Cutaneous Adverse Reactions to COVID-19 Vaccines: Insights from an Immuno-Dermatological Perspective
Abstract
:1. Introduction
2. Literature Review
3. Discussion
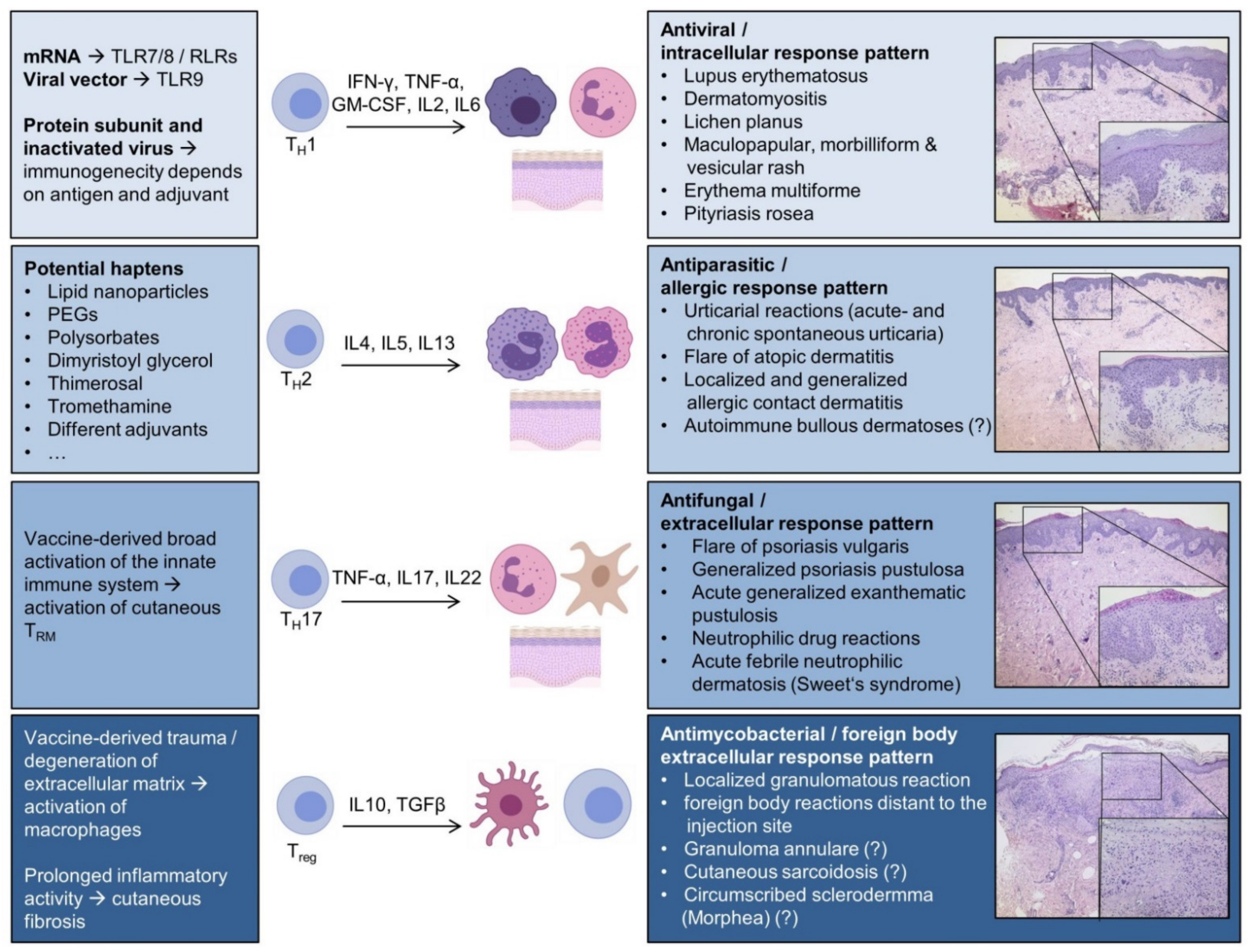
3.1. Th1-Polarized Cutaneous Inflammation
3.1.1. Cutaneous Lupus Erythematosus (CLE)
3.1.2. Dermatomyositis (DM)
3.1.3. Lichen Planus (LP)
3.1.4. Maculopapular, Morbilliform, and Vesicular Rash
3.1.5. Erythema Multiforme (EM)
3.1.6. Pityriasis Rosea (PR)
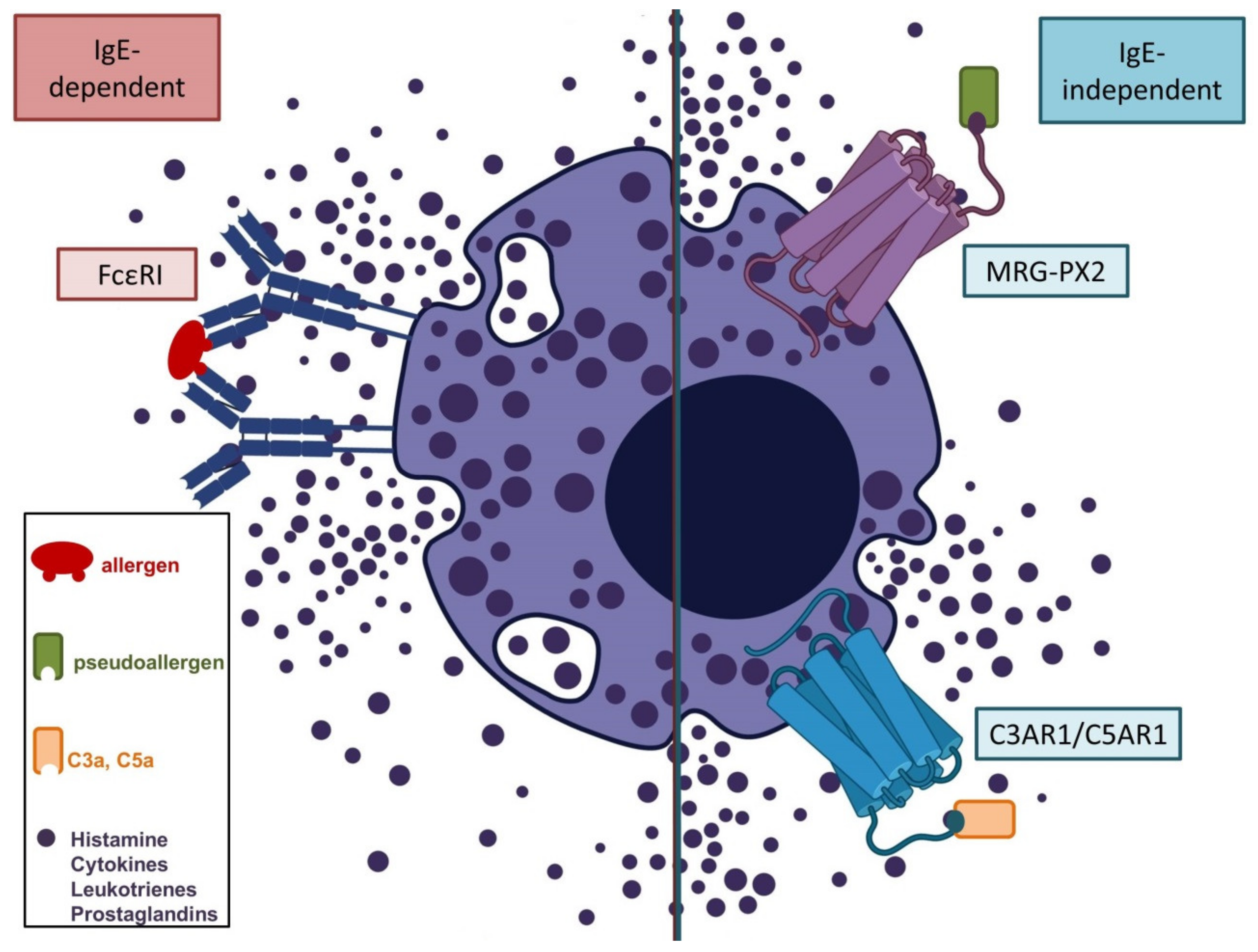
3.2. Th2-Polarized Cutaneous Inflammation
3.2.1. Urticarial Reactions
3.2.2. Atopic Dermatitis (AD)
3.2.3. Injection Site Reactions and Allergic Contact Dermatitis
3.2.4. Autoimmune Bullous Dermatoses
3.3. Th17-Polarized Cutaneous Inflammation
3.3.1. Psoriasis Vulgaris
3.3.2. Neutrophilic and Pustular Drug Reactions
3.4. Granulomatous and Fibrogenic Reactions
3.5. Further Implications, Unanswered Questions, and Future Challenges
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-P.; Yang, M.; Lai, C.-L. COVID-19 Vaccines: A Review of the Safety and Efficacy of Current Clinical Trials. Pharmaceuticals 2021, 14, 406. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Guan, X.-H.; Li, Y.-H.; Huang, J.-Y.; Jiang, T.; Hou, L.-H.; Li, J.-X.; Yang, B.-F.; Wang, L.; Wang, W.-J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Kremsner, P.; Mann, P.; Bosch, J.; Fendel, R.; Gabor, J.J.; Kreidenweiss, A.; Kroidl, A.; Leroux-Roels, I.; Leroux-Roels, G.; Schindler, C.; et al. Phase 1 Assessment of the Safety and Immunogenicity of an mRNA-Lipid Nanoparticle Vaccine Candidate Against SARS-CoV-2 in Human Volunteers. MedRxiv 2020. [Google Scholar] [CrossRef]
- Preissner, K.T.; Fischer, S.; Deindl, E. Extracellular RNA as a Versatile DAMP and Alarm Signal That Influences Leukocyte Recruitment in Inflammation and Infection. Front. Cell Dev. Biol. 2020, 8, 619221. [Google Scholar] [CrossRef]
- Pascolo, S. Synthetic Messenger RNA-Based Vaccines: From Scorn to Hype. Viruses 2021, 13, 270. [Google Scholar] [CrossRef]
- He, Q.; Mao, Q.; Zhang, J.; Bian, L.; Gao, F.; Wang, J.; Xu, M.; Liang, Z. COVID-19 Vaccines: Current Understanding on Immunogenicity, Safety, and Further Considerations. Front. Immunol. 2021, 12, 669339. [Google Scholar] [CrossRef]
- Rosenblatt, A.E.; Stein, S.L. Cutaneous reactions to vaccinations. Clin. Dermatol. 2015, 33, 327–332. [Google Scholar] [CrossRef]
- Fölster-Holst, R.; Zawar, V.P.; Chuh, A. Paraviral exanthems. Expert Rev. Anti Infect. Ther. 2016, 14, 601–611. [Google Scholar] [CrossRef]
- Galván Casas, C.; Català, A.; Carretero Hernández, G.; Rodríguez-Jiménez, P.; Fernández-Nieto, D.; Rodríguez-Villa Lario, A.; Navarro Fernández, I.; Ruiz-Villaverde, R.; Falkenhain-López, D.; Llamas Velasco, M.; et al. Classification of the cutaneous manifestations of COVID-19: A rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020, 183, 71–77. [Google Scholar] [CrossRef]
