Viral Toxin NS1 Implication in Dengue Pathogenesis Making It a Pivotal Target in Development of Efficient Vaccine
Abstract
:1. Dengue Disease
2. Biology and Function of the DENV NS1 Protein
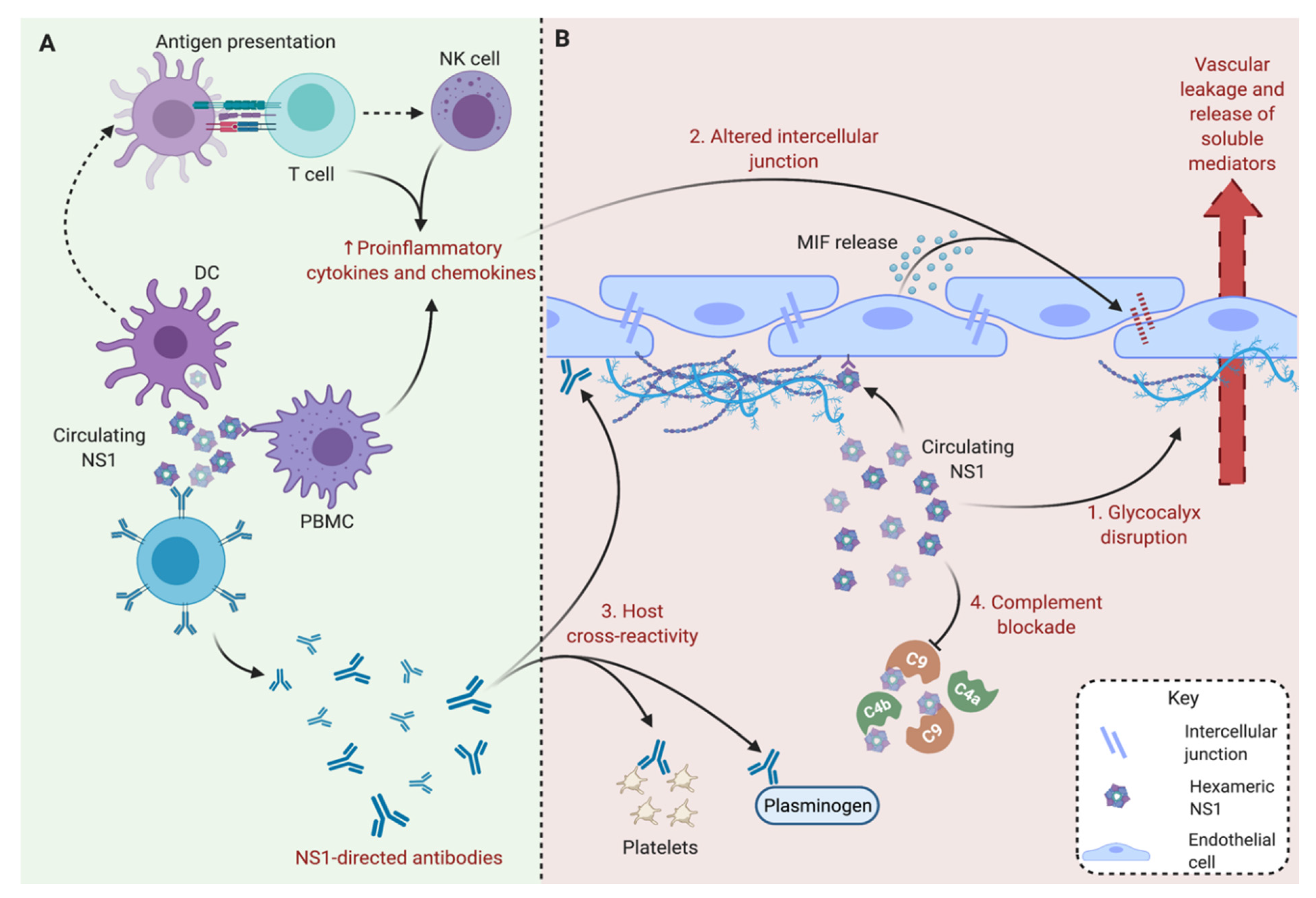
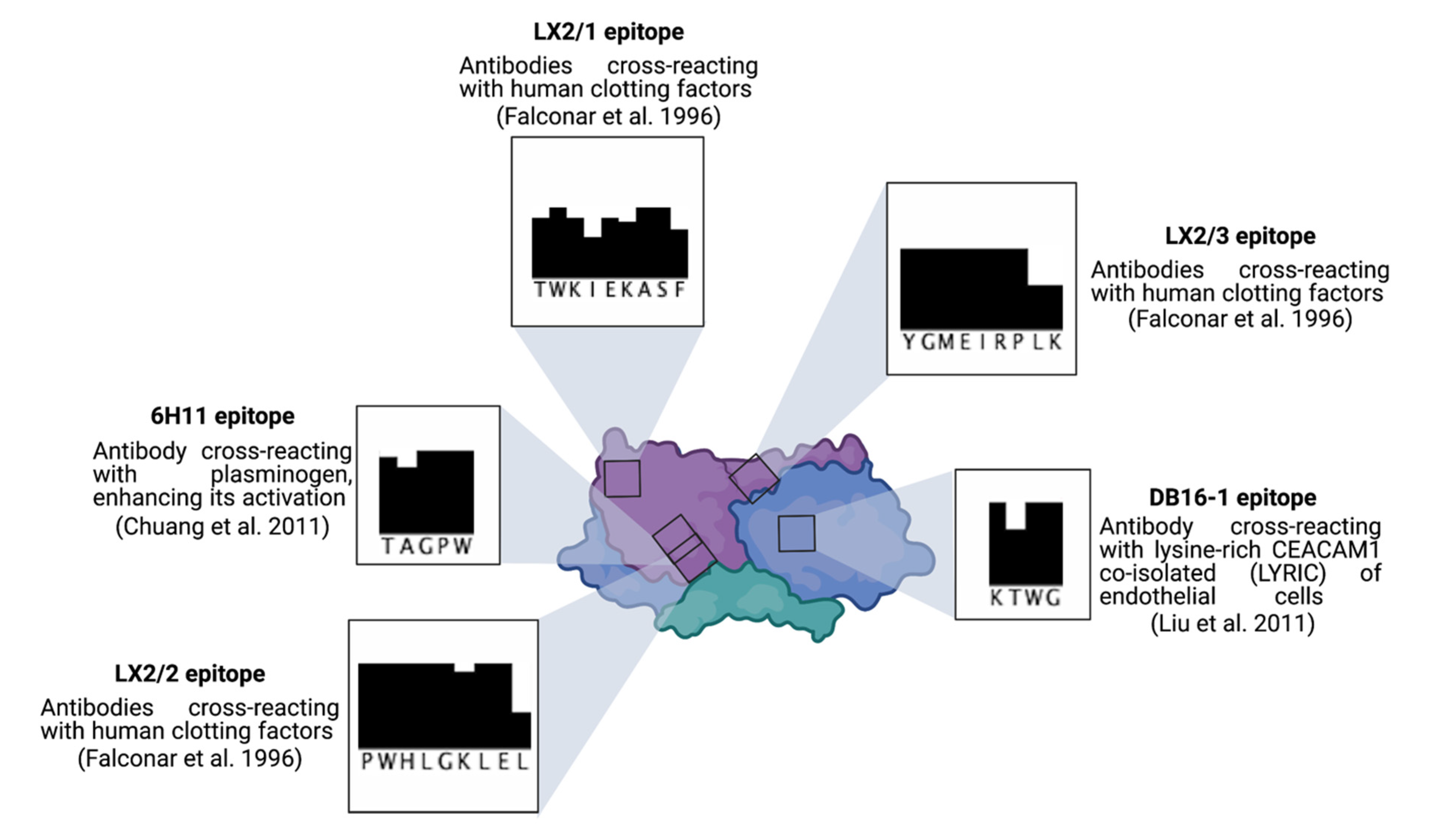
3. Secreted Soluble NS1 Contributes to the Pathogenesis of Severe Dengue
4. Soluble NS1 as a Significant Focus for Dengue Vaccine Strategies
4.1. Immunity to the DENV NS1 Protein
4.2. The Current Challenges for Dengue Vaccine Development
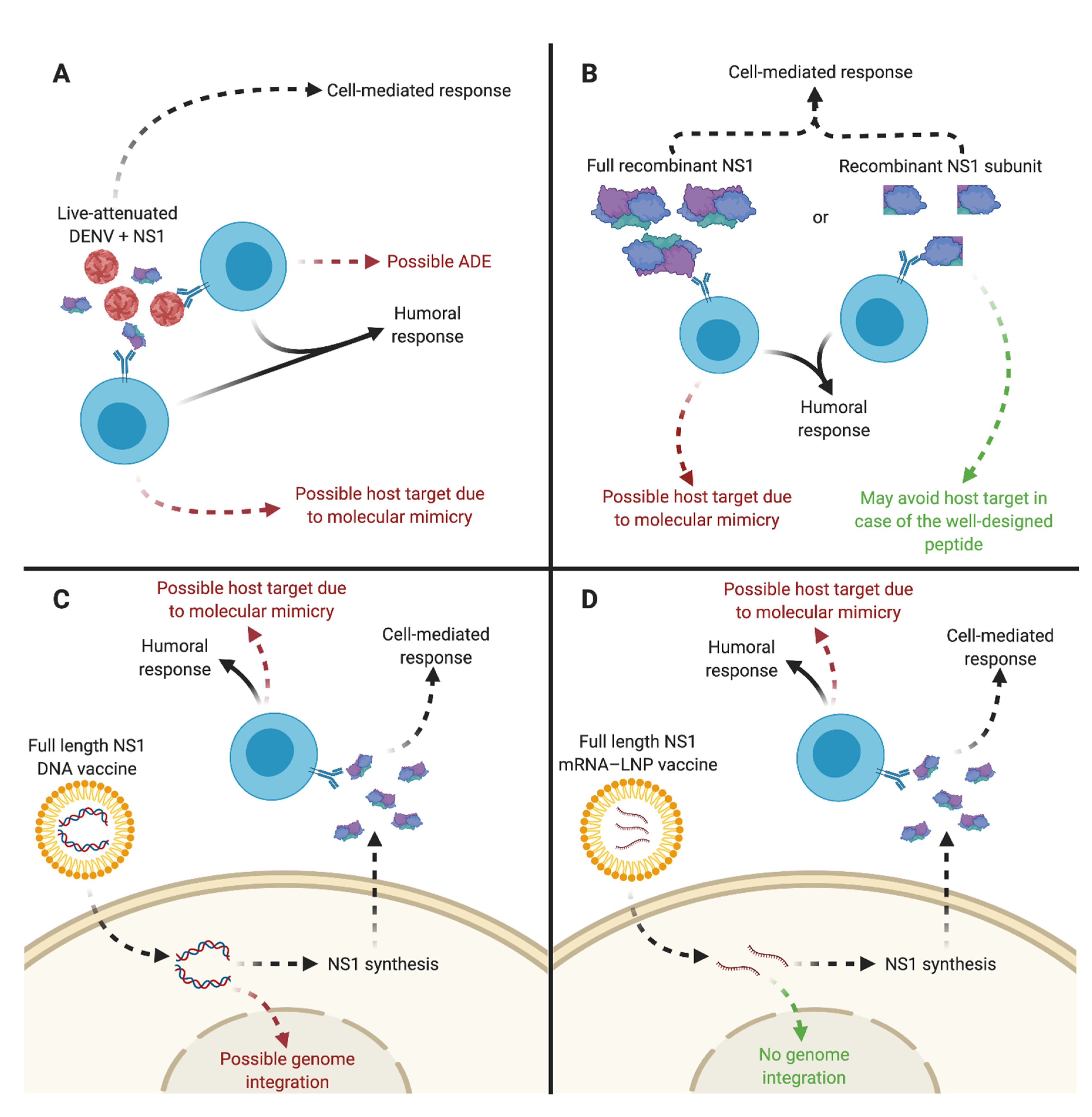
4.3. DENV Vaccine Candidates Expressing sNS1
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADE | Antibody-dependent enhancement |
| DENV | Dengue virus |
| DHF | Dengue hemorrhagic fever |
| DSS | Dengue shock syndrome |
| E | Envelope protein |
| ERGIC | ER–Golgi intermediate compartment |
| FcγR | FC-gamma receptor |
| GMT | Geometric mean titer |
| IL | Interleukin |
| LAV | Live-attenuated virus |
| LNP | Lipid nanoparticle |
| LPS | Lipopolysaccharide |
| mAb | Monoclonal antibody |
| MIF | Macrophage migration inhibitory factor |
| MODCs | Monocyte-derived dendritic cell |
| NS | Nonstructural protein |
| PBMC | Peripheral blood mononuclear cell |
| prM | Precursor of the M protein |
| PRR | Pattern recognition receptor |
| TLR | Toll-like Receptor |
| TMC | Trimethyl chitosan |
| TNF-α | Tumor necrosis factor α |
| VLP | Virus-like particle |
| WD | Wing domain |
| WNV | West Nile virus |
| ZIKV | Zika virus |
References
- Lindenbach, B.D.; Thiel, H.-J.; Rice, C.M. Flaviviridae: The Viruses and Their Replication. In Fields Virology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Slon Campos, J.L.; Mongkolsapaya, J.; Screaton, G.R. The Immune Response against Flaviviruses. Nat. Immunol. 2018, 19, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Roby, J.A.; Hall, R.A.; Setoh, Y.X.; Khromykh, A.A. Post-Translational Regulation and Modifications of Flavivirus Structural Proteins. J. Gen. Virol. 2015, 96, 1551–1569. [Google Scholar] [CrossRef]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Castillo-Medina, L.F.; Rodríguez, Y.; Pacheco, Y.; Halstead, S.; Willison, H.J.; Anaya, J.-M.; Ramírez-Santana, C. Autoimmune Neurological Conditions Associated With Zika Virus Infection. Front. Mol. Neurosci. 2018, 11, 116. [Google Scholar] [CrossRef]
