Phase I Trial Evaluating the Safety and Immunogenicity of Candidate TB Vaccine MVA85A, Delivered by Aerosol to Healthy M.tb-Infected Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
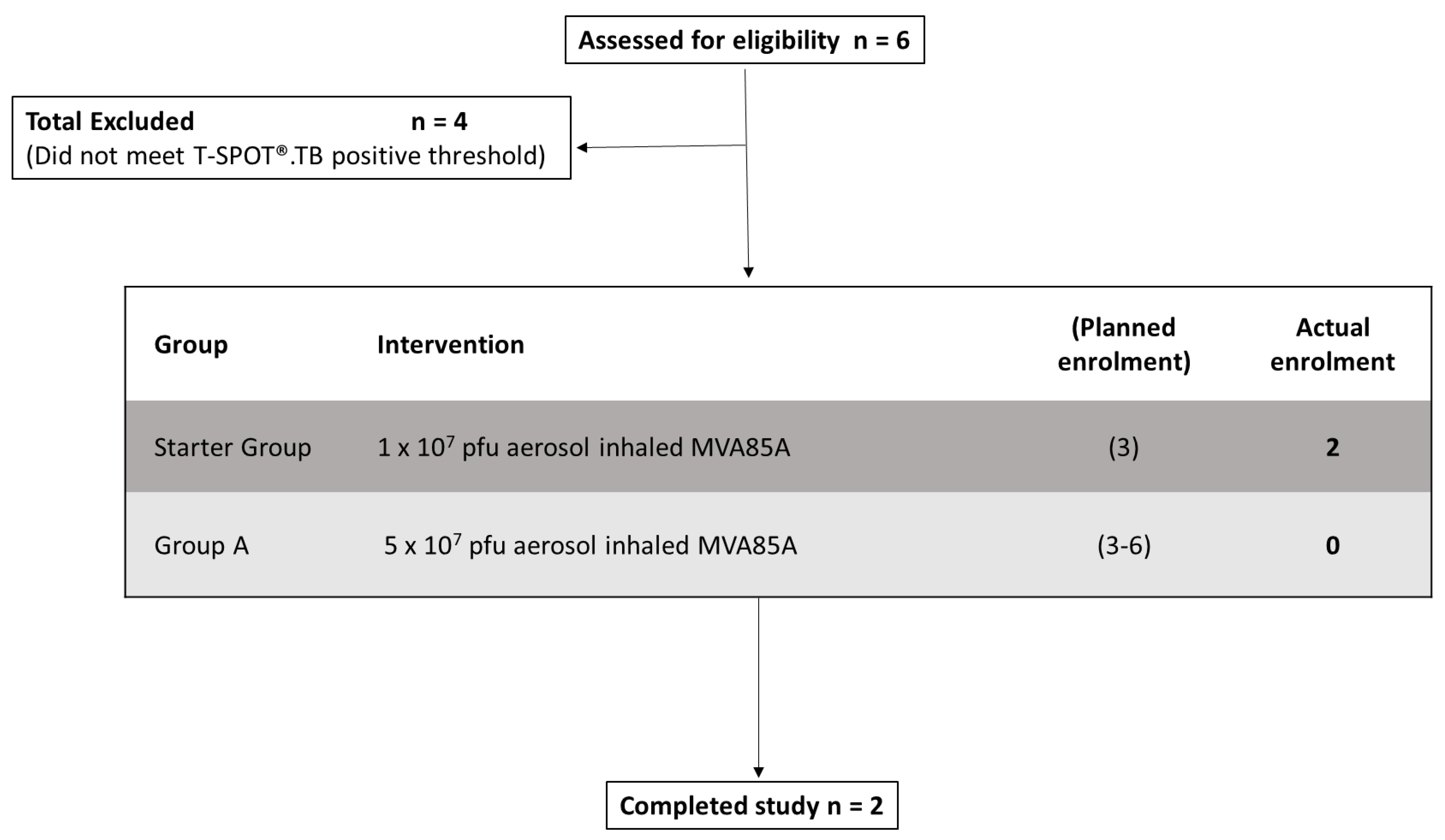
2.2. Participants
2.3. Vaccines
2.4. Clinical Interventions
2.5. Objectives
2.6. Ex Vivo Enzyme-Linked ImmunoSpot (ELISpot)
2.7. Enzyme-Linked-Immunosorbent-Assay (ELISA)