- Novak, N.; Peng, W.; Naegeli, M.C.; Galvan, C.; Kolm-Djamei, I.; Brüggen, C.; Cabanillas, B.; Schmid-Grendelmeier, P.; Catala, A. SARS-CoV-2, COVID-19, skin and immunology—What do we know so far? Allergy 2021, 76, 698–713. [Google Scholar] [CrossRef]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lee, Y.W.; Lim, S.Y.; Lee, J.H.; Lim, J.S.; Lee, S.; Park, S.; Kim, S.K.; Lim, Y.J.; Kim, E.O.; et al. Adverse Reactions Following the First Dose of ChAdOx1 nCoV-19 Vaccine and BNT162b2 Vaccine for Healthcare Workers in South Korea. J. Korean Med. Sci. 2021, 36, e115. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, G.; Bogdanov, I.; Kazandjieva, J.; Tsankov, N. Cutaneous adverse effects of the available COVID-19 vaccines. Clin. Dermatol. 2021. [Google Scholar] [CrossRef]
- Gringeri, M.; Mosini, G.; Battini, V.; Cammarata, G.; Guarnieri, G.; Carnovale, C.; Clementi, E.; Radice, S. Preliminary evidence on the safety profile of BNT162b2 (Comirnaty): New insights from data analysis in EudraVigilance and adverse reaction reports from an Italian health facility. Hum. Vaccin. Immunother. 2021, 1–3. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Freeman, E.E.; Saff, R.R.; Robinson, L.B.; Wolfson, A.R.; Foreman, R.K.; Hashimoto, D.; Banerji, A.; Li, L.; Anvari, S.; et al. Delayed Large Local Reactions to mRNA-1273 Vaccine against SARS-CoV-2. N. Engl. J. Med. 2021, 384, 1273–1277. [Google Scholar] [CrossRef]
- Niebel, D.; Ralser-Isselstein, V.; Jaschke, K.; Braegelmann, C.; Bieber, T.; Wenzel, J. Exacerbation of subacute cutaneous lupus erythematosus following vaccination with BNT162b2 mRNA vaccine. Dermatol. Ther. 2021. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; de Gregorio, C.; Velissaris, D.; Petalas, K.; Brinia, A.; Assimakopoulos, S.F.; Gogos, C.; Kouni, S.N.; Kounis, G.N.; et al. Allergic Reactions to Current Available COVID-19 Vaccinations: Pathophysiology, Causality, and Therapeutic Considerations. Vaccines 2021, 9, 221. [Google Scholar] [CrossRef]
- Banerji, A.; Wolfson, A.R.; Wickner, P.G.; Cogan, A.S.; McMahon, A.E.; Saff, R.; Robinson, L.B.; Phillips, E.; Blumenthal, K.G. COVID-19 Vaccination in Patients with Reported Allergic Reactions: Updated Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 2135–2138. [Google Scholar] [CrossRef]
- Sokolowska, M.; Eiwegger, T.; Ollert, M.; Torres, M.J.; Barber, D.; Del Giacco, S.; Jutel, M.; Nadeau, K.C.; Palomares, O.; Rabin, R.L.; et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID-19 vaccines. Allergy 2021, 76, 1629–1639. [Google Scholar] [CrossRef]
- Cabanillas, B.; Novak, N. Allergy to COVID-19 vaccines: A current update. Allergol. Int. 2021. [Google Scholar] [CrossRef]
- Ring, J.; Worm, M.; Wollenberg, A.; Thyssen, J.P.; Jakob, T.; Klimek, L.; Bangert, C.; Barbarot, S.; Bieber, T.; de Bruin-Weller, M.S.; et al. Risk of severe allergic reactions to COVID-19 vaccines among patients with allergic skin diseases-practical recommendations. A position statement of ETFAD with external experts. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e362–e365. [Google Scholar] [CrossRef]
- Cabanillas, B.; Akdis, C.A.; Novak, N. COVID-19 vaccines and the role of other potential allergenic components different from PEG. A reply to: “Other excipients than PEG might cause serious hypersensitivity reactions in COVID-19 vaccines”. Allergy 2021, 76, 1943–1944. [Google Scholar] [CrossRef]