- Stiasny, K.; Fritz, R.; Pangerl, K.; Heinz, F.X. Molecular Mechanisms of Flavivirus Membrane Fusion. Amino Acids 2011, 41, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Green, A.M.; Beatty, P.R.; Hadjilaou, A.; Harris, E. Innate Immunity to Dengue Virus Infection and Subversion of Antiviral Responses. J. Mol. Biol. 2014, 426, 1148–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avirutnan, P.; Fuchs, A.; Hauhart, R.E.; Somnuke, P.; Youn, S.; Diamond, M.S.; Atkinson, J.P. Antagonism of the Complement Component C4 by Flavivirus Nonstructural Protein NS1. J. Exp. Med. 2010, 207, 793–806. [Google Scholar] [CrossRef] [Green Version]
- Avirutnan, P.; Hauhart, R.E.; Somnuke, P.; Blom, A.M.; Diamond, M.S.; Atkinson, J.P. Binding of Flavivirus Nonstructural Protein NS1 to C4b Binding Protein Modulates Complement Activation. J. Immunol. 2011, 187, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Beatty, P.R.; Puerta-Guardo, H.; Killingbeck, S.S.; Glasner, D.R.; Hopkins, K.; Harris, E. Dengue Virus NS1 Triggers Endothelial Permeability and Vascular Leak That Is Prevented by NS1 Vaccination. Sci. Transl. Med. 2015, 7, 304ra141. [Google Scholar] [CrossRef] [Green Version]
- Alayli, F.; Scholle, F. Dengue Virus NS1 Enhances Viral Replication and Pro-Inflammatory Cytokine Production in Human Dendritic Cells. Virology 2016, 496, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Modhiran, N.; Watterson, D.; Muller, D.A.; Panetta, A.K.; Sester, D.P.; Liu, L.; Hume, D.A.; Stacey, K.J.; Young, P.R. Dengue Virus NS1 Protein Activates Cells via Toll-like Receptor 4 and Disrupts Endothelial Cell Monolayer Integrity. Sci. Transl. Med. 2015, 7, 304ra142. [Google Scholar] [CrossRef] [Green Version]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef] [Green Version]
- Akey, D.L.; Brown, W.C.; Dutta, S.; Konwerski, J.; Jose, J.; Jurkiw, T.J.; DelProposto, J.; Ogata, C.M.; Skiniotis, G.; Kuhn, R.J.; et al. Flavivirus NS1 Structures Reveal Surfaces for Associations with Membranes and the Immune System. Science 2014, 343, 881–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaturro, P.; Cortese, M.; Chatel-Chaix, L.; Fischl, W.; Bartenschlager, R. Dengue Virus Non-Structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins. PLoS Pathog. 2015, 11, e1005277. [Google Scholar] [CrossRef]
- Glasner, D.R.; Puerta-Guardo, H.; Beatty, P.R.; Harris, E. The Good, the Bad, and the Shocking: The Multiple Roles of Dengue Virus Nonstructural Protein 1 in Protection and Pathogenesis. Annu. Rev. Virol. 2018, 5, 227–253. [Google Scholar] [CrossRef]
