A Novel Approach against Salmonella: A Review of Polymeric Nanoparticle Vaccines for Broilers and Layers
Abstract
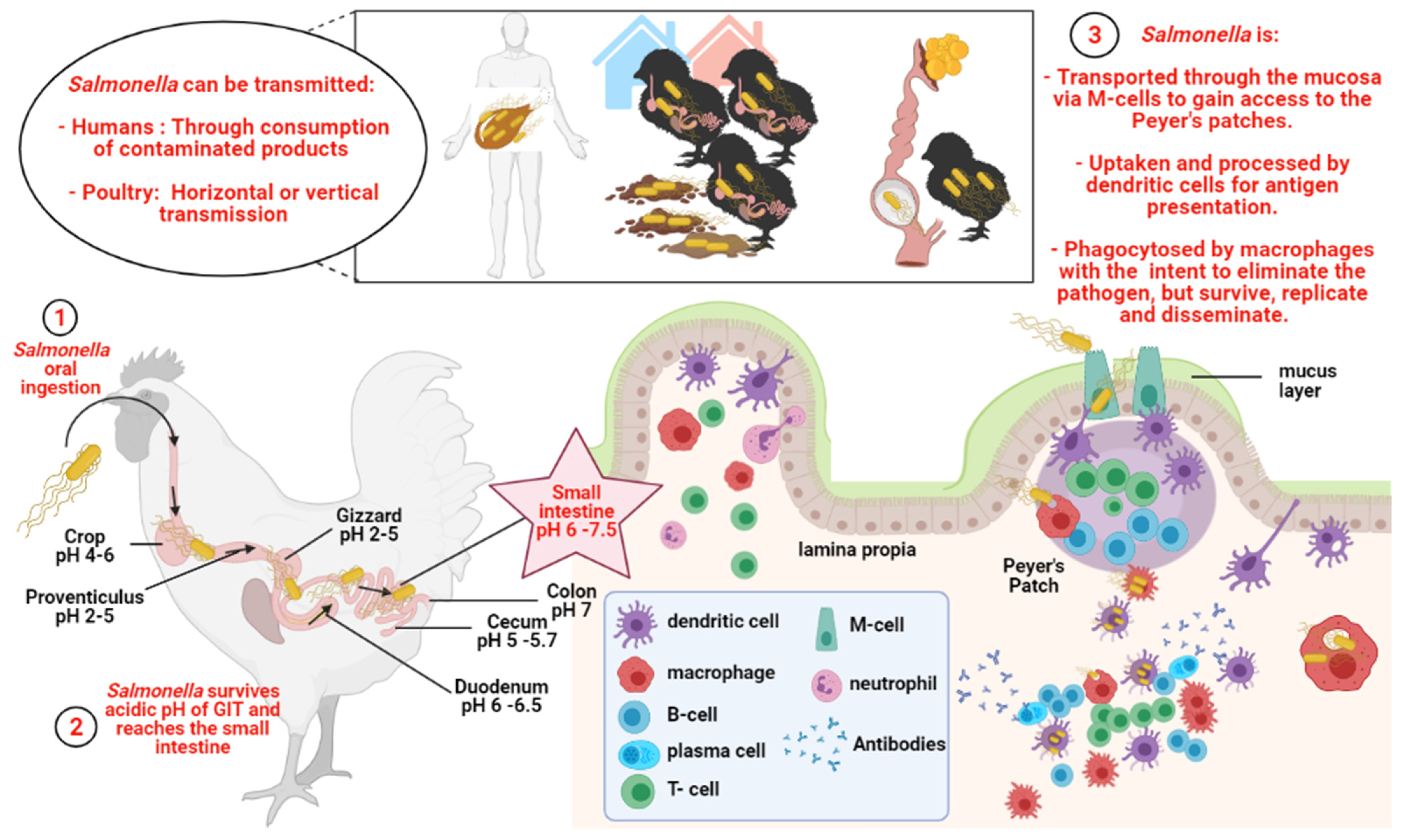
:1. Salmonella
1.1. A Potential Health-Risk Source for Humans
1.2. Salmonella and Poultry
2. Immune Response against Salmonella in Chickens
2.1. Innate Immunity‒Heterophils
2.2. Cellular Immunity‒T-Helper Cells, T-Regulatory Cells, and Th 17 Cells
2.3. Cytokines‒Th1 Proinflammatory and Th2 Anti-Inflammatory Cytokines
2.4. Humoral Immunity‒B-Cells and Antibody Production
3. Mucosal Vaccine Immune Response
3.1. Mucosal Vaccination Practicality for Poultry