- Català, A.; Muñoz-Santos, C.; Galván-Casas, C.; Roncero Riesco, M.; Revilla Nebreda, D.; Solá-Truyols, A.; Giavedoni, P.; Llamas-Velasco, M.; González-Cruz, C.; Cubiró, X.; et al. Cutaneous reactions after SARS-COV-2 vaccination: A cross-sectional Spanish nationwide study of 405 cases. Br. J. Dermatol. 2021. [Google Scholar] [CrossRef]
- Niebel, D.; Wilhelmi, J.; de Vos, L.; Ziob, J.; Jaschke, K.; Bieber, T.; Wenzel, J.; Braegelmann, C. Onset of Rowell’s syndrome in course of covid-19 mRNA vaccines—An overlooked phenomenon? J. Dermatol. 2021. under review. [Google Scholar]
- Eyerich, K.; Eyerich, S. Immune response patterns in non-communicable inflammatory skin diseases. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 692–703. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]
- Sprent, J.; King, C. COVID-19 vaccine side effects: The positives about feeling bad. Sci. Immunol. 2021, 6. [Google Scholar] [CrossRef]
- Rutkowski, K.; Mirakian, R.; Till, S.; Rutkowski, R.; Wagner, A. Adverse reactions to COVID-19 vaccines: A practical approach. Clin. Exp. Allergy 2021, 51, 770–777. [Google Scholar] [CrossRef]
- Cohen, S.R.; Prussick, L.; Kahn, J.S.; Gao, D.X.; Radfar, A.; Rosmarin, D. Leukocytoclastic vasculitis flare following the COVID-19 vaccine. Int. J. Dermatol. 2021. [Google Scholar] [CrossRef]
- Alpalhão, M.; Maia-Silva, J.; Filipe, P. Severe Acute Respiratory Syndrome Coronavirus 2 Vaccines and Cutaneous Adverse Reactions: A Review. Dermatitis 2021, 32, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J. Cutaneous lupus erythematosus: New insights into pathogenesis and therapeutic strategies. Nat. Rev. Rheumatol. 2019, 15, 519–532. [Google Scholar] [CrossRef]
- De Mattos, A.B.N.; Garbo Baroni, L.; Zanotto, L.d.L.; Furian, M.E.A. Subacute cutaneous lupus erythematosus triggered after measles vaccination. Lupus 2021, 30, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Mintoff, D.; Betts, A.; Boffa, M.J. Influenza vaccine-associated cutaneous lupus erythematosus. Clin. Exp. Dermatol. 2020, 45, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Mori, T.; Yamamoto, T. Neonatal lupus erythematosus exacerbated by vaccination. J. Dermatol. 2020, 47, 1450–1453. [Google Scholar] [CrossRef]
- Gambichler, T.; Scholl, L.; Dickel, H.; Ocker, L.; Stranzenbach, R. Prompt onset of Rowell’s syndrome following the first BNT162b2 SARS-CoV-2 vaccination. J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef]
- Song, E.J.; Wong, A.J.S. Widespread annular eruption after Ad26.COV2.S COVID-19 vaccine. JAAD Case Rep. 2021, 13, 30–32. [Google Scholar] [CrossRef]
- Kha, C.; Itkin, A. New-onset chilblains in close temporal association to mRNA-1273 vaccination. JAAD Case Rep. 2021, 12, 12–14. [Google Scholar] [CrossRef]
- Lopez, S.; Vakharia, P.; Vandergriff, T.; Freeman, E.E.; Vasquez, R. Pernio after COVID-19 vaccination. Br. J. Dermatol. 2021. [Google Scholar] [CrossRef]
- Furer, V.; Rondaan, C.; Agmon-Levin, N.; van Assen, S.; Bijl, M.; Kapetanovic, M.C.; de Thurah, A.; Mueller-Ladner, U.; Paran, D.; Schreiber, K.; et al. Point of view on the vaccination against COVID-19 in patients with autoimmune inflammatory rheumatic diseases. RMD Open 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Velikova, T.; Georgiev, T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol. Int. 2021, 41, 509–518. [Google Scholar] [CrossRef]