- Lindenbach, B.D.; Rice, C.M. Genetic Interaction of Flavivirus Nonstructural Proteins NS1 and NS4A as a Determinant of Replicase Function. J. Virol. 1999, 73, 4611–4621. [Google Scholar] [CrossRef] [Green Version]
- Płaszczyca, A.; Scaturro, P.; Neufeldt, C.J.; Cortese, M.; Cerikan, B.; Ferla, S.; Brancale, A.; Pichlmair, A.; Bartenschlager, R. A Novel Interaction between Dengue Virus Nonstructural Protein 1 and the NS4A-2K-4B Precursor Is Required for Viral RNA Replication but Not for Formation of the Membranous Replication Organelle. PLoS Pathog. 2019, 15, e1007736. [Google Scholar] [CrossRef]
- Gutsche, I.; Coulibaly, F.; Voss, J.E.; Salmon, J.; d’Alayer, J.; Ermonval, M.; Larquet, E.; Charneau, P.; Krey, T.; Mégret, F.; et al. Secreted Dengue Virus Nonstructural Protein NS1 Is an Atypical Barrel-Shaped High-Density Lipoprotein. Proc. Natl. Acad. Sci. USA 2011, 108, 8003–8008. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, W.; Wang, J.; Peng, H.; Che, X.; Chen, X.; Zhou, Y. NS1-Based Tests with Diagnostic Utility for Confirming Dengue Infection: A Meta-Analysis. Int. J. Infect. Dis. 2014, 26, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libraty, D.H.; Young, P.R.; Pickering, D.; Endy, T.P.; Kalayanarooj, S.; Green, S.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; Rothman, A.L. High Circulating Levels of the Dengue Virus Nonstructural Protein NS1 Early in Dengue Illness Correlate with the Development of Dengue Hemorrhagic Fever. J. Infect. Dis. 2002, 186, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Glasner, D.R.; Ratnasiri, K.; Puerta-Guardo, H.; Espinosa, D.A.; Beatty, P.R.; Harris, E. Dengue Virus NS1 Cytokine-Independent Vascular Leak Is Dependent on Endothelial Glycocalyx Components. PLoS Pathog. 2017, 13, e1006673. [Google Scholar] [CrossRef]
- Chen, H.-R.; Chuang, Y.-C.; Chao, C.-H.; Yeh, T.-M. Macrophage Migration Inhibitory Factor Induces Vascular Leakage via Autophagy. Biol. Open 2015, 4, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-R.; Chuang, Y.-C.; Lin, Y.-S.; Liu, H.-S.; Liu, C.-C.; Perng, G.-C.; Yeh, T.-M. Dengue Virus Nonstructural Protein 1 Induces Vascular Leakage through Macrophage Migration Inhibitory Factor and Autophagy. PLoS Negl. Trop. Dis. 2016, 10, e0004828. [Google Scholar] [CrossRef]