2.8. Intracellular Cytokine Staining (ICS) on BAL and Peripheral Blood Mononuclear Cells (PBMCs)
2.9. Statistical Analysis
3. Results
3.1. Safety
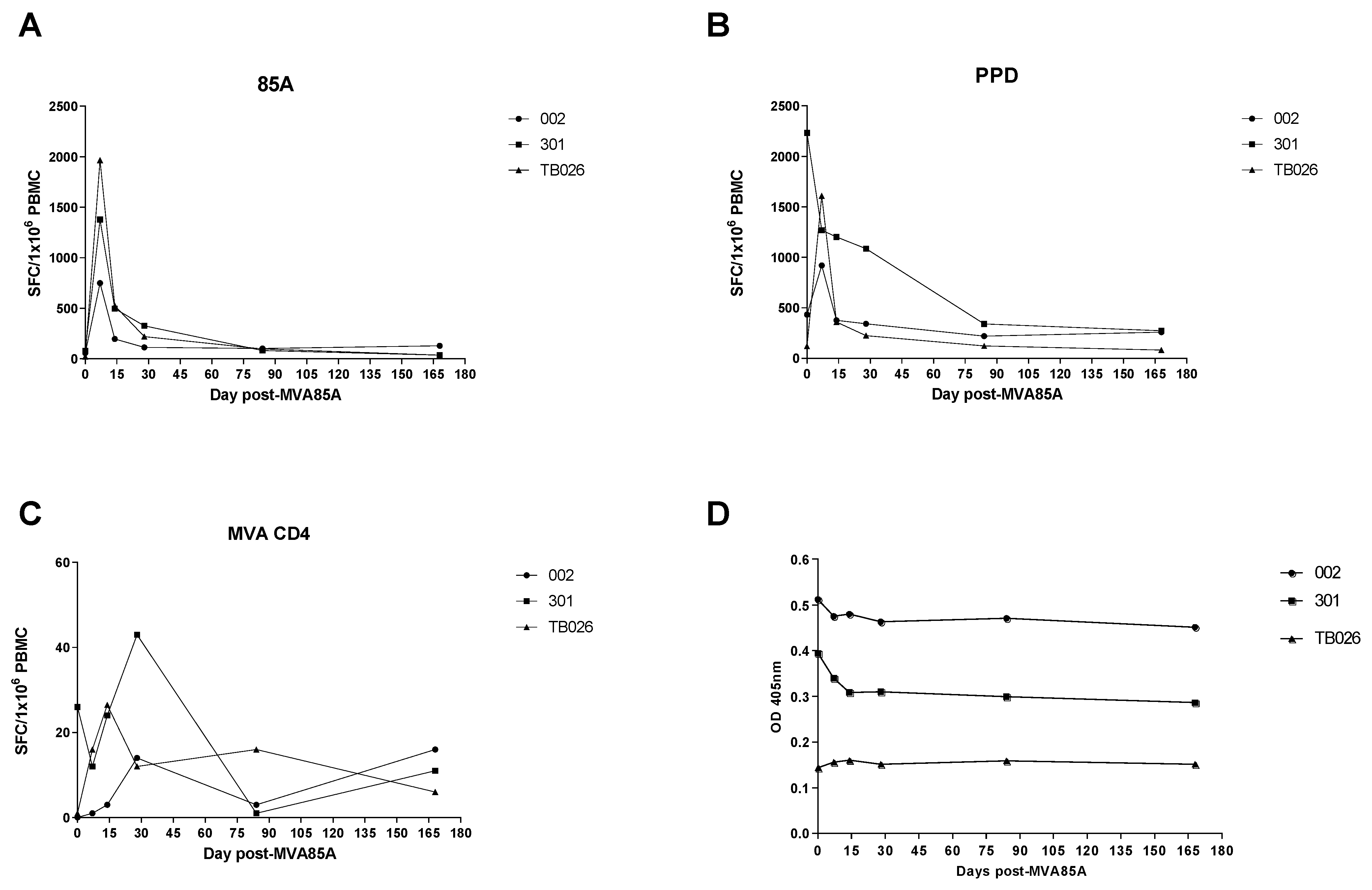
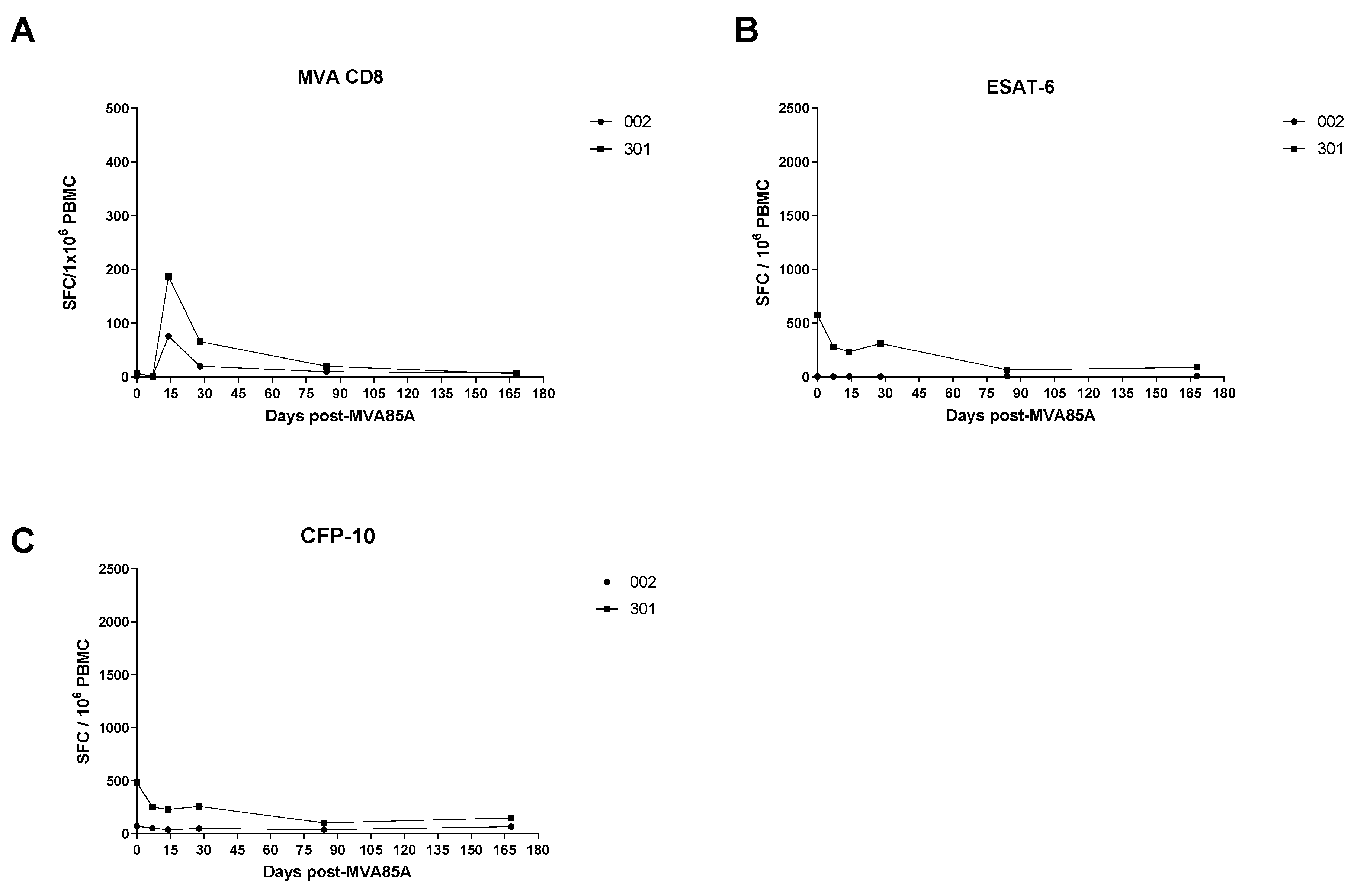