- Curtis, J.R.; Johnson, S.R.; Anthony, D.D.; Arasaratnam, R.J.; Baden, L.R.; Bass, A.R.; Calabrese, C.; Gravallese, E.M.; Harpaz, R.; Sadun, R.E.; et al. American College of Rheumatology Guidance for COVID-19 Vaccination in Patients With Rheumatic and Musculoskeletal Diseases: Version 1. Arthritis Rheumatol. 2021. [Google Scholar] [CrossRef]
- Dietrich, L.L.; Bridges, A.J.; Albertini, M.R. Dermatomyositis after interferon alpha treatment. Med. Oncol. 2000, 17, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, D.J.; Theodorou, S.J.; Axiotis, A.; Gianniki, M.; Tsifetaki, N. COVID-19 vaccine-related myositis. QJM 2021. [Google Scholar] [CrossRef] [PubMed]
- Hiltun, I.; Sarriugarte, J.; Martínez-de-Espronceda, I.; Garcés, A.; Llanos, C.; Vives, R.; Yanguas, J.I. Lichen planus arising after COVID-19 vaccination. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e414–e415. [Google Scholar] [CrossRef] [PubMed]
- Jedlowski, P.M.; Jedlowski, M.F. Morbilliform rash after administration of Pfizer-BioNTech COVID-19 mRNA vaccine. Dermatol. Online J. 2021, 27, 13030/qt4xs486zg. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, M.; Henry, D.; Finon, A.; Binois, R.; Esteve, E. Persistent maculopapular rash after the first dose of Pfizer-BioNTech COVID-19 vaccine. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e423–e425. [Google Scholar] [CrossRef]
- Malayala, S.V.; Mohan, G.; Vasireddy, D.; Atluri, P. Purpuric Rash and Thrombocytopenia After the mRNA-1273 (Moderna) COVID-19 Vaccine. Cureus 2021, 13, e14099. [Google Scholar] [CrossRef]
- Frederiksen, M.S.; Brenøe, E.; Trier, J. Erythema multiforme minor following vaccination with paediatric vaccines. Scand. J. Infect. Dis. 2004, 36, 154–155. [Google Scholar] [CrossRef]
- Busto-Leis, J.M.; Servera-Negre, G.; Mayor-Ibarguren, A.; Sendagorta-Cudós, E.; Feito-Rodríguez, M.; Nuño-González, A.; Montero-Vega, M.D.; Herranz-Pinto, P. Pityriasis rosea, COVID-19 and vaccination: New keys to understand an old acquaintance. J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef]
- Corbeddu, M.; Diociaiuti, A.; Vinci, M.R.; Santoro, A.; Camisa, V.; Zaffina, S.; El Hachem, M. Transient cutaneous manifestations after administration of Pfizer-BioNTech COVID-19 Vaccine: An Italian single-centre case series. J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef]
- Leasure, A.C.; Cowper, S.; McNiff, J.; Cohen, J.M. Generalized eczematous reactions to the Pfizer-BioNTech COVID-19 vaccine. J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef]
- Varma, A.; Levitt, J. Dupilumab-induced phenotype switching from atopic dermatitis to psoriasis. JAAD Case Rep. 2020, 6, 217–218. [Google Scholar] [CrossRef] [Green Version]