- Thiemmeca, S.; Tamdet, C.; Punyadee, N.; Prommool, T.; Songjaeng, A.; Noisakran, S.; Puttikhunt, C.; Atkinson, J.P.; Diamond, M.S.; Ponlawat, A.; et al. Secreted NS1 Protects Dengue Virus from Mannose-Binding Lectin–Mediated Neutralization. J. Immunol. 2016, 197, 4053–4065. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.N.; da Silva, E.M.; Allonso, D.; Coelho, D.R.; da Andrade, I.S.; de Medeiros, L.N.; Menezes, J.L.; Barbosa, A.S.; Mohana-Borges, R. Inhibition of the Membrane Attack Complex by Dengue Virus NS1 through Interaction with Vitronectin and Terminal Complement Proteins. J. Virol. 2016, 90, 9570–9581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, I.-J.; Chiu, C.-Y.; Chen, Y.-C.; Wu, H.-C. Molecular Mimicry of Human Endothelial Cell Antigen by Autoantibodies to Nonstructural Protein 1 of Dengue Virus. J. Biol. Chem. 2011, 286, 9726–9736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falconar, A.K.I.; Young, P.R.; Miles, M.A. Precise Location of Sequential Dengue Virus Subcomplex and Complex B Cell Epitopes on the Nonstructural-1 Glycoprotein. Arch. Virol. 1994, 137, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Falconar, A.K.I. The Dengue Virus Nonstructural-1 Protein (NS1) Generatesantibodies to Common Epitopes on Human Blood Clotting, Integrin/Adhesin Proteins and Binds to Humanendothelial Cells: Potential Implications in Haemorrhagic Fever Pathogenesis. Arch. Virol. 1997, 142, 897–916. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Lei, H.-Y.; Lin, Y.-S.; Liu, H.-S.; Wu, H.-L.; Yeh, T.-M. Dengue Virus-Induced Autoantibodies Bind to Plasminogen and Enhance Its Activation. J. Immunol. 2011, 187, 6483–6490. [Google Scholar] [CrossRef]
- Chen, J.; Ng, M.M.-L.; Chu, J.J.H. Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection. PLoS Pathog. 2015, 11, e1005053. [Google Scholar] [CrossRef]
- Modhiran, N.; Watterson, D.; Blumenthal, A.; Baxter, A.G.; Young, P.R.; Stacey, K.J. Dengue Virus NS1 Protein Activates Immune Cells via TLR4 but Not TLR2 or TLR6. Immunol. Cell Biol. 2017, 95, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Vigne, C.; Dupuy, M.; Richetin, A.; Guy, B.; Jackson, N.; Bonaparte, M.; Hu, B.; Saville, M.; Chansinghakul, D.; Noriega, F.; et al. Integrated Immunogenicity Analysis of a Tetravalent Dengue Vaccine up to 4 y after Vaccination. Hum. Vaccines Immunother. 2017, 13, 2004–2016. [Google Scholar] [CrossRef]