3.2. Gastrointestinal Tract Challenges
3.2.1. pH of the GIT—The Physiochemical Barrier
3.2.2. Mucosal Barrier of the GIT—A Surprising Ally
3.2.3. Microfold Cells—The Sentinels of the Intestinal Epithelium
3.2.4. Killed Mucosal Vaccine Antigens and Antigen Presenting Cells
3.2.5. Killed Mucosal Vaccines and Immunoglobulin A
4. Vaccines against Salmonella
4.1. Live vs. Killed Salmonella Vaccines for Poultry
4.2. Commercially Available Vaccines for Salmonella in Poultry
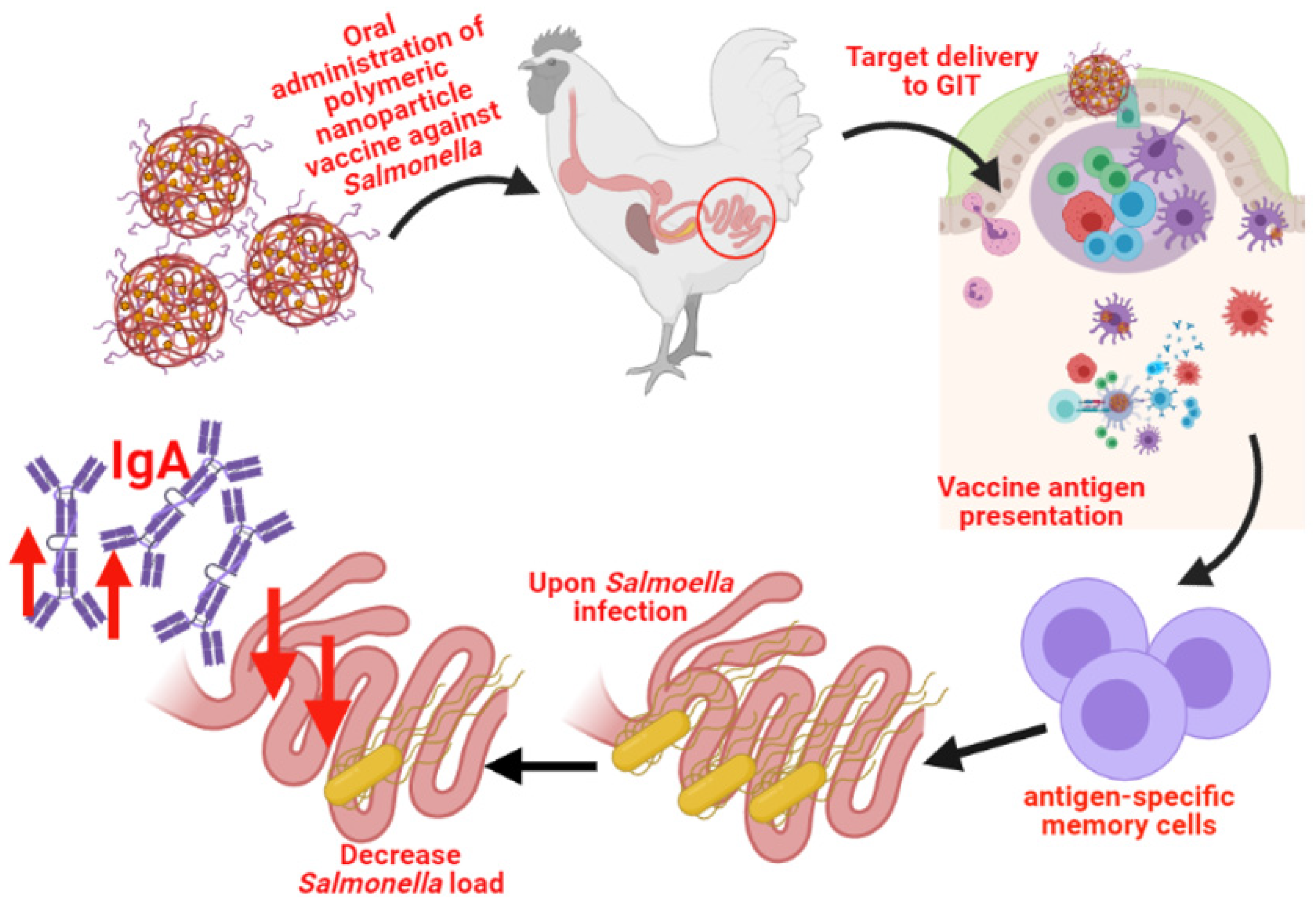
5. A Novel Approach to Poultry Vaccination: Nanoparticles Vaccines against Salmonella for Use in Broilers and Layers