3.2. Ex Vivo IFN-γ Enzyme-Linked Immunospot (ELIspot)
3.3. Enzyme-Linked Immunosorbent Assay (ELISA)
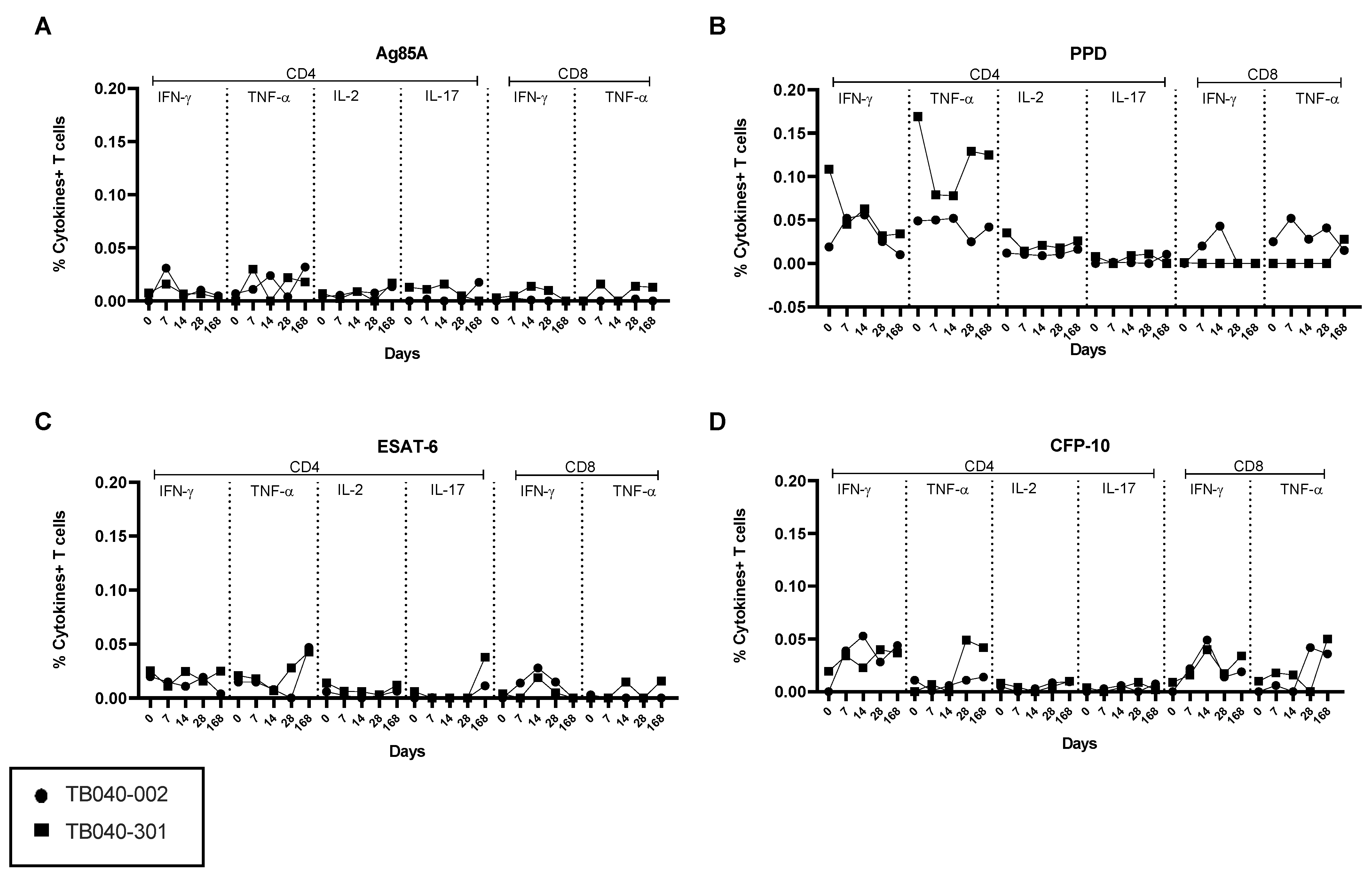
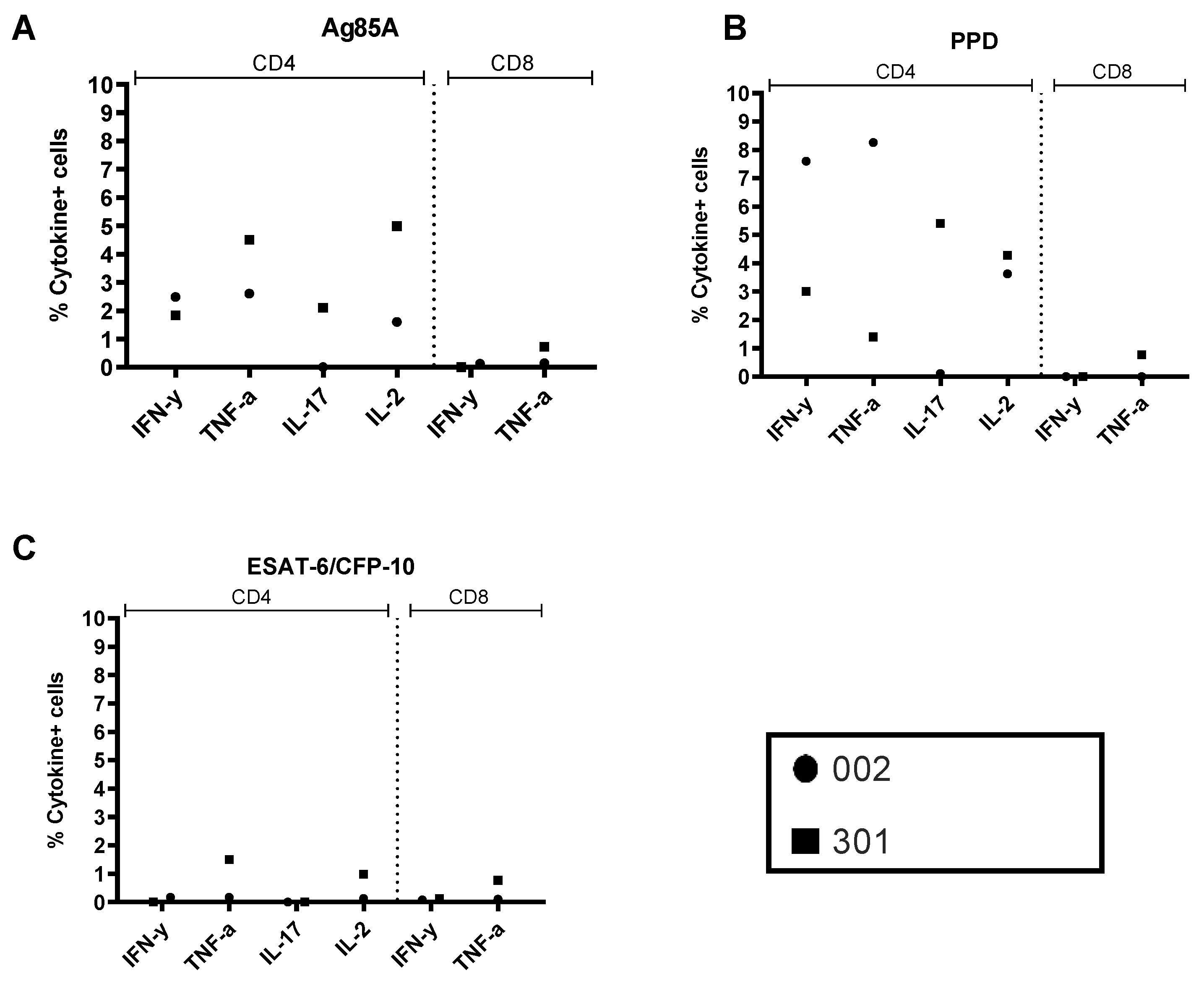
3.4. BAL and PBMC Intracellular Cytokine Responses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Global Tuberculosis Report 2020. Available online: http://www.who.int/tb/publications (accessed on 31 March 2021).