- Guy, B.; Jackson, N. Dengue Vaccine: Hypotheses to Understand CYD-TDV-Induced Protection. Nat. Rev. Microbiol. 2016, 14, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Ismail, H.I.H.M.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govindarajan, D.; Meschino, S.; Guan, L.; Clements, D.E.; ter Meulen, J.H.; Casimiro, D.R.; Coller, B.-A.G.; Bett, A.J. Preclinical Development of a Dengue Tetravalent Recombinant Subunit Vaccine: Immunogenicity and Protective Efficacy in Nonhuman Primates. Vaccine 2015, 33, 4105–4116. [Google Scholar] [CrossRef]
- Pinheiro-Michelsen, J.R.; da Souza, R.S.O.; Santana, I.V.R.; da Silva, P.; Mendez, E.C.; Luiz, W.B.; Amorim, J.H. Anti-Dengue Vaccines: From Development to Clinical Trials. Front. Immunol. 2020, 11, 1252. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.E.; Wallace, D.; Stinchcomb, D.T. A Recombinant, Chimeric Tetravalent Dengue Vaccine Candidate Based on a Dengue Virus Serotype 2 Backbone. Expert Rev. Vaccines 2016, 15, 497–508. [Google Scholar] [CrossRef]
- Waickman, A.T.; Friberg, H.; Gargulak, M.; Kong, A.; Polhemus, M.; Endy, T.; Thomas, S.J.; Jarman, R.G.; Currier, J.R. Assessing the Diversity and Stability of Cellular Immunity Generated in Response to the Candidate Live-Attenuated Dengue Virus Vaccine TAK-003. Front. Immunol. 2019, 10, 1778. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Glasner, D.R.; Watkins, H.; Puerta-Guardo, H.; Kassa, Y.; Egan, M.A.; Dean, H.; Harris, E. Magnitude and Functionality of the NS1-Specific Antibody Response Elicited by a Live-Attenuated Tetravalent Dengue Vaccine Candidate. J. Infect. Dis. 2020, 221, 867–877. [Google Scholar] [CrossRef] [Green Version]
- Ambuel, Y.; Young, G.; Brewoo, J.N.; Paykel, J.; Weisgrau, K.L.; Rakasz, E.G.; Haller, A.A.; Royals, M.; Huang, C.Y.-H.; Capuano, S.; et al. A Rapid Immunization Strategy with a Live-Attenuated Tetravalent Dengue Vaccine Elicits Protective Neutralizing Antibody Responses in Non-Human Primates. Front. Immunol. 2014, 5, 363. [Google Scholar] [CrossRef] [Green Version]
- Jearanaiwitayakul, T.; Sunintaboon, P.; Chawengkittikul, R.; Limthongkul, J.; Midoeng, P.; Warit, S.; Ubol, S. Nanodelivery System Enhances the Immunogenicity of Dengue-2 Nonstructural Protein 1, DENV-2 NS1. Vaccine 2020, 38, 6814–6825. [Google Scholar] [CrossRef]