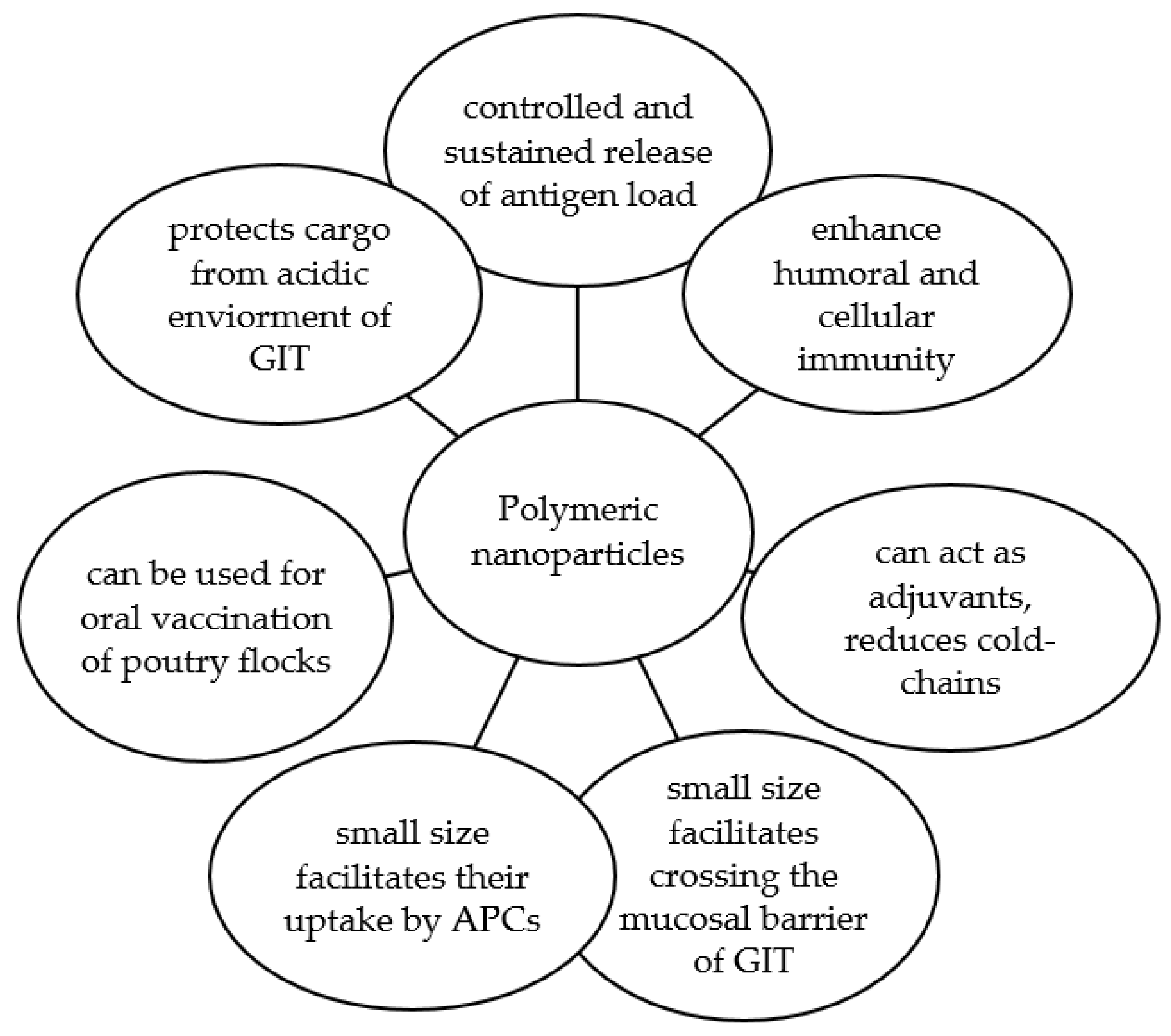
5.1. Nanoparticles
5.2. Nanoparticle Vaccines against Salmonella
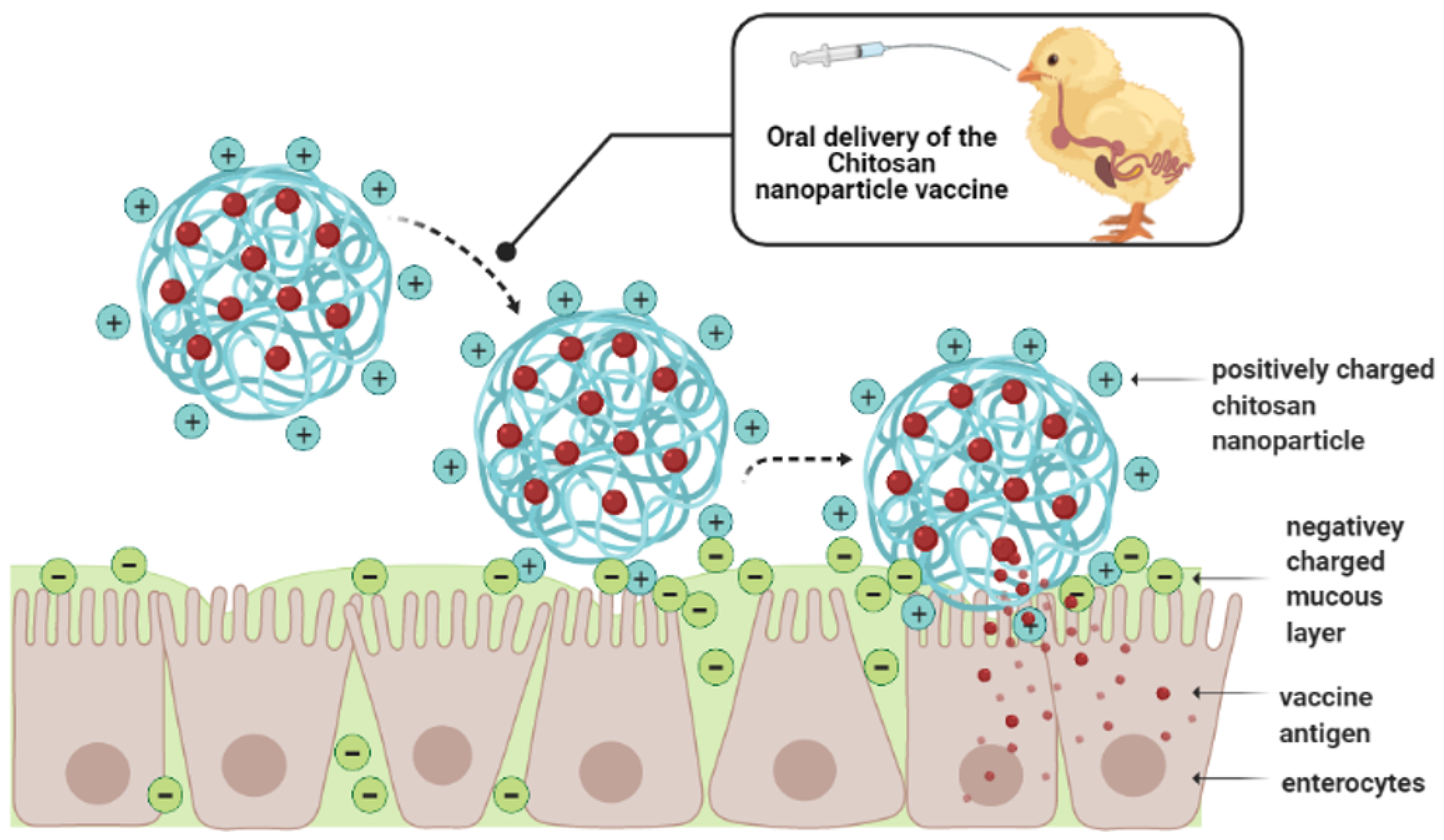
5.3. Chitosan-Based Nanoparticles
Salmonella Chitosan Nanoparticle Vaccines in Chickens
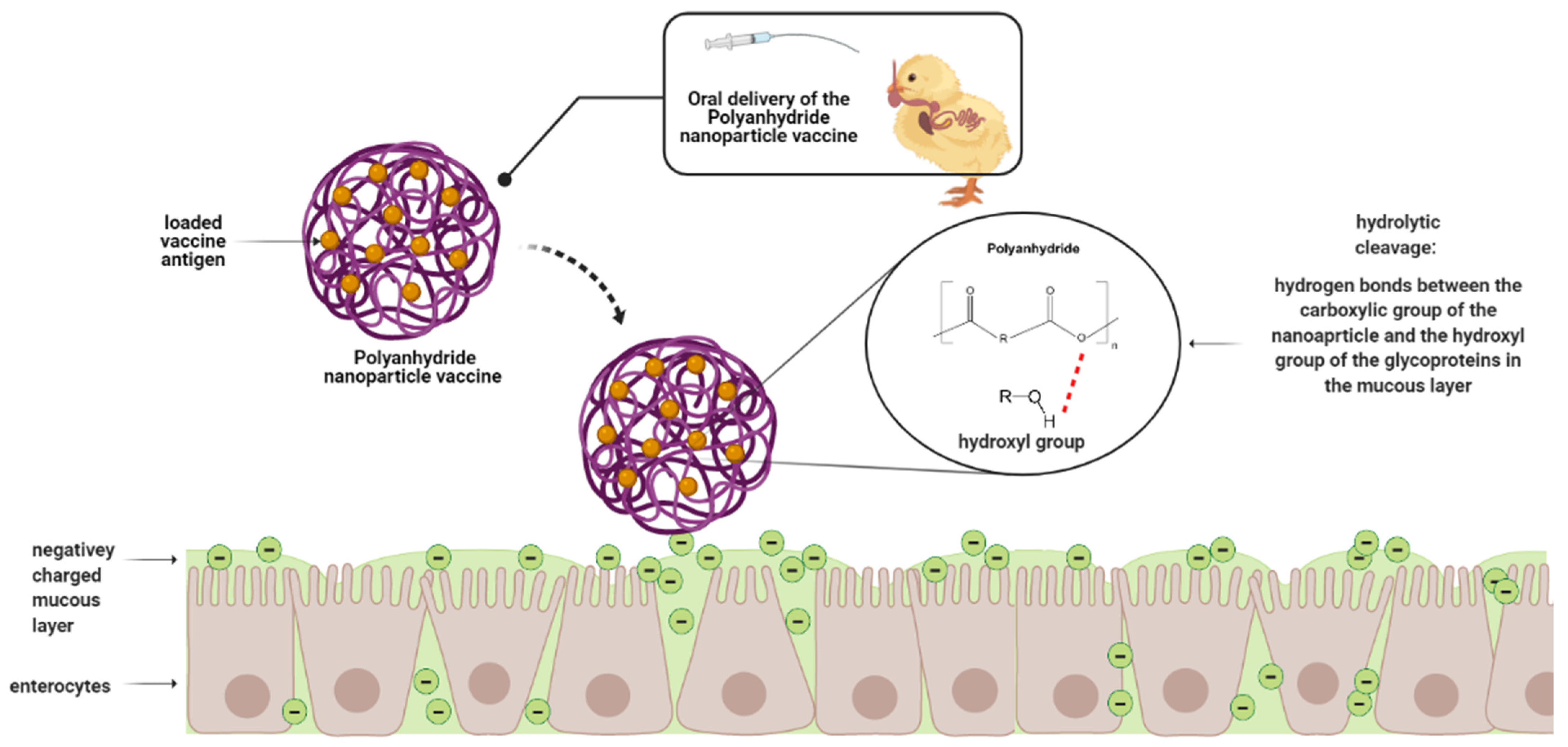
5.4. Polyanhydride-Based Nanoparticles
Salmonella Polyanhydride Nanoparticle Vaccines in Chickens
6. Conclusions and Recommendations: Future of Polymeric Nanoparticles in the Broiler Industry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention 8 Zoonotic Diseases Shared Between Animals and People