- Al-Janabi, A.; Foulkes, A.C.; Mason, K.; Smith, C.H.; Griffiths, C.E.M.; Warren, R.B. Phenotypic switch to eczema in patients receiving biologics for plaque psoriasis: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Pfaar, O.; Klimek, L.; Hamelmann, E.; Kleine-Tebbe, J.; Taube, C.; Wagenmann, M.; Werfel, T.; Brehler, R.; Novak, N.; Mülleneisen, N.; et al. COVID-19 vaccination of patients with allergies and type-2 inflammation with concurrent antibody therapy (biologicals)—A Position Paper of the German Society of Allergology and Clinical Immunology (DGAKI) and the German Society for Applied Allergology (AeDA). Allergol. Select 2021, 5, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.M.; Ferree, S.D.; Mesinkovska, N.A.; Kourosh, A.S. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int. J. Womens. Dermatol. 2021, 7, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.L.; Kelso, J.M. “COVID Arm”: Very delayed large injection site reactions to mRNA COVID-19 vaccines. J. Allergy Clin. Immunol. Pract. 2021, 9, 2480–2481. [Google Scholar] [CrossRef]
- Wei, N.; Fishman, M.; Wattenberg, D.; Gordon, M.; Lebwohl, M. “COVID arm”: A reaction to the Moderna vaccine. JAAD Case Rep. 2021, 10, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, H.; Paik, S.S.; Moon, J.-Y.; Yoon, H.J.; Kim, S.-H. Delayed cutaneous reaction to ChAdOx1 nCoV-19 vaccine: Is it an ‘AstraZeneca arm’? J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef]
- Fernandez-Nieto, D.; Hammerle, J.; Fernandez-Escribano, M.; Moreno-Del Real, C.M.; Garcia-Abellas, P.; Carretero-Barrio, I.; Solano-Solares, E.; de-la-Hoz-Caballer, B.; Jimenez-Cauhe, J.; Ortega-Quijano, D.; et al. Skin manifestations of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers. ‘COVID-arm’: A clinical and histological characterization. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e425–e427. [Google Scholar] [CrossRef]
- Johnston, M.S.; Galan, A.; Watsky, K.L.; Little, A.J. Delayed Localized Hypersensitivity Reactions to the Moderna COVID-19 Vaccine: A Case Series. JAMA Dermatol. 2021, 157, 716–720. [Google Scholar] [CrossRef]
- Samarakoon, U.; Alvarez-Arango, S.; Blumenthal, K.G. Delayed Large Local Reactions to mRNA Covid-19 Vaccines in Blacks, Indigenous Persons, and People of Color. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, L.S.; McFadden, J.P.; Basketter, D.A.; Ferguson, F.J.; White, I.R.; Kimber, I. Harnessing co-operative immune augmentation by contact allergens to enhance the efficacy of viral vaccines. Contact Dermatitis 2020, 83, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Solimani, F.; Mansour, Y.; Didona, D.; Dilling, A.; Ghoreschi, K.; Meier, K. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, P.K.; Matusiak, Ł.; Szepietowski, J.C. Psoriasis flare-up associated with second dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine. J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef]
- Simonetti, O.; Rizzetto, G.; Molinelli, E.; Diotallevi, F.; Radi, G.; Cirioni, O.; D’Errico, M.M.; Offidani, A. Safety and Efficacy of Vaccines during COVID-19 Pandemic in Patients Treated with Biological Drugs in a Dermatological Setting. Healthcare 2021, 9, 401. [Google Scholar] [CrossRef]
- Gisondi, P.; Geat, D.; Naldi, L.; Piaserico, S. Insights into Sars-CoV-2 vaccination in patients with chronic plaque psoriasis on systemic treatments. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e361–e362. [Google Scholar] [CrossRef]