- Gonçalves, A.J.S.; Oliveira, E.R.A.; Costa, S.M.; Paes, M.V.; Silva, J.F.A.; Azevedo, A.S.; Mantuano-Barradas, M.; Nogueira, A.C.M.A.; Almeida, C.J.; Alves, A.M.B. Cooperation between CD4+ T Cells and Humoral Immunity Is Critical for Protection against Dengue Using a DNA Vaccine Based on the NS1 Antigen. PLoS Negl. Trop. Dis. 2015, 9, e0004277. [Google Scholar] [CrossRef] [Green Version]
- Costa, S.M.; Freire, M.S.; Alves, A.M.B. DNA Vaccine against the Non-Structural 1 Protein (NS1) of Dengue 2 Virus. Vaccine 2006, 24, 4562–4564. [Google Scholar] [CrossRef]
- Costa, S.M.; Azevedo, A.S.; Paes, M.V.; Sarges, F.S.; Freire, M.S.; Alves, A.M.B. DNA Vaccines against Dengue Virus Based on the Ns1 Gene: The Influence of Different Signal Sequences on the Protein Expression and Its Correlation to the Immune Response Elicited in Mice. Virology 2007, 358, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Pinto, P.B.A.; Assis, M.L.; Vallochi, A.L.; Pacheco, A.R.; Lima, L.M.; Quaresma, K.R.L.; Pereira, B.A.S.; Costa, S.M.; Alves, A.M.B. T Cell Responses Induced by DNA Vaccines Based on the DENV2 E and NS1 Proteins in Mice: Importance in Protection and Immunodominant Epitope Identification. Front. Immunol. 2019, 10, 1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.-F.; Liao, C.-L.; Lin, Y.-L.; Yeh, C.-T.; Chen, L.-K.; Huang, Y.-F.; Chou, H.-Y.; Huang, J.-L.; Shaio, M.-F.; Sytwu, H.-K. Evaluation of Protective Efficacy and Immune Mechanisms of Using a Non-Structural Protein NS1 in DNA Vaccine against Dengue 2 Virus in Mice. Vaccine 2003, 21, 3919–3929. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, J.; Li, M.; Jin, X. Modified MRNA-LNP Vaccines Confer Protection against Experimental DENV-2 Infection in Mice. Mol. Ther. -Methods Clin. Dev. 2020, 18, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.H.; Diniz, M.O.; Cariri, F.A.M.O.; Rodrigues, J.F.; Bizerra, R.S.P.; Gonçalves, A.J.S.; de Barcelos Alves, A.M.; de Souza Ferreira, L.C. Protective Immunity to DENV2 after Immunization with a Recombinant NS1 Protein Using a Genetically Detoxified Heat-Labile Toxin as an Adjuvant. Vaccine 2012, 30, 837–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, S.-W.; Lu, Y.-T.; Huang, C.-H.; Lin, C.-F.; Anderson, R.; Liu, H.-S.; Yeh, T.-M.; Yen, Y.-T.; Wu-Hsieh, B.A.; Lin, Y.-S. Protection against Dengue Virus Infection in Mice by Administration of Antibodies against Modified Nonstructural Protein 1. PLoS ONE 2014, 9, e92495. [Google Scholar] [CrossRef]