of Most Concern in the U.S. Available online: https://www.cdc.gov/media/releases/2019/s0506-zoonotic-diseases-shared.html (accessed on 6 August 2021).
- Crum-Cianflone, N.F. Salmonellosis and the gastrointestinal tract: More than just peanut butter. Curr. Gastroenterol. Rep. 2008, 10, 424–431. [Google Scholar] [CrossRef]
- Buddingh, G.J. Bergey’s Manual of Determinative Bacteriology. Am. J. Trop. Med. Hyg. 1975, 24, 550. [Google Scholar] [CrossRef] [Green Version]
- Eswarappa, S.M.; Janice, J.; Balasundaram, S.V.; Dixit, N.M.; Chakravortty, D. Host-specificity of Salmonella enterica serovar Gallinarum: Insights from comparative genomics. Infect. Genet. Evol. 2009, 9, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Salmonella species. In “Bad Bug Book” Handbook of Foodborne Pathogenic Microorganisms and Natural Toxins; Food and Drug Administration: Silver Spring, MD, USA, 2012; Volume 2, pp. 9–13. ISBN 9780323401814. [Google Scholar]
- Kothary, M.H.; Babu, U.S. Infective dose of foodborne pathogens in volunteers: A review. J. Food Saf. 2001, 21, 49–68. [Google Scholar] [CrossRef]
- Kasuga, F.; Hirota, M.; Wada, M.; Yunokawa, T.; Toyofuku, H.; Shibatsuji, M.; Michino, H.; Kuwasaki, T.; Yamamoto, S.; Kumagai, S. Archiving of food samples from restaurants and caterers-Quantitative profiling of outbreaks of foodborne salmonellosis in Japan. J. Food Prot. 2004, 67, 2024–2032. [Google Scholar] [CrossRef]
- Hara-Kudo, Y.; Takatori, K. Contamination level and ingestion dose of foodborne pathogens associated with infections. Epidemiol. Infect. 2011, 139, 1505–1510. [Google Scholar] [CrossRef] [Green Version]
- Klochko, A. What Is the Infectious Dose of Salmonella? Available online: https://www.medscape.com/answers/228174-77482/what-is-the-infectious-dose-of-salmonella (accessed on 9 March 2021).