- Pacifico, A.; d’Arino, A.; Pigatto, P.D.M.; Malagoli, P.; Young, D.I.N.; Damiani, G. COVID-19 vaccines do not trigger psoriasis flares in patients with psoriasis treated with apremilast. Clin. Exp. Dermatol. 2021. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Armstrong, A.W.; Bell, S.; Anesi, G.L.; Blauvelt, A.; Calabrese, C.; Dommasch, E.D.; Feldman, S.R.; Gladman, D.; Kircik, L.; et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: Version 2-Advances in psoriatic disease management, COVID-19 vaccines, and COVID-19 treatments. J. Am. Acad. Dermatol. 2021, 84, 1254–1268. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Park, S.-Y.; Kim, J.-H.; Lee, S.M.; Lee, S.P. COVID-19 vaccine-induced acute generalized exanthematous pustulosis. Korean J. Intern. Med. 2021. [Google Scholar] [CrossRef]
- Onsun, N.; Kaya, G.; Işık, B.G.; Güneş, B. A generalized pustular psoriasis flare after CoronaVac COVID-19 vaccination: Case report. Health Promot. Perspect. 2021, 11, 261–262. [Google Scholar] [CrossRef]
- Lospinoso, K.; Nichols, C.S.; Malachowski, S.J.; Mochel, M.C.; Nutan, F. A case of severe cutaneous adverse reaction following administration of the Janssen Ad26.COV2.S COVID-19 vaccine. JAAD Case Rep. 2021, 13, 134–137. [Google Scholar] [CrossRef]
- Jovanović, M.; Poljacki, M.; Vujanović, L.; Duran, V. Acute febrile neutrophilic dermatosis (Sweet’s syndrome) after influenza vaccination. J. Am. Acad. Dermatol. 2005, 52, 367–369. [Google Scholar] [CrossRef]
- Darrigade, A.-S.; Théophile, H.; Sanchez-Pena, P.; Milpied, B.; Colbert, M.; Pedeboscq, S.; Pistone, T.; Jullié, M.-L.; Seneschal, J. Sweet syndrome induced by SARS-CoV-2 Pfizer-BioNTech mRNA vaccine. Allergy 2021. [Google Scholar] [CrossRef] [PubMed]
- Munavalli, G.G.; Guthridge, R.; Knutsen-Larson, S.; Brodsky, A.; Matthew, E.; Landau, M. “COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: A challenging clinical conundrum in diagnosis and treatment”. Arch. Dermatol. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lopatynsky-Reyes, E.Z.; Acosta-Lazo, H.; Ulloa-Gutierrez, R.; Ávila-Aguero, M.L.; Chacon-Cruz, E. BCG Scar Local Skin Inflammation as a Novel Reaction Following mRNA COVID-19 Vaccines in Two International Healthcare Workers. Cureus 2021, 13, e14453. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.; Grezard, P.; Berard, F.; Clavel, G.; Perrot, H. Generalized granuloma annulare and hepatitis B vaccination. Eur. J. Dermatol. 1998, 8, 435–436. [Google Scholar]
- Osborne, G.E.N.; Mallon, E.; Mayou, S.C. Juvenile sarcoidosis after BCG vaccination. J. Am. Acad. Dermatol. 2003, 48, S99–S102. [Google Scholar] [CrossRef]
- Torrelo, A.; Suárez, J.; Colmenero, I.; Azorín, D.; Perera, A.; Zambrano, A. Deep morphea after vaccination in two young children. Pediatr. Dermatol. 2006, 23, 484–487. [Google Scholar] [CrossRef]
- Manansala, M.; Chopra, A.; Baughman, R.P.; Novak, R.; Lower, E.E.; Culver, D.A.; Korsten, P.; Drake, W.P.; Judson, M.A.; Sweiss, N. COVID-19 and Sarcoidosis, Readiness for Vaccination: Challenges and Opportunities. Front. Med. 2021, 8, 672028. [Google Scholar] [CrossRef]