- The Global Plan to End TB: The Paradigm Shift 2016–2020. 2016, Stop TB Partnership. Available online: http://www.stoptb.org (accessed on 31 March 2021).
- Scriba, T.J.; Netea, M.G.; Ginsberg, A.M. Key recent advances in TB vaccine development and understanding of protective immune responses against Mycobacterium tuberculosis. Semin. Immunol. 2020, 50, 101431. [Google Scholar] [CrossRef] [PubMed]
- Morrison, H.; McShane, H. Local Pulmonary Immunological Biomarkers in Tuberculosis. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Cooper, A.M. Cell-Mediated Immune Responses in Tuberculosis. Annu. Rev. Immunol. 2009, 27, 393–422. [Google Scholar] [CrossRef]
- Comstock, G.W.; Livesay, V.T.; Woolpert, S.F. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am. J. Epidemiol. 1974, 99, 131–138. [Google Scholar] [CrossRef]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.M.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F.; et al. Protection by BCG Vaccine Against Tuberculosis: A Systematic Review of Randomized Controlled Trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Trunz, B.B.; Fine, P.; Dye, C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet 2006, 367, 1173–1180. [Google Scholar] [CrossRef]
- Roth, Y.; Chapnik, J.S.; Cole, P. Feasibility of Aerosol Vaccination in Humans. Ann. Otol. Rhinol. Laryngol. 2003, 112, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, C.; Zedler, U.; Kühl, A.A.; Lozza, L.; Saikali, P.; Sander, L.E.; Vogelzang, A.; Kaufmann, S.H.E.; Kupz, A. Mucosal BCG Vaccination Induces Protective Lung-Resident Memory T Cell Populations against Tuberculosis. mBio 2016, 7, e01686-16. [Google Scholar] [CrossRef]
- Verreck, F.A.; Tchilian, E.Z.; Vervenne, R.A.; Sombroek, C.C.; Kondova, I.; Eissen, O.A.; Sommandas, V.; van der Werff, N.M.; Verschoor, E.; Braskamp, G.; et al. Variable BCG efficacy in rhesus populations: Pulmonary BCG provides protection where standard intra-dermal vaccination fails. Tuberc 2017, 104, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Cutts, F.T.; Clements, C.J.; Bennett, J.V. Alternative routes of measles immunization: A review. Biologicals 1997, 25, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Goonetilleke, N.P.; McShane, H.; Hannan, C.M.; Anderson, R.J.; Brookes, R.H.; Hill, A.V. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacilli Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccine virus Ankara. J. Immunol. 2003, 171, 1602–1609. [Google Scholar] [CrossRef]
- Low, N.; Kraemer, S.; Schneider, M.; Restrepo, A.M. Immunogenicity and safety of aerosolized measles vaccine: Systematic review and meta-analysis. Vaccine 2008, 26, 383–398. [Google Scholar] [CrossRef]
- Santosuosso, M.; McCormick, S.; Zhang, X.; Zganiacz, A.; Xing, Z. Intranasal boosting with an adenovirus-vectored vaccine markedly enhances protection by parenteral Mycobacterium bovis BCG immunization against pulmonary tuberculosis. Infect. Immun. 2006, 74, 4634–4643. [Google Scholar] [CrossRef] [PubMed]
