Age and Smoking Predict Antibody Titres at 3 Months after the Second Dose of the BNT162b2 COVID-19 Vaccine
Abstract
:1. Introduction
2. Methods
2.1. Population and Study Design
2.2. Data Analysis
3. Results
3.1. Study Population
3.2. Distribution of Ab Titres against SARS-CoV-2 Spike Antigen 3 Months after Vaccination According to Age and Sex
3.3. Relationship between Ab Titres against SARS-CoV-2 Spike Antigen 3 Months after Vaccination and Risk Factors
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Song, Z.; Xu, Y.; Bao, L.; Zhang, L.; Yu, P.; Qu, Y.; Zhu, H.; Zhao, W.; Han, Y.; Qin, C. From SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses 2019, 11, 59. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Lu, L.; Liu, Q.; Xu, W.; Du, L. Receptor-binding domains of spike proteins of emerging or re-emerging viruses as targets for development of antiviral vaccines. Emerg. Microbes Infect. 2012, 1, e13. [Google Scholar] [CrossRef] [PubMed]
- Dispinseri, S.; Secchi, M.; Pirillo, M.F.; Tolazzi, M.; Borghi, M.; Brigatti, C.; de Angelis, M.L.; Baratella, M.; Bazzigaluppi, E.; Venturi, G.; et al. Neutralizing antibody responses to SARSCoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat. Commun. 2021, 12, 2670. [Google Scholar] [CrossRef]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; Warren, F.; et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N. Engl. J. Med. 2021, 384, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, K.; Jeremiah, S.S.; Kato, H.; Yamaoka, Y.; Go, H.; Yamanaka, T.; Ryo, A. Rapid detection of neutralizing antibodies to SARS-CoV-2 variants in post-vaccination sera. medRxiv 2021. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [Green Version]
- Iyer, A.S.; Jones, F.K.; Nodoushani, A.; Kelly, M.; Becker, M.; Slater, D.; Mills, R.; Teng, E.; Kamruzzaman, M.; Garcia-Beltran, W.F.; et al. Dynamics and significance of the antibody response to SARS-CoV-2 infection. medRxiv 2020. [Google Scholar] [CrossRef]
- Chen, R.E.; Zhang, X.; Case, J.B.; Winkler, E.S.; Liu, Y.; VanBlargan, L.A.; Liu, J.; Errico, J.M.; Xie, X.; Suryadevara, N.; et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat. Med. 2021, 27, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Ikeda, K.; Tanaka, S.; Taniguchi, T.; Igari, H.; Onouchi, Y.; Kaneda, A.; Matsushita, K.; Hanaoka, H.; Nakada, T.; et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine in 2015 healthcare workers in a single tertiary referral hospital in Japan. medRxiv 2021. [Google Scholar] [CrossRef]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Terpos, E.; Trougakos, I.P.; Apostolakou, F.; Charitaki, I.; Sklirou, A.D.; Mavrianou, N.; Papanagnou, E.-D.; Liacos, C.-I.; Gumeni, S.; Rentziou, G.; et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am. J. Hematol. 2021, 96, E257–E259. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.A.; Lin, S.; Goodhand, J.R.; Chanchlani, N.; Hamilton, B.; Bewshea, C.; Nice, R.; Chee, D.; Cummings, J.F.; Fraser, A.; et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021, 70, 1884–1893. [Google Scholar] [CrossRef]
- Wei, J.; Stoesser, N.; Matthews, P.C.; Ayoubkhani, D.; Studley, R.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. COVID-19 Infection Survey team. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat. Microbiol. 2021, 6, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. How obesity could create problems for a COVID vaccine. Nature 2020, 586, 488–489. [Google Scholar] [CrossRef]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2021, e3465. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; Elmer, A.; Kingston, N.; et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021, 596. [Google Scholar] [CrossRef]
- Monin-Aldama, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; del Molino del Barrio, I.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Cooper, J.; et al. Interim results of the safety and immune-efficacy of 1 versus 2 doses of COVID-19 vaccine BNT162b2 for cancer patients in the context of the UK vaccine priority guidelines. medRxiv 2021. [Google Scholar] [CrossRef]
- Perkmann, T.; Perkmann-Nagele, N.; Breyer, M.K.; Breyer-Kohansal, R.; Burghuber, O.C.; Hartl, S.; Aletaha, D.; Sieghart, D.; Quehenberger, P.; Marculescu, R.; et al. Side-by-side comparison of three fully automated SARS-CoV-2 antibody assays with a focus on specificity. Clin. Chem. 2020, 66, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Callegaro, A.; Borleri, D.; Farina, C.; Napolitano, G.; Valenti, D.; Rizzi, M.; Maggiolo, F. Antibody response to SARS-CoV-2 vaccination is extremely vivacious in subjects with previous SARS-CoV-2 infection. J. Med. Virol. 2021, 93, 4612–4615. [Google Scholar] [CrossRef] [PubMed]
- Pfizer. Pfizer and BioNTech Confirm High Efficacy and No Serious Safety Concerns Through Up to Six Months Following Second Dose in Updated Topline Analysis of Landmark COVID-19 Vaccine Study. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-confirm-high-efficacy-and-no-serious (accessed on 15 June 2021).