- Fischinger, S.; Boudreau, C.M.; Butler, A.L.; Streeck, H.; Alter, G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 2019, 41, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Cebeci, F.; Kartal, İ. Petechial skin rash associated with CoronaVac vaccination: First cutaneous side effect report before phase 3 results. Eur. J. Hosp. Pharm. 2021. [Google Scholar] [CrossRef]
- Akdaş, E.; Öğüt, B.; Erdem, Ö.; Öztaş, M.O.; İlter, N. Cutaneous reactions following CoronaVac COVID-19 vaccination: A case series of six healthcare workers from a single center. J. Eur. Acad. Dermatol. Venereol. 2021. [Google Scholar] [CrossRef]
- Sun, Q.; Fathy, R.; McMahon, D.E.; Freeman, E.E. COVID-19 Vaccines and the Skin: The landscape of cutaneous vaccine reactions worldwide. Dermatol. Clin. 2021. [Google Scholar] [CrossRef]
- Schattner, A. Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine 2005, 23, 3876–3886. [Google Scholar] [CrossRef]
- Akinosoglou, K.; Tzivaki, I.; Marangos, M. Covid-19 vaccine and autoimmunity: Awakening the sleeping dragon. Clin. Immunol. 2021, 226, 108721. [Google Scholar] [CrossRef]
- Pulsipher, K.J.; Presley, C.L.; Waller, J.D.; Szeto, M.D.; Laughter, M.R.; Dellavalle, R.P. Coronavirus Vaccination Adverse Reactions and the Role of the Dermatologist. J. Drugs Dermatol. 2021, 20, 351–352. [Google Scholar] [CrossRef]
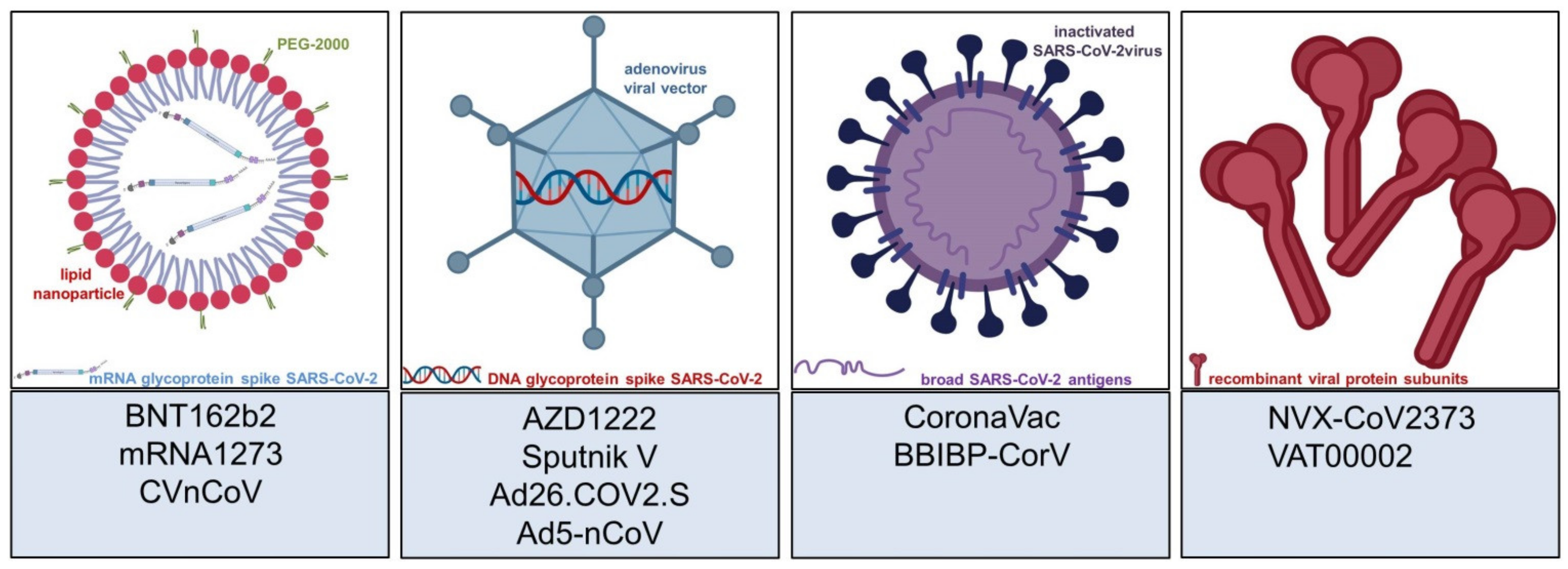
| Vaccine (Developing Institution) | Vaccine Type | First Approval | General ADRs | Cutaneous ADRs |
|---|---|---|---|---|
| BNT162b2 Tozinameran, Comirnaty (BioNTech/Pfizer) | mRNA | December 2020 in UK | >10%: Fatigue, headache, musculoskeletal pain, fever [4] | 1–10%: Local injection site reaction: erythema, swelling; <1%: delayed local reactions (“COVID-arm”), morbilliform rash, urticarial reactions, pityriasis rosea; singular cases: Rowell’s syndrome, lichen planus |
| mRNA-1273 Spikevax (Moderna) | mRNA | December 2020 in USA | >10%: Fever, headache, fatigue, myalgia, arthralgia, nausea, chills [5] | 1–10%: Local injection site reaction: erythema, swelling; <1%: delayed local reactions (“COVID-arm”), morbilliform rash, urticarial reactions, pityriasis rosea, erythema multiforme, erythromelalgia, herpes simplex, herpes zoster, perniones/chilblains; singular cases: reactions to cosmetic fillers, purpuric/petechial rash |
| AZD1222/ChAdOx1 nCoV-19 Vaxzevria, Covishield (AstraZeneca) | Viral vector vaccine (adenovirus) | December 2020 in UK | >10%: Fatigue, nausea, musculoskeletal pain, headache, subfebrile temperatures [6] | 1–10%: Local injection site reaction: erythema, swelling; <1%: itch, rash, sweating; singular cases: psoriasis, rosacea, vitiligo, Raynaud’s phenomenon, cellulitis, pityriasis rosea, delayed large local reactions |
| Gam-COVID-Vac/Sputnik V (Gamaleya Research Institute) | Viral vector vaccine (adenovirus) | August 2020 in Russia | 1–10%: Flu-like illness, headache, asthenia [7] | 1–10%: Local injection site reactions, not further specified; <1%: indeterminate rash, petechial rash, “allergic rash”, itching, eczema/dermatitis; singular cases: abscess, alopecia, acneiform dermatitis |