- White, A.D.; Sibley, L.; Dennis, M.J.; Gooch, K.; Betts, G.; Edwards, N.; Reyes-Sandoval, A.; Carroll, M.W.; Williams, A.; Marsh, P.D.; et al. Evaluation of the safety and immunogenicity of a candidate tuberculosis vaccine, MVA85A, delivered by aerosol to the lungs of macaques. Clin. Vaccine Immunol. 2013, 20, 663–672. [Google Scholar] [CrossRef] [PubMed]
- White, A.D.; Sarfas, C.; West, K.; Sibley, L.S.; Wareham, A.S.; Clark, S.; Dennis, M.J.; Williams, A.; Marsh, P.D.; Sharpe, S.A. Evaluation of the safety and immunogenicity of Mycobacterium bovis BCG delivered by aerosol to the lungs of macaques. Clin. Vaccine Immunol. 2015, 22, 992–1003. [Google Scholar] [CrossRef]
- McShane, H.; Pathan, A.A.; Sander, C.R.; Keating, S.M.; Gilbert, S.C.; Huygen, K.; Fletcher, H.A.; Hill, A.V. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 2004, 10, 1240–1244. [Google Scholar] [CrossRef]
- Minassian, A.M.; Rowland, R.; Beveridge, N.E.R.; Poulton, I.D.; Satti, I.; Harris, S.; Poyntz, H.; Hamill, M.; Griffiths, K.; Sander, C.R.; et al. A Phase I study evaluating the safety and immunogenicity of MVA85A, a candidate TB vaccine, in HIV-infected adults. BMJ Open 2011, 1, e000223. [Google Scholar] [CrossRef]
- Sheehan, S.; Harris, S.A.; Satti, I.; Hokey, D.A.; Dheenadhayalan, V.; Stockdale, L.; Thomas, Z.-R.M.; Minhinnick, A.; Wilkie, M.; Vermaak, S.; et al. A Phase I, open-label trial, evaluating the safety and immunogenicity of candidate tuberculosis vaccines AERAS-402 and MVA85A, administered by prime-boost regime in BCG-vaccinated healthy adults. PLoS ONE 2015, 10, e0141687. [Google Scholar] [CrossRef]
- Meyer, J.; Harris, S.A.; Satti, I.; Poulton, I.D.; Poyntz, H.C.; Tanner, R.; Rowland, R.; Griffiths, K.L.; Fletcher, H.A.; McShane, H. Comparing the safety and immunogenicity of a candidate TB vaccine MVA85A administered by intramuscular and intradermal delivery. Vaccine 2013, 31, 1026–1033. [Google Scholar] [CrossRef]
- Minhinnick, A.; Satti, I.; Harris, S.; Wilkie, M.; Sheehan, S.; Stockdale, L.; Thomas, Z.-R.M.; Lopez-Ramon, R.; Poulton, I.; Lawrie, A.; et al. A first-in-human phase 1 trial to evaluate the safety and immunogenicity of the candidate tuberculosis vaccine MVA85A-IMX313, administered to BCG-vaccinated adults. Vaccine 2016, 34, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, M.; Satti, I.; Minhinnick, A.; Harris, S.; Riste, M.; Ramon, R.L.; Sheehan, S.; Thomas, Z.-R.M.; Wright, D.; Stockdale, L.; et al. A phase I trial evaluating the safety and immunogenicity of a candidate tuberculosis vaccination regimen, ChAdOx1 85A prime—MVA85A boost in healthy UK adults. Vaccine 2020, 38, 779–789. [Google Scholar] [CrossRef]
- Pathan, A.A.; Minassian, A.M.; Sander, C.R.; Rowland, R.; Porter, D.W.; Poulton, I.D.; Hill, A.V.; Fletcher, H.A.; McShane, H. Effect of vaccine dose on the safety and immunogenicity of a candidate TB vaccine, MVA85A, in BCG vaccinated UK adults. Vaccine 2012, 30, 5616–5624. [Google Scholar] [CrossRef]
- Tameris, M.D.; Hatherill, M.; Landry, B.S.; Scriba, T.J.; Snowden, M.A.; Lockhart, S.; Shea, J.E.; McClain, J.B.; Hussey, G.D.; Hanekom, W.A.; et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: A randomised, placebo-controlled phase 2b trial. Lancet 2013, 381, 1021–1028. [Google Scholar] [CrossRef]