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, J.S.; MacKenzie, I.H.; Holt, P.G. The effect of cigarette smoking on susceptibility to epidemic influenza and on serological responses to live attenuated and killed subunit influenza vaccines. J. Hyg. 1976, 77, 409–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, P.; Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
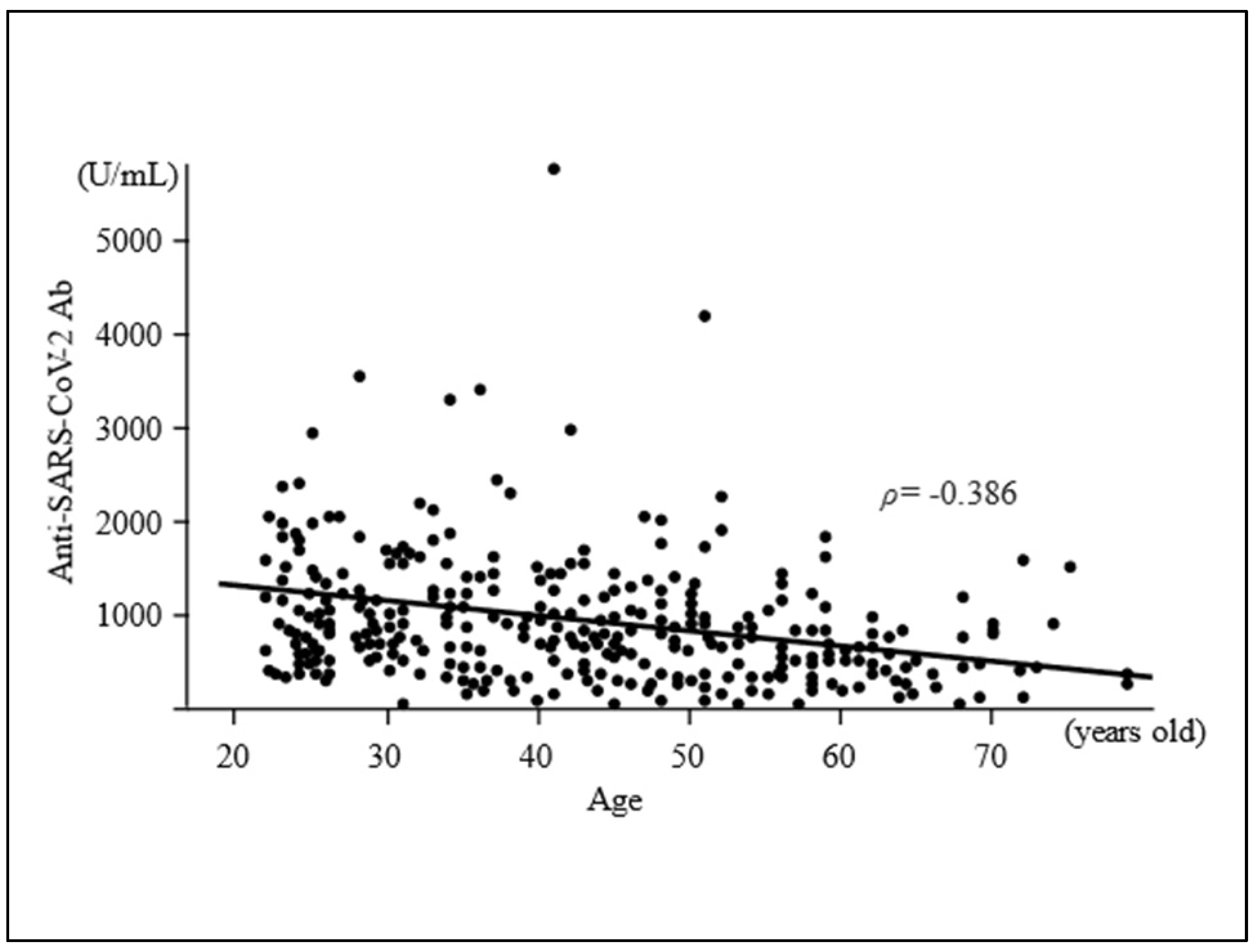

| Variable | Total | Antibody Titre, Median (IQR), U/mL | Correlation Coefficient ρ | p-Value |
|---|---|---|---|---|
| Age, median (IQR), y | 44 (32–54) | −0.386 | <0.0001 # | |
| Sex (Male/female), n | 123/255 | 652 (388–1025)/825 (458–1210) | 0.0193 * | |
| Body mass index, median (IQR), kg/m2 | 22.4 (20.3–24.8) | −0.007 | 0.8975 # | |
| Occupation, n (%) | ||||
| Physician | 38 (10.1) | 690 (550–952) | 0.1669 (vs. Nurse) * | |
| Nurse | 177 (46.8) | 875 (500–1240) | 0.1015 (vs. Clerical) * | |
| Clerical work | 34 (9.0) | 669 (424–1033) | 0.7181 (vs. Physician) * | |
| Pharmacist | 10 (2.6) | |||
| Clinical laboratory technician | 7 (1.9) | |||
| Radiologist | 7 (1.9) | |||
| Rehabilitation staff | 18 (4.8) | |||
| Caregiver | 11 (2.9) | |||
| Others | 76 (20.1) | |||
| Smoking (ever/never), n | 154/224 | 528 (320–910)/919 (587–1373) | <0.0001 * | |
| Brinkman index, n | 134 §§§ | −0.237 | 0.0062 # | |
| Drinking, n | 235/140/3 § | 758 (418–1175)/823 (471–1133) §§ | 0.7244 * | |
| Allergy, n | ||||
| Food Drug | 40/302/36 § 38/303/37 § | 745 (374–1208)/757 (435–1098) §§ 757 (439–1103)/758 (434–1130) §§ | 0.8223 * 0.7988 * | |
| Allergic disease, n | ||||
| Allergic rhinitis including pollinosis Bronchial asthma Skin allergy including atopic dermatitis | 178/176/24 § 46/308/24 § 49/305/24 § | 780 (385–1138)/696 (446–1103) §§ 876 (449–1135)/742 (418–1133) §§ 924 (582–1410)/733 (415–1080) §§ | 0.7839 * 0.5095 * 0.0413 * | |
| Diabetes mellitus, n | 12/353/13 § | 382 (211–741)/768 (436–1150) §§ | 0.0189 * | |
| Hypertension, n | 27/338/13 § | 521 (285–869)/777 (443–1158) §§ | 0.0120 * | |
| Dyslipidaemia, n | 18/347/13 § | 569 (340–878)/768 (434–1155) §§ | 0.0741 * | |
| Collagen disease, n | 13/345/20 § | 548 (256–1030)/766 (435–1140) §§ | 0.3170 * |
| Variable | Total | Antibody Titre, Median (IQR), U/mL | Correlation Coefficient ρ | p-Value |
|---|---|---|---|---|
| Male/female | 123/255 | −69 (−373 to 216)/55 (−266 to 426) | 0.0170 * | |
| Occupation, n (%) | ||||
| Physician Nurse Clerical work | 38 (10.1) 177 (46.8) 34 (9.0) | 31 (−263 to 144) 46 (−335 to 440) −91 (−309 to 301) | 0.6069 (vs. Nurse) * 0.5018 (vs. Clerical) * 0.7479 (vs. Physician) * | |
| Smoking (ever/never), n | 154/224 | −174 (−378 to 145)/90 (−174 to 512) | <0.0001 * | |
| Brinkman index | 134 §§§ | −0.003 | 0.97010 # | |
| Drinking, n | 235/140/3 § | −18 (−329 to 371)/32 (−267 to 318) §§ | 0.4955 * | |
| Allergy, n | ||||
| Food Drug | 40/302/36 § 38/303/37 § | 36 (−393 to 357)/−7 (−296 to 319) §§ 114 (−165 to 317)/−15 (−309 to 320) §§ | 0.7336 * 0.3018 * | |
| Allergic disease, n | ||||
| Allergic rhinitis including pollinosis Bronchial asthma Skin allergy including atopic dermatitis | 178/176/24 § 46/308/24 § 49/305/24 § | 10 (−334 to 331)/−19 (−289 to 320) §§ 93 (−324 to 329)/−23 (−303 to 321) §§ 129 (−257 to 522)/−28 (−308 to 299) §§ | 0.8541 * 0.6102 * 0.0826 * | |
| Diabetes mellitus, n | 12/353/13 § | −169 (−315 to 82)/0 (−300 to 342) §§ | 0.3105 * | |
| Hypertension, n | 27/338/13 § | −88 (−361 to 284)/0 (−286 to 338) §§ | 0.3771 * | |
| Dyslipidaemia, n | 18/347/13 § | 24 (−280 to 114)/−3 (−300 to 340) §§ | 0.7484 * | |