- Espinosa, D.A.; Beatty, P.R.; Reiner, G.L.; Sivick, K.E.; Glickman, L.H.; Dubensky, T.W.; Harris, E. Cyclic Dinucleotide–Adjuvanted Dengue Virus Nonstructural Protein 1 Induces Protective Antibody and T Cell Responses. J. Immunol. 2019, 202, 1153–1162. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.-C.; Chuang, Y.-C.; Liu, C.-C.; Ho, T.-S.; Lin, Y.-S.; Anderson, R.; Yeh, T.-M. Antibodies Against Modified NS1 Wing Domain Peptide Protect Against Dengue Virus Infection. Sci. Rep. 2017, 7, 6975. [Google Scholar] [CrossRef] [PubMed]
- Redoni, M.; Yacoub, S.; Rivino, L.; Giacobbe, D.R.; Luzzati, R.; Di Bella, S. Dengue: Status of Current and Under-development Vaccines. Rev. Med. Virol. 2020, 30, e201. [Google Scholar] [CrossRef] [PubMed]
- Grgacic, E.V.L.; Anderson, D.A. Virus-like Particles: Passport to Immune Recognition. Methods 2006, 40, 60–65. [Google Scholar] [CrossRef]
- Zhang, N.; Li, C.; Jiang, S.; Du, L. Recent Advances in the Development of Virus-Like Particle-Based Flavivirus Vaccines. Vaccines 2020, 8, 481. [Google Scholar] [CrossRef]
- Reyes-Sandoval, A.; Ludert, J.E. The Dual Role of the Antibody Response Against the Flavivirus Non-Structural Protein 1 (NS1) in Protection and Immuno-Pathogenesis. Front. Immunol. 2019, 10, 1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-F.; Lei, H.-Y.; Shiau, A.-L.; Liu, C.-C.; Liu, H.-S.; Yeh, T.-M.; Chen, S.-H.; Lin, Y.-S. Antibodies from Dengue Patient Sera Cross-React with Endothelial Cells and Induce Damage. J. Med. Virol. 2003, 69, 82–90. [Google Scholar] [CrossRef]
- Lin, C.-F.; Chiu, S.-C.; Hsiao, Y.-L.; Wan, S.-W.; Lei, H.-Y.; Shiau, A.-L.; Liu, H.-S.; Yeh, T.-M.; Chen, S.-H.; Liu, C.-C.; et al. Expression of Cytokine, Chemokine, and Adhesion Molecules during Endothelial Cell Activation Induced by Antibodies against Dengue Virus Nonstructural Protein 1. J. Immunol. 2005, 174, 395–403. [Google Scholar] [CrossRef]
- Lin, S.-W.; Chuang, Y.-C.; Lin, Y.-S.; Lei, H.-Y.; Liu, H.-S.; Yeh, T.-M. Dengue Virus Nonstructural Protein NS1 Binds to Prothrombin/Thrombin and Inhibits Prothrombin Activation. J. Infect. 2012, 64, 325–334. [Google Scholar] [CrossRef] [PubMed]

| Platform | Methods | Preclinical | Outcome | Reference |
|---|---|---|---|---|
| LAV | Tetravalent dengue vaccine (TDV) | NHP | Neutralizing anti-DENV antibodies | [40] |
| VLPs | NS11–279TMC NPs | BALB/c mice | Protection efficacy of 97.47% | [41] |
| DNA | pcTPANS1 * | BALB/c mice | Protection efficacy of 100% | [42,43] |