- Andino, A.; Hanning, I. Salmonella enterica: Survival, colonization, and virulence differences among serovars. Sci. World J. 2015, 2015, 520179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertasi, F.; Filipello, P.; D’Incau, L. Contamination of Poultry Meat with Salmonella infantis should be considered a Risk for Food Safety? Eur. J. Public Health 2019, 22, 2019. [Google Scholar]
- McMillan, E.A.; Wasilenko, J.L.; Tagg, K.A.; Chen, J.C.; Simmons, M.; Gupta, S.K.; Tillman, G.E.; Folster, J.; Jackson, C.R.; Frye, J.G. Carriage and gene content variability of the pesi-like plasmid associated with salmonella infantis recently established in united states poultry production. Genes 2020, 11, 1516. [Google Scholar] [CrossRef] [PubMed]
- van Oort, R. Salmonella control: A global perspective. Poult. World 2021, 1, 18. [Google Scholar]
- Whitworth, J. FSA Renews Chicken Warning; Board Discusses Deadly Salmonella Outbreak. Food Safety News. 2021. Available online: https://www.foodsafetynews.com/2021/03/fsa-renews-chicken-warning-board-discusses-deadly-salmonella-outbreak/ (accessed on 12 April 2021).
- Whitworth, J. Nearly 400 People Sick from Salmonella in UK.; Nearly Half Are Children. Food Safety News. 2020. Available online: https://www.foodsafetynews.com/2020/10/nearly-400-people-sick-from-salmonella-in-uk-nearly-half-are-children/ (accessed on 13 April 2021).
- Scharff, R.L. Food Attribution and Economic Cost Estimates for Meat- andPoultry-Related Illnesses. J. Food Prot. 2020, 83, 959–967. [Google Scholar] [CrossRef]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, food safety and food handling practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Barbut, S. The Science of Poultry and Meat Processing; McLaughlin Library: Guelph, ON, Canada, 2015; ISBN 9781855737273. [Google Scholar]
- Northcutt, J.K.; Russell, S.M. General Guidelines for Implementation of HACCP in a Poultry Processing Plant. Available online: Https://Studylib.Net/Doc/12948473/M-General-Guidelines-for-Implementation-of-Haccp-in-a-Pou (accessed on 21 May 2020).
- Centers for Disease Control and Prevention. Salmonella and Food. Available online: https://www.cdc.gov/foodsafety/communication/salmonella-food.html (accessed on 16 July 2021).
- McDermid, A.S.; Lever, M.S. Survival of Salmonella enteritidis PT4 and Salm. typhimurium Swindon in aerosols. Lett. Appl. Microbiol. 1996, 23, 107–109. [Google Scholar] [CrossRef]
- Spickler, A.R.; Leedom Larson, K.R. Salmonellosis Paratyphoid, Nontyphoidal Salmonellosis. 2013. Available online: http://www.cfsph.iastate.edu/DiseaseInfo/factsheets.php (accessed on 21 May 2021).
- Foley, S.L.; Nayak, R.; Hanning, I.B.; Johnson, T.J.; Han, J.; Ricke, S.C. Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Appl. Environ. Microbiol. 2011, 77, 4273–4279. [Google Scholar] [CrossRef] [Green Version]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Gast, R.; Humphrey, T.J.; Van Immerseel, F. Mechanisms of egg contamination by Salmonella Enteritidis: Review article. FEMS Microbiol. Rev. 2009, 33, 718–738. [Google Scholar] [CrossRef] [Green Version]
- Stern, N.J.; Cox, N.A.; Bailey, J.S.; Berrang, M.E.; Musgrove, M.T. Comparison of mucosal competitive exclusion and competitive exclusion treatment to reduce Salmonella and Campylobacter spp. colonization in broiler chickens. Poult. