TB and SIV Coinfection; a Model for Evaluating Vaccine Strategies against TB Reactivation in Asian Origin Cynomolgus Macaques: A Pilot Study Using BCG Vaccination
Abstract
:1. Introduction
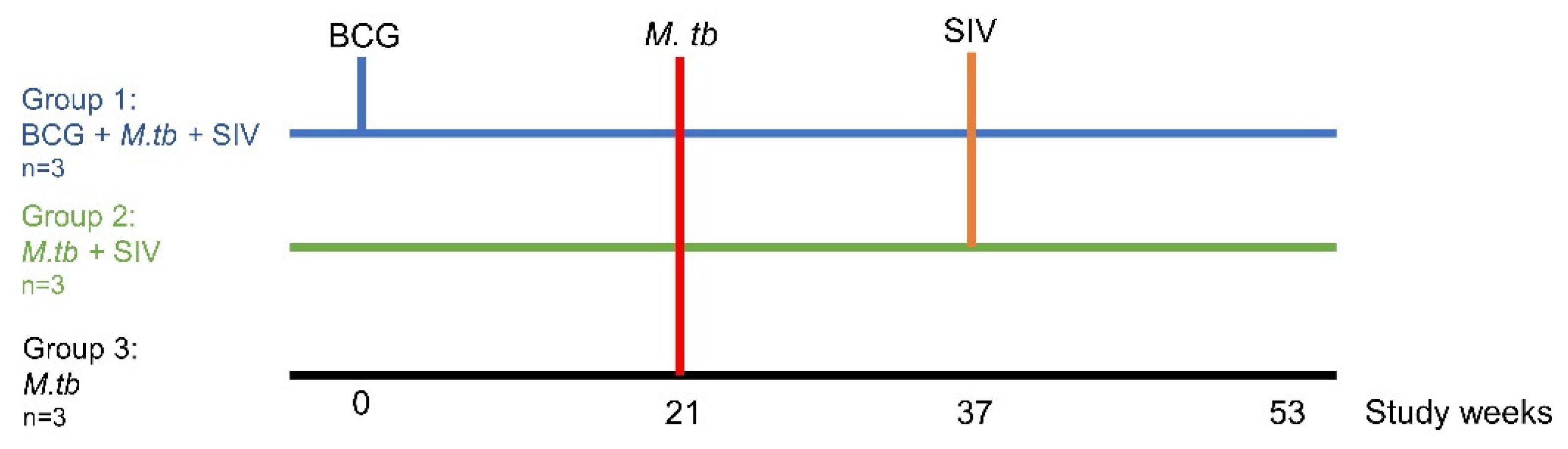
2. Materials and Methods
2.1. Experimental Animals
2.2. BCG Vaccination
2.3. Administration of M. tb Aerosol Challenge
2.4. Administration of SIV Intravenous Challenge
2.5. Plasma RNA Load
2.6. Postchallenge Clinical Assessment
2.7. Computed Tomography (CT) Imaging
2.8. Necropsy
2.9. Gross Pathology
2.10. Histopathology
2.11. Bacteriology
2.12. IFNγ ELISPOT
2.13. Whole-Blood Immunophenotyping Assay
2.14. Statistical Analysis
3. Results
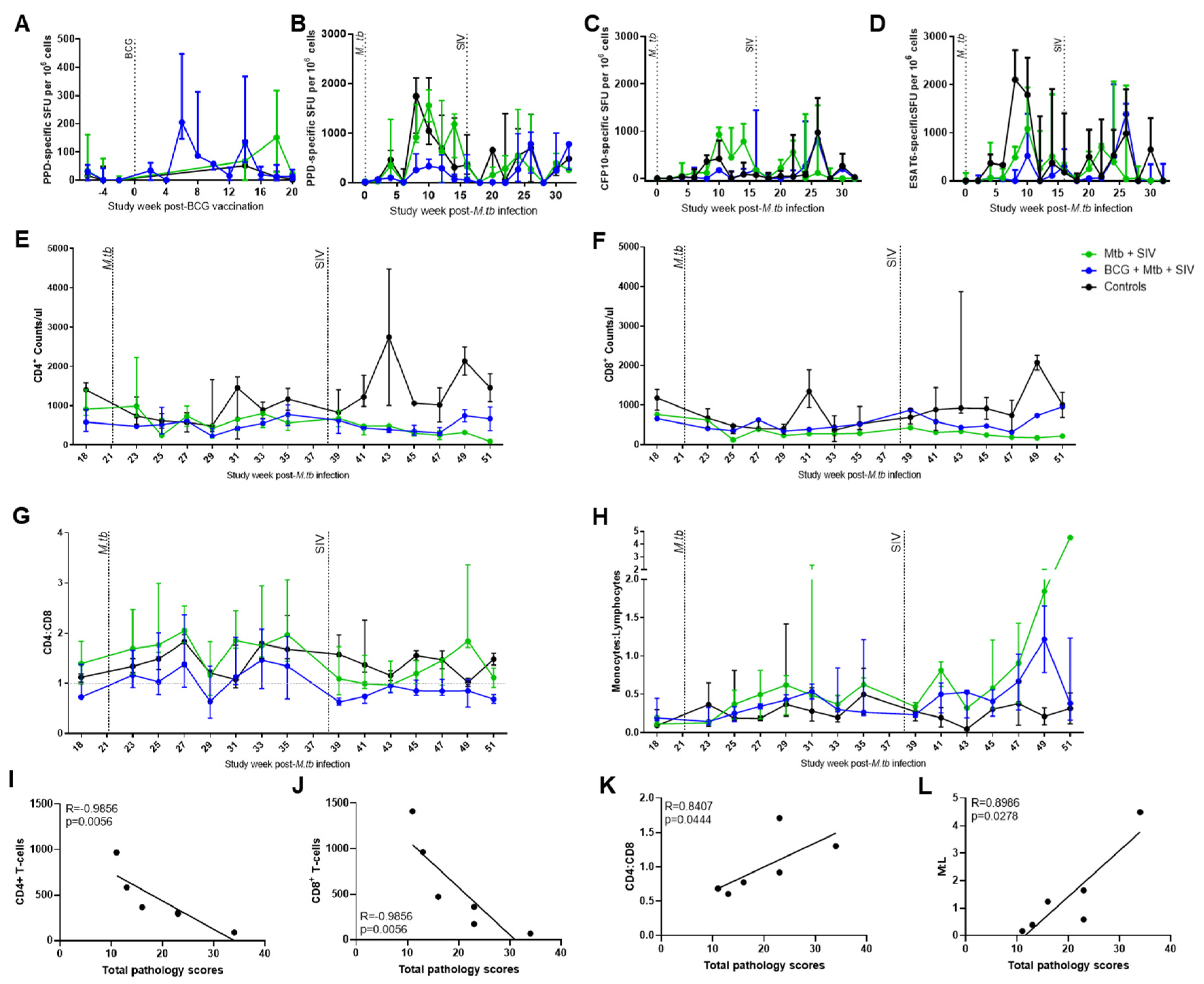
3.1. TB and SIV Disease Progression
3.2. Immune Responses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Tuberculosis Report 2020. 2020. Available online: http://apps.who.int/bookorders (accessed on 24 February 2021).
- Chaisson, R.E.; Churchyard, G.J. Recurrent tuberculosis: Relapse, reinfection, and HIV. J. Infect. Dis. 2010, 201, 653–655. [Google Scholar] [CrossRef] [Green Version]
- Unis, G.; Ribeiro, A.W.; Souza Esteves, L.; Spies, F.S.; Picon, P.D.; Costa, E.R.D.; Rossetti, M.L.R. Tuberculosis Recurrence in a High Incidence Setting for HIV and Tuberculosis in Brazil. BMC Infect. Dis. 2014, 14, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Colditz, G.A.; Brewer, T.F.; Berkey, C.S.; Wilson, M.E.; Burdick, E.; Fineberg, H.V.; Mosteller, F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 1994, 271, 698–702. [Google Scholar] [CrossRef]
- Tait, D.R.; Hatherill, M.; Van Der Meeren, O.; Ginsberg, A.M.; Van Brakel, E.; Salaun, B.; Scriba, T.; Akite, E.J.; Ayles, H.M.; Bollaerts, A.; et al. Final Analysis of a Trial of M72/AS01 E Vaccine to Prevent Tuberculosis. N. Engl. J. Med. 2019, 381, 2429–2439. [Google Scholar] [CrossRef]