| pcENS1 ** | BALB/c mice | Protection efficacy < 90% | [44] | |
| pE11D2 *** and pcTRANS1 **** | BALB/c mice | Protection efficacy < 90% | [45] | |
| pD2NS1/pD2NS1 + pIL-2 | C3H mice | Protection efficacy of 50–80% | [46] | |
| mRNA | DENV-2 E80-mRNA, NS1-mRNA | BALB/c mice | n.a. | [47] |
| Viral antigen | rNS1 + LTG33D adjuvant | BALB/c mice | Protection efficacy of 50% | [48] |
| ΔC NS1 # + CFA adjuvant | C3H/HeN mice | Protection efficacy of 65% | [49] | |
| Chimeric DJ NS1 ## + CFA adjuvant | C3H/HeN mice | Protection efficacy of 65% | [49] | |
| Full DENV1–4 NS1+ MPLA/AddaVax adjuvant | Ifnar−/− C57BL6 mice | Protection efficacy of 60–100% | [9] | |
| Recombinant DENV NS1 + cyclic dinucleotides (CDNs) adjuvant | Ifnar−/− C57BL6 mice | Protection efficacy of 60–70% | [50] | |
| Synthetic peptide | Modified NS1-WD ### + CFA adjuvant | C3H/HeN: STAT1−/− C57BL6 mice | Protection efficacy of 100% | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebeau, G.; Lagrave, A.; Ogire, E.; Grondin, L.; Seriacaroupin, S.; Moutoussamy, C.; Mavingui, P.; Hoarau, J.-J.; Roche, M.; Krejbich-Trotot, P.; et al. Viral Toxin NS1 Implication in Dengue Pathogenesis Making It a Pivotal Target in Development of Efficient Vaccine. Vaccines 2021, 9, 946. https://doi.org/10.3390/vaccines9090946
Lebeau G, Lagrave A, Ogire E, Grondin L, Seriacaroupin S, Moutoussamy C, Mavingui P, Hoarau J-J, Roche M, Krejbich-Trotot P, et al. Viral Toxin NS1 Implication in Dengue Pathogenesis Making It a Pivotal Target in Development of Efficient Vaccine. Vaccines. 2021; 9(9):946. https://doi.org/10.3390/vaccines9090946
Chicago/Turabian StyleLebeau, Grégorie, Alisé Lagrave, Eva Ogire, Lauriane Grondin, Soundary Seriacaroupin, Cédric Moutoussamy, Patrick Mavingui, Jean-Jacques Hoarau, Marjolaine Roche, Pascale Krejbich-Trotot, and et al. 2021. "Viral Toxin NS1 Implication in Dengue Pathogenesis Making It a Pivotal Target in Development of Efficient Vaccine" Vaccines 9, no. 9: 946. https://doi.org/10.3390/vaccines9090946
APA StyleLebeau, G., Lagrave, A., Ogire, E., Grondin, L., Seriacaroupin, S., Moutoussamy, C., Mavingui, P., Hoarau, J.-J., Roche, M., Krejbich-Trotot, P., Desprès, P., & Viranaicken, W. (2021). Viral Toxin NS1 Implication in Dengue Pathogenesis Making It a Pivotal Target in Development of Efficient Vaccine. Vaccines, 9(9), 946. https://doi.org/10.3390/vaccines9090946







