang, X.-Z.; Jo, J.; Tan, A.T.; Sandalova, E.; Chia, A.; Tan, K.C.; Lee, K.H.; Gehring, A.; De Libero, G.; Bertoletti, A. IL-7 Licenses Activation of Human Liver Intrasinusoidal Mucosal-Associated Invariant T Cells. J. Immunol. 2013, 190, 3142–3152. [Google Scholar] [CrossRef]
- Loh, L.; Wang, Z.; Sant, S.; Koutsakos, M.; Jegaskanda, S.; Corbett, A.J.; Liu, L.; Fairlie, D.; Crowe, J.; Rossjohn, J.; et al. Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation. Proc. Natl. Acad. Sci. USA 2016, 113, 10133–10138. [Google Scholar] [CrossRef] [PubMed]
- Le Bourhis, L.; Martin, E.; Péguillet, I.; Guihot, A.; Froux, N.; Coré, M.; Lévy, E.; Dusseaux, M.; Meyssonnier, V.; Premel, V.; et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 2010, 11, 701–708. [Google Scholar] [CrossRef]
- Salio, M.; Silk, J.D.; Jones, Y.; Cerundolo, V. Biology of CD1- and MR1-Restricted T Cells. Annu. Rev. Immunol. 2014, 32, 323–366. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.C.; Cerri, S.; Smyk-Pearson, S.; Cansler, M.E.; Vogt, T.M.; Delepine, J.; Winata, E.; Swarbrick, G.M.; Chua, W.-J.; Yu, Y.Y.L.; et al. Human Mucosal Associated Invariant T Cells Detect Bacterially Infected Cells. PLoS Biol. 2010, 8, e1000407. [Google Scholar] [CrossRef] [PubMed]
- Lepore, M.; Kalinichenko, A.; Colone, A.; Paleja, B.; Singhal, A.; Tschumi, A.; Lee, B.; Poidinger, M.; Zolezzi, F.; Quagliata, L.; et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat. Commun. 2014, 5, 3866. [Google Scholar] [CrossRef]
- Morita, M.; Motoki, K.; Akimoto, K.; Natori, T.; Sakai, T.; Sawa, E.; Yamaji, K.; Koezuka, Y.; Kobayashi, E.; Fukushima, H. Structure-Activity relationship of α-Galactosylceramide against B-16 bearing mice. J. Med. Chem. 1995, 38, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.C.W.; Penaranda, C.; Kashyap, P.C.; Williams, B.B.; Clardy, J.; Kronenberg, M.; Sonnenburg, J.L.; Comstock, L.E.; Bluestone, J.A.; Fischbach, M.A. Production of α-Galactosylceramide by a Prominent Member of the Human Gut Microbiota. PLoS Biol. 2013, 11, e1001610. [Google Scholar] [CrossRef]
- von Gerichten, J.; Schlosser, K.; Lamprecht, D.; Morace, I.; Eckhardt, M.; Wachten, D.; Jennemann, R.; Gröne, H.-J.; Mack, M.; Sandhoff, R. Diastereomer-specific quantification of bioactive hexosylceramides from bacteria and mammals. J. Lipid Res. 2017, 58, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Sriram, V.; Du, W.; Gervay-Hague, J.; Brutkiewicz, R.R. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur. J. Immunol. 2005, 35, 1692–1701. [Google Scholar] [CrossRef]
- Brutkiewicz, R.R. CD1d ligands: The good, the bad, and the ugly. J. Immunol. 2006, 177, 769–775. [Google Scholar] [CrossRef]
- Albacker, L.A.; Chaudhary, V.; Chang, Y.J.; Kim, H.Y.; Chuang, Y.T.; Pichavant, M.; DeKruyff, R.H.; Savage, P.B.; Umetsu, D.T. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyper reactivity. Nat. Med. 2013, 19, 1297–1304. [Google Scholar] [CrossRef]
- Dhodapkar, M.V.; Kumar, V. Type II NKT Cells and Their Emerging Role in Health and Disease. J. Immunol. 2017, 198, 1015–1021. [Google Scholar] [CrossRef]
- Brutkiewicz, R.R.; Lin, Y.; Cho, S.; Hwang, Y.K.; Sriram, V.; Roberts, T.J. CD1d-mediated antigen presentation to natural killer T (NKT) cells. Crit. Rev. Immunol. 2003, 23, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Coquet, J.; Chakravarti, S.; Kyparissoudis, K.; McNab, F.W.; Pitt, L.A.; McKenzie, B.S.; Berzins, S.P.; Smyth, M.; Godfrey, D.I. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc. Natl. Acad. Sci. USA 2008, 105, 11287–11292. [Google Scholar] [CrossRef] [PubMed]
- James, E.E.; Andrew, J.K.; Webb, T.J. Raising the roof: The preferential pharmacological stimulation of Th1 and Th2 responses mediated by NKT cells. Med. Res. Rev. 2014, 34, 45–76. [Google Scholar]
- Liu, Z.; Guo, J. NKT-Cell glycolipid agonist as adjuvant in synthetic vaccine. Carbohydr. Res. 2017, 452, 78–90. [Google Scholar] [CrossRef]
- Kakimi, K.; Guidotti, L.G.; Koezuka, Y.; Chisari, F. Natural Killer T Cell Activation Inhibits Hepatitis B Virus Replication in Vivo. J. Exp. Med. 2000, 192, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Glosson, N.L.; Du, W.; Gervay-Hague, J.; Brutkiewicz, R.R. A Thr/Ser dual residue motif in the cytoplasmic tail of human CD1d is important for the down-regulation of antigen presentation following a herpes simplex virus 1 infection. Immunology 2013, 140, 191–201. [Google Scholar] [CrossRef]
- Exley, M.A.; Bigley, N.J.; Cheng, O.; Tahir, S.M.; Smiley, S.T.; Carter, Q.L.; Stills, H.F.; Grusby, M.J.; Koezuka, Y.; Taniguchi, M.; et al. CD1d-Reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis viruses. J. Leukoc. Biol. 2001, 69, 713–718. [Google Scholar] [PubMed]
- Diana, J.; Griseri, T.; Lagaye, S.; Beaudoin, L.; Autrusseau, E.; Gautron, A.-S.; Tomkiewicz, C.; Herbelin, A.; Barouki, R.; von Herrath, M.; et al. NKT Cell-Plasmacytoid Dendritic Cell Cooperation via OX40 Controls Viral Infection in a Tissue-Specific Manner. Immunity 2009, 30, 289–299. [Google Scholar] [CrossRef]
- Ho, L.; Denney, L.; Luhn, K.; Teoh, D.; Clelland, C.; McMichael, A.J. Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza A virus infection. Eur. J. Immunol. 2008, 38, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- van Dommelen, S.L.H.; Tabarias, H.A.; Smyth, M.J.; Degli-Esposti, M.A.; Law, L.M.J.; Everitt, J.C.; Beatch, M.D.; Holmes, C.F.B.; Hobman, T.C. Activation of Natural Killer (NK) T Cells during Murine Cytomegalovirus Infection Enhances the Antiviral Response Mediated by NK Cells. J. Virol. 2003, 77, 1764–1771. [Google Scholar] [CrossRef]
- Wu, C.Y.; Feng, Y.; Qian, G.C.; Wu, J.H.; Luo, J.; Wang, Y.; Chen, G.J.; Guo, X.-K.; Wang, Z.J. α-Galactosylceramide protects mice from lethal Coxsackievirus B3 infection and subsequent myocarditis. Clin. Exp. Immunol. 2010, 162, 178–187. [Google Scholar] [CrossRef]
- Johnson, T.R.; Hong, S.; Van Kaer, L.; Koezuka, Y.; Graham, B.S. NK T Cells Contribute to Expansion of CD8 + T Cells and Amplification of Antiviral Immune Responses to Respiratory Syncytial Virus. J. Virol. 2002, 76, 4294–4303. [Google Scholar] [CrossRef]
- De Santo, C.; Salio, M.; Masri, S.H.; Lee, L.Y.-H.; Dong, T.; Speak, A.; Porubsky, S.; Booth, S.; Veerapen, N.; Besra, G.; et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus–induced myeloid-derived suppressor cells in mice and humans. J. Clin. Investig. 2008, 118, 4036–4048. [Google Scholar] [CrossRef]
- Singh, D.; Ghate, M.; Godbole, S.; Kulkarni, S.; Thakar, M. Functional Invariant Natural Killer T Cells Secreting Cytokines Are Associated with Non-Progressive Human Immunodeficiency Virus-1 Infection but Not With Suppressive Anti-Retroviral Treatment. Front. Immunol. 2018, 9, 1152. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.E.; Hom, J.; Gong, S.; Ganguly, A.; Ma, C.; Cannons, J.L.; Tangye, P.S.; Schwartzberg, P.L.; Koretzky, G.A.; Stein, P.L. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat. Med. 2005, 11, 340–345. [Google Scholar] [CrossRef]
- Rigaud, S.; Fondanèche, M.-C.; Lambert, N.C.; Pasquier, B.; Mateo, V.; Soulas-Sprauel, P.; Galicier, L.; Le Deist, F.; Rieux-Laucat, F.; Revy, P.; et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature 2006, 444, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Locci, M.; Draghici, E.; Marangoni, F.; Bosticardo, M.; Catucci, M.; Aiuti, A.; Cancrini, C.; Marodi, L.; Espanol, T.; Bredius, R.G.; et al. The Wiskott-Aldrich syndrome protein is required for iNKT cell maturation and function. J. Exp. Med. 2009, 206, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Orange, J.S.; Hibberd, P.; Steinberg, S.; LaRussa, P.; Weinberg, A.; Wilson, S.B.; Shaulov, A.; Fleisher, G.; Geha, R.S.; et al. Disseminated varicella infection due to the vaccine strain of varicella-zoster virus, in a patient with a novel deficiency in natural killer T cells. J. Infect. Dis. 2003, 188, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Grubor-Bauk, B.; Simmons, A.; Mayrhofer, G.; Speck, P.G. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J alpha 281 TCR. J. Immunol. 2003, 170, 1430–1434. [Google Scholar] [CrossRef]
- Yuan, W.; Dasgupta, A.; Cresswell, P. Herpes simplex virus evades naturalkiller T cell recognition by suppressing CD1d recycling. Nat. Immunol. 2006, 7, 835–842. [Google Scholar] [CrossRef]
- Tyznik, A.J.; Tupin, E.; Nagarajan, N.A.; Her, M.J.; Benedict, C.A.; Kronenberg, M. Cutting edge: The mechanism of invariant NKT cell responses to viral danger signals. J. Immunol. 2008, 181, 4452–4456. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; Dent, A.; Cooper, S.; Hangoc, G.; Wang, Z.-Y.; Du, W.; Gervay-Haque, J.; Sriram, V.; Renukaradhya, G.J.; Brutkiewicz, R.R. A role for natural killer T cells and CD1d molecules in counteracting suppression of hematopoiesis in mice induced by infection with murine cytomegalovirus. Exp. Hematol. 2007, 35, 87–93. [Google Scholar] [CrossRef]
- Paget, C.; Ivanov, S.; Fontaine, J.; Blanc, F.; Pichavant, M.; Renneson, J.; Bialecki, E.; Pothlichet, J.; Vendeville, C.; Barba-Speath, G.; et al. Potential Role of Invariant NKT Cells in the Control of Pulmonary Inflammation and CD8+ T Cell Response during Acute Influenza A Virus H3N2 Pneumonia. J. Immunol. 2011, 186, 5590–5602. [Google Scholar] [CrossRef] [PubMed]
- Ajuebor, M.N. Role of NKT cells in the digestive system. I. Invariant NKT cells and liver diseases: Is there strength in numbers? Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G651–G656. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Lei, Y.; Chen, M.; Chen, C.B.; Ren, H.; Shi, T.D. PD-1/PDL1 and CD28/CD80 pathways modulate naturalkiller T cell function to inhibit hepatitis B virus replication. J. Viral. Hepat. 2013, 20, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Zeissig, S.; Murata, K.; Sweet, L.; Publicover, J.; Hu, Z.; Kaser, A.; Bosse, E.; Iqbal, J.; Hussain, M.M.; Balschun, K.; et al. Hepatitis B virus-induced lipid alterations contribute to naturalkiller T cell-dependent protective immunity. Nat. Med. 2012, 18, 1060–1068. [Google Scholar] [CrossRef]
- Villanueva, A.I.; Haeryfar, S.M.; Mallard, B.A.; Kulkarni, R.R.; Sharif, S. Functions of NKT cells are modulated by TLR ligands and IFNα. Innate Immun. 2015, 21, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.G.; Haga, I.R. The role of Toll-like receptors in the host response to viruses. Mol. Immunol. 2005, 42, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Hühr, J.; Schwaiger, T.; Dorhoi, A.; Mettenleiter, T.C.; Blome, S.; Schröder, C.; Blohm, U. Porcine Invariant Natural Killer T Cells: Functional Profiling and Dynamics in Steady State and Viral Infections. Front. Immunol. 2019, 10, 1380. [Google Scholar] [CrossRef] [PubMed]
- Renukaradhya, G.J.; Manickam, C.; Khatri, M.; Rauf, A.; Li, X.; Tsuji, M.; Rajashekara, G.; Dwivedi, V. Functional invariant NKT cells in pig lungs regulate the airway hyperreactivity: A potential animal model. J. Clin. Immunol. 2010, 31, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Artiaga, B.L.; Yang, G.; Hutchinson, T.E.; Loeb, J.C.; Richt, J.A.; Lednicky, J.A.; Salek-Ardakani, S.; Driver, J.P. Rapid control of pandemic H1N1 influenza by targeting NKT-cells. Sci. Rep. 2016, 6, 37999. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, V.; Manickam, C.; Dhakal, S.; Binjawadagi, B.; Ouyang, K.; Hiremath, J.; Khatri, M.; Hague, J.G.; Lee, C.W.; Renukaradhya, G.J. Adjuvant effects of invariant NKT cell ligand potentiates the innate and adaptive immunity to an inactivated H1N1 swine influenza virus vaccine in pigs. Veter Microbiol. 2016, 186, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Pegu, A.; Asokan, M.; Wu, L.; Wang, K.; Hataye, J.; Casazza, J.P.; Guo, X.; Shi, W.; Georgiev, I.; Zhou, T.; et al. Activation and lysis of human CD4 cells latently infected with HIV-1. Nat. Commun. 2015, 6, 8447. [Google Scholar] [CrossRef]
- Courtney, A.N.; Nehete, P.; Nehete, B.P.; Thapa, P.; Zhou, D.; Sastry, K.J. Alpha-galactosylceramide is an effective mucosal adjuvant for repeated intranasal or oral delivery of HIV peptide antigens. Vaccine 2009, 27, 3335–3341. [Google Scholar] [CrossRef]
- Sandberg, J.K.; Fast, N.M.; Palacios, E.H.; Fennelly, G.; Dobroszycki, J.; Palumbo, P.; Wiznia, A.; Grant, R.M.; Bhardwaj, N.; Rosenberg, M.G.; et al. Selective loss of innate CD4+ V alpha 24 natural killer T cells in human immunodeficiency virus infection. J. Virol. 2002, 76, 7528–7534. [Google Scholar] [CrossRef]
- Moll, M.; Kuylenstierna, C.; Gonzalez, V.D.; Andersson, S.K.; Bosnjak, L.; Sönnerborg, A.; Quigley, M.F.; Sandberg, J.K. Severe functional impairment and elevated PD-1 expression in CD1d-restricted NKT cells retained during chronic HIV-1 infection. Eur. J. Immunol. 2009, 39, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Knox, K.S.; Kohli, L.M.; He, J.J.; Exley, M.A.; Wilson, S.B.; Brutkiewicz, R.R. Impaired cell surface expression of human CD1d by the formation of an HIV-1 Nef/CD1d complex. Virology 2005, 337, 242–252. [Google Scholar] [CrossRef]
- Chen, N.; McCarthy, C.; Drakesmith, H.; Li, D.; Cerundolo, V.; McMichael, A.J.; Screaton, G.R.; Xu, X.-N. HIV-1 down-regulates the expression of CD1d via Nef. Eur. J. Immunol. 2006, 36, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Motsinger-Reif, A.; Haas, D.W.; Stanic-Kostic, A.; Van Kaer, L.; Joyce, S.; Unutmaz, D. CD1d-restricted Human Natural Killer T Cells Are Highly Susceptible to Human Immunodeficiency Virus 1 Infection. J. Exp. Med. 2002, 195, 869–879. [Google Scholar] [CrossRef]
- Fernandez, C.S.; Kelleher, A.D.; Finlayson, R.; Godfrey, D.I.; Kent, S.J. NKT cell depletion in humans during early HIV infection. Immunol. Cell Biol. 2014, 92, 578–590. [Google Scholar] [CrossRef]
- Mureithi, M.W.; Cohen, K.; Moodley, R.; Poole, D.; Mncube, Z.; Kasmar, A.; Moody, D.B.; Goulder, P.J.; Walker, B.D.; Altfeld, M.; et al. Impairment of CD1d-Restricted Natural Killer T Cells in Chronic HIV Type 1 Clade C Infection. AIDS Res. Hum. Retroviruses 2011, 27, 501–509. [Google Scholar] [CrossRef]
- Brutkiewicz, R.R. Cell Signaling Pathways That Regulate Antigen Presentation. J. Immunol. 2016, 197, 2971–2979. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Webb, T.; Khan, M.A.; Lin, Y.L.; Du, W.; Gervay-Hague, J.; Brutkiewicz, R. Virus-Induced Inhibition of CD1d1-Mediated Antigen Presentation: Reciprocal Regulation by p38 and ERK. J. Immunol. 2005, 175, 4301–4308. [Google Scholar] [CrossRef]
- Liu, J.; Gallo, R.M.; Khan, M.A.; Iyer, A.K.; Kratzke, I.M.; Brutkiewicz, R.R. JNK2 modulates the CD1d-Dependent and -Independent activation of iNKT cells. Eur. J. Immunol. 2018, 49, 255–265. [Google Scholar] [CrossRef]
- Iyer, A.K.; Liu, J.; Gallo, R.M.; Kaplan, M.H.; Brutkiewicz, R.R. STAT3 promotes CD1d-mediated lipid antigen presentation by regulating a critical gene in glycosphingolipid biosynthesis. Immunology 2015, 146, 444–455. [Google Scholar] [CrossRef]
- He, Y.; Fisher, R.; Chowdhury, S.; Sultana, I.; Pereira, C.P.; Bray, M.; Reed, J.L. Vaccinia Virus Induces Rapid Necrosis in Keratinocytes by a STAT3-Dependent Mechanism. PLoS ONE 2014, 9, e113690. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Khan, M.A.; Shaji, D.; Brutkiewicz, R. Vesicular Stomatitis Virus Matrix Protein Impairs CD1d-Mediated Antigen Presentation through Activation of the p38 MAPK Pathway. J. Virol. 2008, 82, 12535–12542. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, Y.; Roberts, T.J.; Wang, C.; Cho, S.; Brutkiewicz, R. Long-term loss of canonical NKT cells following an acute virus infection. Eur. J. Immunol. 2005, 35, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, J.A.; Cho, S.; Roberts, T.J.; Sriram, V.; Zhang, J.; Xu, M.; Brutkiewicz, R.R. Selective Loss of Natural Killer T Cells by Apoptosis following Infection with Lymphocytic Choriomeningitis Virus. J. Virol. 2001, 75, 10746–10754. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S.; Turner, S.; Simmons, A.; Powe, T.; Chakravarty, E.; Alberola-Ila, J. West Nile virus-Infected human dendritic cells fail to fully activate invariant natural killer T cells. Clin. Exp. Immunol. 2016, 186, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.; Pham, H.T.; Kulkarni, A.; Yang, Y.; Liu, X.; Knipe, D.M.; Cresswell, P.; Yuan, W. Herpes Simplex Virus 1 Glycoprotein B and US3 Collaborate To Inhibit CD1d Antigen Presentation and NKT Cell Function. J. Virol. 2011, 85, 8093–8104. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.; Freeman, G.J.; Sharpe, A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Parekh, V.V.; Lalani, S.; Kim, S.; Halder, R.; Azuma, M.; Yagita, H.; Kumar, V.; Wu, L.; Van Kaer, L. PD-1/PD-L Blockade Prevents Anergy Induction and Enhances the Anti-Tumor Activities of Glycolipid-Activated Invariant NKT Cells. J. Immunol. 2009, 182, 2816–2826. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.; DePierres, C.; et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Zingaropoli, M.A.; Perri, V.; Pasculli, P.; Dezza, F.C.; Nijhawan, P.; Savelloni, G.; La Torre, G.; D’Agostino, C.; Mengoni, F.; Lichtner, M.; et al. Major reduction of NKT cells in patients with severe COVID-19 pneumonia. Clin. Immunol. 2020, 222, 108630. [Google Scholar] [CrossRef] [PubMed]
- Fifis, T.; Gamvrellis, A.; Crimeen-Irwin, B.; Pietersz, G.A.; Li, J.; Mottram, P.L.; McKenzie, I.F.C.; Plebanski, M. Size-Dependent Immunogenicity: Therapeutic and Protective Properties of Nano-Vaccines against Tumors. J. Immunol. 2004, 173, 3148–3154. [Google Scholar] [CrossRef]
- Syed, F.M.; Khan, M.A.; Nasti, T.H.; Ahmad, N.; Mohammad, O. Antigen entrapped in escheriosomes leads to the generation of CD4+ helper and CD8+ cytotoxic T cell response. Vaccine 2003, 21, 2383–2393. [Google Scholar] [CrossRef]
- Ahmad, N.; Khan, M.A.; Owais, M. Fusogenic potential of prokaryotic membrane lipids: Implication in vaccine development. Eur. J. Biochem. 2001, 268, 1–10. [Google Scholar] [CrossRef]
- Kumari, S.; Chatterjee, K. Biomaterials-based formulations and surfaces to combat viral infectious diseases. APL Bioeng. 2021, 5, 011503. [Google Scholar] [CrossRef]
- Tao, W.; Gill, H.S. M2e-immobilized gold nanoparticles as influenza A vaccine: Role of soluble M2e and longevity of protection. Vaccine 2015, 33, 2307–2315. [Google Scholar] [CrossRef]
- Guo, H.-C.; Feng, X.-M.; Sun, S.-Q.; Wei, Y.-Q.; Sun, D.-H.; Liu, X.-T.; Liu, Z.-X.; Luo, J.-X.; Yin, H. Immunization of mice by Hollow Mesoporous Silica Nanoparticles as carriers of Porcine Circovirus Type 2 ORF2 Protein. Virol. J. 2012, 9, 108. [Google Scholar] [CrossRef]
- Pantarotto, D.; Partidos, C.D.; Hoebeke, J.; Brown, F.; Kramer, E.; Briand, J.P.; Muller, S.; Prato, M.; Bianco, A. Immunization with peptide-functionalized carbon nanotubes enhances virus-specific neutralizing antibody responses. Chem. Biol. 2003, 10, 61–66. [Google Scholar] [CrossRef]
- Khan, M.A.; Ahmad, N.; Moin, S.; Mannan, A.; Wajahul, H.; Pasha, S.T.; Khan, A.; Owais, M. Tuftsin-mediated immunoprophylaxis against an isolate of Aspergillus fumigatus shows less in vivo susceptibility to amphotericin B. FEMS Immunol. Med. Microbiol. 2005, 44, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Almatroudi, A.; Rahmani, A.H. Recent strategies towards the surface modification of liposomes: An innovative approach for different clinical applications. 3 Biotech 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Brunel, F.; Darbouret, A.; Ronco, J. Cationic lipid DC-Chol induces an improved and balanced immunity able to overcome the unresponsiveness to the hepatitis B vaccine. Vaccine 1999, 17, 2192–2203. [Google Scholar] [CrossRef]
- Joseph, A.; Itskovitz-Cooper, N.; Samira, S.; Flasterstein, O.; Eliyahu, H.; Simberg, D.; Goldwaser, I.; Barenholz, Y.; Kedar, E. A new intranasal influenza vaccine based on a novel polycationic lipid—Ceramide carbamoyl-spermine (CCS): I. Immunogenicity and efficacy studies in mice. Vaccine 2006, 24, 3990–4006. [Google Scholar] [CrossRef] [PubMed]
- Blom, R.A.M.; Erni, S.T.; Krempaská, K.; Schaerer, O.; Van Dijk, R.M.; Amacker, M.; Moser, C.; Hall, S.R.R.; Von Garnier, C.; Blank, F. A Triple Co-Culture Model of the Human Respiratory Tract to Study Immune-Modulatory Effects of Liposomes and Virosomes. PLoS ONE 2016, 11, e0163539. [Google Scholar] [CrossRef]
- Jin, Z.; Gao, S.; Cui, X.; Sun, D.; Zhao, K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019, 572, 118731. [Google Scholar] [CrossRef]
- Mehrabi, M.; Montazeri, H.; Dounighi, N.M.; Rashti, A.; Vakili-Ghartavol, R. Chitosan-Based Nanoparticles in Mucosal Vaccine Delivery. Arch. Razi Inst. 2018, 73, 165–176. [Google Scholar]
- Liu, Q.; Zheng, X.; Zhang, C.; Shao, X.; Zhang, X.; Zhang, Q.; Jiang, X. Conjugating influenza a (H1N1) antigen to n-trimethylaminoethylmethacrylate chitosan nanoparticles improve the immunogenicity of the antigen after nasal administration. J. Med. Virol. 2015, 87, 1807–1815. [Google Scholar] [CrossRef]
- Gu, P.; Wusiman, A.; Zhang, Y.; Liu, Z.; Bo, R.; Hu, Y.; Liu, J.; Wang, D. Rational Design of PLGA Nanoparticle Vaccine Delivery Systems to Improve Immune Responses. Mol. Pharm. 2019, 16, 5000–5012. [Google Scholar] [CrossRef]
- Dhakal, S.; Hiremath, J.; Bondra, K.; Lakshmanappa, Y.S.; Shyu, D.-L.; Ouyang, K.; Kang, K.-I.; Binjawadagi, B.; Goodman, J.; Tabynov, K.; et al. Biodegradable nanoparticle delivery of inactivated swine influenza virus vaccine provides heterologous cell-mediated immune response in pigs. J. Control. Release 2017, 247, 194–205. [Google Scholar] [CrossRef]
- Akondy, R.S.; Monson, N.D.; Miller, J.D.; Edupuganti, S.; Teuwen, D.; Wu, H.; Quyyumi, F.; Garg, S.; Altman, J.D.; Del Rio, C.; et al. The Yellow Fever Virus Vaccine Induces a Broad and Polyfunctional Human Memory CD8+ T Cell Response. J. Immunol. 2009, 183, 7919–7930. [Google Scholar] [CrossRef]
- Sei, J.J.; Cox, K.S.; Dubey, S.A.; Antonello, J.M.; Krah, D.L.; Casimiro, D.R.; Vora, K.A. Effector and Central Memory Poly-Functional CD4+ and CD8+ T Cells are Boosted upon ZOSTAVAX® Vaccination. Front. Immunol. 2015, 6, 553. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Haeryfar, S.M.; Sharif, S. The invariant NKT cell subset in anti-viral defenses: A dark horse in anti-influenza immunity? J. Leukoc. Biol. 2010, 88, 635–643. [Google Scholar] [CrossRef]
- Kok, W.L.; Denney, L.; Benam, K.; Cole, S.; Clelland, C.; McMichael, A.J.; Ho, L.-P. Pivotal Advance: Invariant NKT cells reduce accumulation of inflammatory monocytes in the lungs and decrease immune-pathology during severe influenza A virus infection. J. Leukoc. Biol. USA 2011, 91, 357–368. [Google Scholar] [CrossRef]
- Fujii, S.-I.; Shimizu, K.; Hemmi, H.; Fukui, M.; Bonito, A.J.; Chen, G.; Franck, R.W.; Tsuji, M.; Steinman, R.M. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc. Natl. Acad. Sci. USA 2006, 103, 11252–11257. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Nuti, S.; Tavarini, S.; Galli-Stampino, L.; De Lalla, C.; Casorati, G.; Dellabona, P.; Abrignani, S. CD1d-restricted Help to B Cells By Human Invariant Natural Killer T Lymphocytes. J. Exp. Med. 2003, 197, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Artiaga, B.L.; Yang, G.; Hackmann, T.J.; Liu, Q.; Richt, J.A.; Salek-Ardakani, S.; Castleman, W.L.; Lednicky, J.A.; Driver, J.P. α-Galactosylceramide protects swine against influenza infection when administered as a vaccine adjuvant. Sci. Rep. 2016, 6, 23593. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.P.; Madrid, D.M.D.C.; Gu, W.; Artiaga, B.L.; Richt, J.A. Modulation of Immune Responses to Influenza A Virus Vaccines by Natural Killer T Cells. Front. Immunol. 2020, 11, 2172. [Google Scholar] [CrossRef] [PubMed]
- Tefit, J.N.; Crabé, S.; Orlandini, B.; Nell, H.; Bendelac, A.; Deng, S.; Savage, P.B.; Teyton, L.; Serra, V. Efficacy of ABX196, a new NKT agonist, in prophylactic human vaccination. Vaccine 2014, 32, 6138–6145. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, A.; Li, X.; Chen, Z.; Zhang, W.; Song, Y.; Gurner, D.; Gardiner, D.; Basu, S.; Ho, D.D.; et al. Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, α-galactosylceramide. Vaccine 2008, 26, 1807–1816. [Google Scholar] [CrossRef]
- Fujii, S.-I.; Shimizu, K.; Smith, C.L.; Bonifaz, L.; Steinman, R.M. Activation of Natural Killer T Cells by α-Galactosylceramide Rapidly Induces the Full Maturation of Dendritic Cells In Vivo and Thereby Acts as an Adjuvant for Combined CD4 and CD8 T Cell Immunity to a Coadministered Protein. J. Exp. Med. 2003, 198, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Neumann, S.; Young, K.; Compton, B.; Anderson, R.; Painter, G.; Hook, S. Synthetic TRP2 long-peptide and α-galactosylceramide formulated into cationic liposomes elicit CD8 + T-cell responses and prevent tumour progression. Vaccine 2015, 33, 5838–5844. [Google Scholar] [CrossRef] [PubMed]
- Dolen, Y.; Kreutz, M.; Gileadi, U.; Tel, J.; Vasaturo, A.; Van Dinther, E.A.W.; Van Hout-Kuijer, M.A.; Cerundolo, V.; Figdor, C.G. Co-delivery of PLGA encapsulated invariant NKT cell agonist with antigenic protein induce strong T cell-mediated antitumor immune responses. OncoImmunology 2015, 5, e1068493. [Google Scholar] [CrossRef]
- Singh, A.K.; Gaur, P.; Das, S.N. Natural killer T cell anergy, co-stimulatory molecules and immunotherapeutic interventions. Hum. Immunol. 2014, 75, 250–260. [Google Scholar] [CrossRef]
- Thapa, P.; Zhang, G.; Xia, C.; Gelbard, A.; Overwijk, W.W.; Liu, C.; Hwu, P.; Chang, D.Z.; Courtney, A.; Sastry, J.K.; et al. Nanoparticle formulated alpha-galactosylceramide activates NKT cells without inducing anergy. Vaccine 2009, 27, 3484–3488. [Google Scholar] [CrossRef] [PubMed]
- Inoue, J.; Ideue, R.; Takahashi, D.; Kubota, M.; Kumazawa, Y. Liposomal glycosphingolipids activate natural killer T cell-mediated immune responses through the endosomal pathway. J. Control. Release 2009, 133, 18–23. [Google Scholar] [CrossRef]
- Khan, M.A.; Aljarbou, A.N.; Aldebasi, Y.H.; Alorainy, M.S.; Rahmani, A.H.; Younus, H.; Khan, A. Liposomal formulation of glycosphingolipids from Sphingomonas paucimobilis induces antitumor immunity in mice. J. Drug Target. 2018, 26, 709–719. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.A.; Khan, A. Role of NKT Cells during Viral Infection and the Development of NKT Cell-Based Nanovaccines. Vaccines 2021, 9, 949. https://doi.org/10.3390/vaccines9090949
Khan MA, Khan A. Role of NKT Cells during Viral Infection and the Development of NKT Cell-Based Nanovaccines. Vaccines. 2021; 9(9):949. https://doi.org/10.3390/vaccines9090949
Chicago/Turabian StyleKhan, Masood Alam, and Arif Khan. 2021. "Role of NKT Cells during Viral Infection and the Development of NKT Cell-Based Nanovaccines" Vaccines 9, no. 9: 949. https://doi.org/10.3390/vaccines9090949
APA StyleKhan, M. A., & Khan, A. (2021). Role of NKT Cells during Viral Infection and the Development of NKT Cell-Based Nanovaccines. Vaccines, 9(9), 949. https://doi.org/10.3390/vaccines9090949