- Ji, Z.; Jian, M.; Chen, T.; Luo, L.; Li, L.; Dai, X.; Bai, R.; Ding, Z.; Bi, Y.; Wen, S.; et al. Immunogenicity and Safety of the M72/AS01E Candidate Vaccine Against Tuberculosis: A Meta-Analysis. Front. Immunol. 2019, 10, 2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardona, P.-J. RUTI: A new chance to shorten the treatment of latent tuberculosis infection. Tuberculosis 2006, 86, 273–289. [Google Scholar] [CrossRef]
- Nell, A.S.; D’lom, E.; Bouic, P.; Sabaté, M.; Bosser, R.; Picas, J.; Amat, M.; Churchyard, G.; Cardona, P.-J. Safety, tolerability, and immunogenicity of the novel antituberculous vaccine RUTI: Randomized, placebo-controlled phase II clinical trial in patients with latent tuberculosis infection. PLoS ONE 2014, 9, e89612. [Google Scholar] [CrossRef]
- Flynn, J.L.; Gideon, H.P.; Mattila, J.T.; Lin, P.L. Immunology studies in non-human primate models of tuberculosis. Immunol. Rev. 2015, 264, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Capuano, S.V.; Croix, D.A.; Pawar, S.; Zinovik, A.; Myers, A.; Lin, P.L.; Bissel, S.; Fuhrman, C.; Klein, E.; Flynn, J.L. Experimental Mycobacterium tuberculosis Infection of Cynomolgus Macaques Closely Resembles the Various Manifestations of Human M. tuberculosis Infection. Infect. Immun. 2003, 71, 5831–5844. [Google Scholar] [CrossRef] [Green Version]
- Diedrich, C.R.; Mattila, J.T.; Klein, E.; Janssen, C.; Phuah, J.Y.; Sturgeon, T.J.; Montelaro, R.C.; Lin, P.L.; Flynn, J.L. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS ONE 2010, 5, e9611. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, S.; White, A.; Gleeson, F.; McIntyre, A.; Smyth, D.; Clark, S.; Sarfas, C.; Laddy, D.; Rayner, E.; Hall, G.; et al. Ultra low dose aerosol challenge with Mycobacterium tuberculosis leads to divergent outcomes in rhesus and cynomolgus macaques. Tuberculosis 2016, 96, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharpe, S.A.; Eschelbach, E.; Basaraba, R.J.; Gleeson, F.; Hall, G.; McIntyre, A.; Williams, A.; Kraft, S.; Clark, S.; Gooch, K.; et al. Determination of lesion volume by MRI and stereology in a macaque model of tuberculosis. Tuberculosis 2009, 89, 405–416. [Google Scholar] [CrossRef]
- Mitchell, J.L.; Mee, E.T.; Almond, N.M.; Cutler, K.; Rose, N.J. Characterisation of MHC haplotypes in a breeding colony of Indonesian cynomolgus macaques reveals a high level of diversity. Immunogenetics 2012, 64, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Code of Practice for the Housing and Care of Animals Bred, Supplied or Used for Scientific Purposes. GOV.UK. Available online: https://www.gov.uk/government/publications/code-of-practice-for-the-housing-and-care-of-animals-bred-supplied-or-used-for-scientific-purposes (accessed on 10 July 2017).
- National Committee for Refinement, Reduction and Replacement (NC3Rs). Non-Human Primate Accommodation, Care and Use|NC3Rs.
- Sharpe, S.A.; Smyth, D.; McIntyre, A.; Gleeson, F.; Dennis, M.J. Refinement and reduction through application of a quantitative score system for estimation of TB-induced disease burden using computed tomography. Lab. Anim. 2018, 52, 599–610. [Google Scholar] [CrossRef]
- Cranage, M.P.; Whatmore, A.M.; Sharpe, S.A.; Cook, N.; Polyanskaya, N.; Leech, S.; Smith, J.D.; Rud, E.W.; Dennis, M.J.; Hall, G.A. Macaques Infected with Live Attenuated SIVmac Are Protected against Superinfection via the Rectal Mucosa. Virology 1997, 229, 143–154. [Google Scholar] [CrossRef]
- Kitchen, P.A.; Cranage, M.P.; Almond, N.; Barnard, A.; Baskerville, A.; Corcoran, T.F.C.; Greenaway, P.; Grief, C.; Jenkins, A. Titration of SIVmac251 (32H isolate) in cynomolgus macaques for use as a challenge in vaccination studies. In International TNO Meeting on Animal Models in AIDS; Elsevier: Amsterdam, The Netherlands, 1990; pp. 115–129. [Google Scholar]
- Atkinson, G.F. The Spearman-Karber Method of Estimating 50% Endpoints. Cornell University. Biometrics Unit.; Biometrics Unit Technical Reports; Number BU-141-M, 1961. Available online: https://ecommons.cornell.edu/handle/1813/32006 (accessed on 24 August 2021).
- Cranage, M.; Sharpe, S.; Herrera, C.; Cope, A.; Dennis, M.; Berry, N.; Ham, C.; Heeney, J.; Rezk, N.; Kashuba, A.; et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 2008, 5, e157. [Google Scholar] [CrossRef]
- Leutenegger, C.M.; Higgins, J.; Matthews, T.B.; Tarantal, A.F.; Luciw, P.A.; Pedersen, N.C.; North, T.W. Real-time TaqMan PCR as a specific and more sensitive alternative to the branched-chain DNA assay for quantitation of simian immunodeficiency virus RNA. AIDS Res. Hum. Retroviruses 2001, 17, 243–251. [Google Scholar] [CrossRef]
- White, A.D.; Sarfas, C.; Sibley, L.S.; Gullick, J.; Clark, S.; Rayner, E.; Gleeson, F.; Català, M.; Nogueira, I.; Cardona, P.-J.; et al. Protective Efficacy of Inhaled BCG Vaccination Against Ultra-Low Dose Aerosol M. tuberculosis Challenge in Rhesus Macaques. Pharmaceutics 2020, 12, 394. [Google Scholar] [CrossRef]
- Sharpe, S.A.; McShane, H.; Dennis, M.J.; Basaraba, R.J.; Gleeson, F.; Hall, G.; McIntyre, A.; Gooch, K.; Clark, S.; Beveridge, N.; et al. Establishment of an aerosol challenge model of tuberculosis in rhesus macaques and an evaluation of endpoints for vaccine testing. Clin. Vaccine Immunol. 2010, 17, 1170–1182. [Google Scholar] [CrossRef] [Green Version]
- White, A.D.; Sibley, L.; Sarfas, C.; Morrison, A.; Gullick, J.; Clark, S.; Gleeson, F.; McIntyre, A.; Arlehamn, C.L.; Sette, A.; et al. OPEN MTBVAC vaccination protects rhesus macaques against aerosol challenge with M. tuberculosis and induces immune signatures analogous to those observed in clinical studies. npj Vaccines 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Rayner, E.L.; Pearson, G.R.; Hall, G.A.; Gleeson, F.; McIntyre, A.; Smyth, D.; Dennis, M.; Sharpe, S. Early lesions following aerosol challenge of rhesus macaques (Macaca mulatta) with Mycobacterium tuberculosis (Erdman strain). J. Comp. Pathol. 2015, 152, 217–226. [Google Scholar] [CrossRef]
- Sharpe, S.A.; White, A.D.; Sibley, L.; Gleeson, F.; Hall, G.A.; Basaraba, R.J.; McIntyre, A.; Clark, S.; Gooch, K.; Marsh, P.D.; et al. An aerosol challenge model of tuberculosis in Mauritian cynomolgus macaques. PLoS ONE 2017, 12, e0171906. [Google Scholar] [CrossRef]
- Okamura, T.; Tsujimura, Y.; Soma, S.; Takahashi, I.; Matsuo, K.; Yasutomi, Y. Simian immunodeficiency virus SIVmac239 infection and simian human immunodeficiency virus SHIV89.6P infection result in progression to AIDS in cynomolgus macaques of Asian origin. J. Gen. Virol. 2016, 97, 3413–3426. [Google Scholar] [CrossRef]
- Abubakar, I.; Pimpin, L.; Ariti, C.; Beynon, R.; Mangtani, P.; Sterne, J.; Fine, P.E.M.; Smith, P.G.; Lipman, M.; Elliman, D.; et al. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guérin vaccination against tuberculosis. Health Technol. Assess 2013, 17, 1–4. [Google Scholar] [CrossRef]
- Lin, P.L.; Dietrich, J.; Tan, E.; Abalos, R.M.; Burgos, J.; Bigbee, C.; Bigbee, M.; Milk, L.; Gideon, H.; Rodgers, M.; et al. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J. Clin. Investig. 2012, 122, 303–314. [Google Scholar] [CrossRef]
- Sharpe, S.; White, A.; Sarfas, C.; Sibley, L.; Gleeson, F.; McIntyre, A.; Basaraba, R.; Clark, S.; Hall, G.; Rayner, E.; et al. Alternative BCG delivery strategies improve protection against Mycobacterium tuberculosis in non-human primates: Protection associated with mycobacterial antigen-specific CD4 effector memory T-cell populations. Tuberculosis 2016, 101, 174–190. [Google Scholar] [CrossRef] [Green Version]
- Sibley, L.; White, A.D.; Gooch, K.; Stevens, L.M.; Tanner, R.; Jacobs, A.; Daykin-Pont, O.; Gleeson, F.; McIntyre, A.; Basaraba, R. High dose aerosol challenge with Mycobacterium tuberculosis fails to overcome BCG vaccination-induced protection in cynomolgus macaques of Chinese origin: Implications of natural resistance for TB vaccine evaluation. Sci. Rep. 2021. [Google Scholar] [CrossRef]