| Ad26.COV2.S/JNJ-78436735 COVID-19 Vaccine Janssen (Johnson & Johnson) | Viral vector vaccine (adenovirus) | February 2021 in USA | >10%: fatigue, headache, myalgia, nausea, pyrexia [8] | 1–10%: Local injection site reaction: erythema, swelling; singular cases: widespread annular eruption, DRESS-syndrome |
| Ad5-nCoV Convidecia (CanSinoBIO) | Viral vector vaccine (adenovirus) | February 2021 in China | >10%: fatigue, fever, headache, muscle pain, joint pain [9] | 1–10%: Local injection site reaction: redness, swelling, itch; singular cases: non-infective gingivitis, buccal ulcerations, herpes simplex |
| CoronaVac (Sinovac) | Inactivated whole virus (aluminum adjuvant) | February 2021 in China | 1–10%: fatigue, diarrhea, fever, muscle pain, headache, nausea, cough [10] | 1–10%: Local injection site reaction: swelling, redness, pruritus, discoloration, induration; <1%: urticaria, petechial rash, flare of pustular psoriasis |
| BBIBP-CorV (Sinopharm) | Inactivated whole virus (aluminum adjuvant) | December 2020 in China | 1–10%: fever, fatigue, inappetence, nausea, constipation, headache [11] | 1–10%: Local injection site reaction: erythema, swelling, induration, “mucocutaneous abnormalities”; <1%: “rash”, itch, herpes simplex, buccal ulcer |
| NVX-CoV2373 (Novavax) | Recombinant protein subunit (saponin adjuvant) | Not yet approved | >10%: arthralgia, fatigue, headache, myalgia, nausea, malaise [12] | 1–10%: Local injection site reaction: erythema, induration or swelling |
| CVnCoV Zorecimeran (CureVac) | mRNA | Not yet approved | “Dose dependent effects included fever, headache, fatigue, chills, myalgia, arthralgia, nausea/vomiting, diarrhea” [13] | 1–10% local injection site reaction: swelling and itching (preliminary data) |
| VAT00002 Sanofi–GSK COVID-19 vaccine (Sanofi/ GlaxoSmithKline) | Recombinant protein subunit (AS03 adjuvant) | Not yet approved | No data available, yet (NCT04762680) Phase III trial launched in May 2021 | No data available, yet |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niebel, D.; Novak, N.; Wilhelmi, J.; Ziob, J.; Wilsmann-Theis, D.; Bieber, T.; Wenzel, J.; Braegelmann, C. Cutaneous Adverse Reactions to COVID-19 Vaccines: Insights from an Immuno-Dermatological Perspective. Vaccines 2021, 9, 944. https://doi.org/10.3390/vaccines9090944
Niebel D, Novak N, Wilhelmi J, Ziob J, Wilsmann-Theis D, Bieber T, Wenzel J, Braegelmann C. Cutaneous Adverse Reactions to COVID-19 Vaccines: Insights from an Immuno-Dermatological Perspective. Vaccines. 2021; 9(9):944. https://doi.org/10.3390/vaccines9090944
Chicago/Turabian StyleNiebel, Dennis, Natalija Novak, Jasmin Wilhelmi, Jana Ziob, Dagmar Wilsmann-Theis, Thomas Bieber, Joerg Wenzel, and Christine Braegelmann. 2021. "Cutaneous Adverse Reactions to COVID-19 Vaccines: Insights from an Immuno-Dermatological Perspective" Vaccines 9, no. 9: 944. https://doi.org/10.3390/vaccines9090944
APA StyleNiebel, D., Novak, N., Wilhelmi, J., Ziob, J., Wilsmann-Theis, D., Bieber, T., Wenzel, J., & Braegelmann, C. (2021). Cutaneous Adverse Reactions to COVID-19 Vaccines: Insights from an Immuno-Dermatological Perspective. Vaccines, 9(9), 944. https://doi.org/10.3390/vaccines9090944