| Collagen disease, n | 13/345/20 § | −90 (−516 to 252)/0 (−299 to 334) §§ | 0.3085 * |
| Male, Antibody Titre, Median (IQR), U/mL | Female, Antibody Titre, Median (IQR), U/mL | p-Value between Sexes | |
|---|---|---|---|
| Ever smokers (men, 75; women, 79) | −246 (−398 to 65) | −140 (−304 to 217) | 0.1175 * |
| Never smokers (men, 48; women, 176) | 49 (−186 to 621) | 95 (−151 to 503) | 0.9970 * |
| p-value between ever and never smokers | 0.0007 * | 0.0023 * |
| Antibody Titre, Median (IQR), U/mL | p-Value (vs. Never Smokers) | |
|---|---|---|
| Current smokers (n = 49) | −271 (−475 to 33) | <0.0001 * |
| Ex-smokers (n = 91) | −162 (−332 to 285) | 0.0019 * |
| P-value (current vs. ex-smokers) | 0.0188 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, Y.; Sawahata, M.; Nakamura, Y.; Kurihara, M.; Koike, R.; Katsube, O.; Hagiwara, K.; Niho, S.; Masuda, N.; Tanaka, T.; et al. Age and Smoking Predict Antibody Titres at 3 Months after the Second Dose of the BNT162b2 COVID-19 Vaccine. Vaccines 2021, 9, 1042. https://doi.org/10.3390/vaccines9091042
Nomura Y, Sawahata M, Nakamura Y, Kurihara M, Koike R, Katsube O, Hagiwara K, Niho S, Masuda N, Tanaka T, et al. Age and Smoking Predict Antibody Titres at 3 Months after the Second Dose of the BNT162b2 COVID-19 Vaccine. Vaccines. 2021; 9(9):1042. https://doi.org/10.3390/vaccines9091042
Chicago/Turabian StyleNomura, Yushi, Michiru Sawahata, Yosikazu Nakamura, Momoko Kurihara, Ryousuke Koike, Otohiro Katsube, Koichi Hagiwara, Seiji Niho, Norihiro Masuda, Takaaki Tanaka, and et al. 2021. "Age and Smoking Predict Antibody Titres at 3 Months after the Second Dose of the BNT162b2 COVID-19 Vaccine" Vaccines 9, no. 9: 1042. https://doi.org/10.3390/vaccines9091042