- Vordermeier, H.M.; Chambers, M.A.; Cockle, P.J.; Whelan, A.O.; Simmons, J.; Hewinson, R.G. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 2002, 70, 3026–3032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, H.A.; The BCG Study Team; Filali-Mouhim, A.; Nemes, E.; Hawkridge, A.; Keyser, A.; Njikan, S.; Hatherill, M.; Scriba, T.; Abel, B.; et al. Human newborn bacille Calmette-Guérin vaccination and risk of tuberculosis disease: A case-control study. BMC Med. 2016, 29, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naranbhai, V.; Moodley, D.; Chipato, T.; Stranix-Chibanda, L.; Nakabaiito, C.; Kamateeka, M.; Musoke, P.; Manji, K.; George, K.; Emel, L.M.; et al. The association between the ratio of monocytes:lymphocytes and risk of tuberculosis (TB) amongst HIV infected postpartum women. J. Acquir. Immune Defic. Syndr. 2014, 67, 573–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yin, Y.; Wang, X.; Pei, H.; Kuai, S.; Gu, L.; Xing, H.; Zhang, Y.; Huang, Q.; Guan, B. Ratio of monocytes to lymphocytes in peripheral blood in patients diagnosed with active tuberculosis. Braz. J. Infect. Dis. 2015, 19, 125–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LA Manna, M.P.; Orlando, V.; Dieli, F.; Di Carlo, P.; Cascio, A.; Cuzzi, G.; Palmieri, F.; Goletti, D.; Caccamo, N. Quantitative and qualitative profiles of circulating monocytes may help identifying tuberculosis infection and disease stages. PLoS ONE 2017, 12, e0171358. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Hill, A.V.S.; Karim, S.A.; Naidoo, K.; Karim, Q.A.; Warimwe, G.M.; McShane, H.; Fletcher, H. Ratio of monocytes to lymphocytes in peripheral blood identifies adults at risk of incident tuberculosis among HIV-infected adults initiating antiretroviral therapy. J. Infect. Dis. 2013, 209, 500–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Animal ID | Discrete Lesions | Coalescing | Consolidation | |
|---|---|---|---|---|
| Group 1: BCG + M. tb + SIV | 053GFD | 3 | 0 | 0 |
| 768EIF | 27 | 0 | 0 | |
| 357GHD | 73 | 0 | 0 | |
| Group 2: M. tb + SIV | 037KCHA | 6 | 0 | 0 |
| 436FIC | 73 | 1 | 3 | |
| 817DHB | 454 | 0 | 3 | |
| Group 3: M. tb | 962DFC | 4 | 0 | 0 |
| 980ABAGC | 222 | 0 | 0 | |
| N31DC | 1 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, A.D.; Sibley, L.; Gullick, J.; Sarfas, C.; Clark, S.; Fagrouch, Z.; Verschoor, E.; Salguero, F.J.; Dennis, M.; Sharpe, S. TB and SIV Coinfection; a Model for Evaluating Vaccine Strategies against TB Reactivation in Asian Origin Cynomolgus Macaques: A Pilot Study Using BCG Vaccination. Vaccines 2021, 9, 945. https://doi.org/10.3390/vaccines9090945
White AD, Sibley L, Gullick J, Sarfas C, Clark S, Fagrouch Z, Verschoor E, Salguero FJ, Dennis M, Sharpe S. TB and SIV Coinfection; a Model for Evaluating Vaccine Strategies against TB Reactivation in Asian Origin Cynomolgus Macaques: A Pilot Study Using BCG Vaccination. Vaccines. 2021; 9(9):945. https://doi.org/10.3390/vaccines9090945
Chicago/Turabian StyleWhite, Andrew D., Laura Sibley, Jennie Gullick, Charlotte Sarfas, Simon Clark, Zahra Fagrouch, Ernst Verschoor, Francisco J. Salguero, Mike Dennis, and Sally Sharpe. 2021. "TB and SIV Coinfection; a Model for Evaluating Vaccine Strategies against TB Reactivation in Asian Origin Cynomolgus Macaques: A Pilot Study Using BCG Vaccination" Vaccines 9, no. 9: 945. https://doi.org/10.3390/vaccines9090945
APA StyleWhite, A. D., Sibley, L., Gullick, J., Sarfas, C., Clark, S., Fagrouch, Z., Verschoor, E., Salguero, F. J., Dennis, M., & Sharpe, S. (2021). TB and SIV Coinfection; a Model for Evaluating Vaccine Strategies against TB Reactivation in Asian Origin Cynomolgus Macaques: A Pilot Study Using BCG Vaccination. Vaccines, 9(9), 945. https://doi.org/10.3390/vaccines9090945