- Thomas, Z.-R.M.; Satti, I.; Marshall, J.L.; Harris, S.A.; Ramon, R.L.; Hamidi, A.; Minhinnick, A.; Riste, M.; Stockdale, L.; Lawrie, A.M.; et al. Alternate aerosol and systemic immunisation with a recombinant viral vector for tuberculosis, MVA85A: A phase I randomised controlled trial. PLoS Med 2019, 16, e1002790. [Google Scholar]
- Satti, I.; Meyer, J.; Harris, S.A.; Thomas, Z.-R.M.; Griffiths, K.; Antrobus, R.D.; Rowland, R.; Ramon, R.L.; Smith, M.; Sheehan, S.; et al. Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: A phase 1, double-blind, randomised controlled trial. Lancet Infect. Dis. 2014, 14, 939–946. [Google Scholar] [CrossRef]
- Sander, C.R.; Pathan, A.A.; Beveridge, N.E.R.; Poulton, I.; Minassian, A.; Alder, N.; Van Wijgerden, J.; Hill, A.V.S.; Gleeson, F.V.; Davies, R.J.O.; et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals. Am. J. Respir. Crit. Care Med. 2009, 179, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Scriba, T.J.; Tameris, M.; Smit, E.; Van Der Merwe, L.; Hughes, E.J.; Kadira, B.; Mauff, K.; Moyo, S.; Brittain, N.; Lawrie, A.; et al. A phase IIa trial of the new tuberculosis vaccine, MVA85A, in HIV- and/or Mycobacterium tuberculosis–infected Adults. Am. J. Respir. Crit. Care Med. 2012, 185, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, B.P.; Thienemann, F.; Ota, M.; Landry, B.S.; Camara, M.; Dièye, S.; Dieye, T.N.; Esmail, H.; Goliath, R.; Huygen, K.; et al. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: A randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2015, 3, 190–200. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riste, M.; Marshall, J.L.; Satti, I.; Harris, S.A.; Wilkie, M.; Lopez Ramon, R.; Wright, D.; Wittenberg, R.E.; Vermaak, S.; Powell Doherty, R.; et al. Phase I Trial Evaluating the Safety and Immunogenicity of Candidate TB Vaccine MVA85A, Delivered by Aerosol to Healthy M.tb-Infected Adults. Vaccines 2021, 9, 396. https://doi.org/10.3390/vaccines9040396
Riste M, Marshall JL, Satti I, Harris SA, Wilkie M, Lopez Ramon R, Wright D, Wittenberg RE, Vermaak S, Powell Doherty R, et al. Phase I Trial Evaluating the Safety and Immunogenicity of Candidate TB Vaccine MVA85A, Delivered by Aerosol to Healthy M.tb-Infected Adults. Vaccines. 2021; 9(4):396. https://doi.org/10.3390/vaccines9040396
Chicago/Turabian StyleRiste, Michael, Julia L. Marshall, Iman Satti, Stephanie A. Harris, Morven Wilkie, Raquel Lopez Ramon, Danny Wright, Rachel E. Wittenberg, Samantha Vermaak, Rebecca Powell Doherty, and et al. 2021. "Phase I Trial Evaluating the Safety and Immunogenicity of Candidate TB Vaccine MVA85A, Delivered by Aerosol to Healthy M.tb-Infected Adults" Vaccines 9, no. 4: 396. https://doi.org/10.3390/vaccines9040396
APA StyleRiste, M., Marshall, J. L., Satti, I., Harris, S. A., Wilkie, M., Lopez Ramon, R., Wright, D., Wittenberg, R. E., Vermaak, S., Powell Doherty, R., Lawrie, A., Conlon, C. P., Cosgrove, C., Gleeson, F., Lipman, M., Moss, P., Perrin, F., Dedicoat, M., Bettinson, H., & McShane, H. (2021). Phase I Trial Evaluating the Safety and Immunogenicity of Candidate TB Vaccine MVA85A, Delivered by Aerosol to Healthy M.tb-Infected Adults. Vaccines, 9(4), 396. https://doi.org/10.3390/vaccines9040396





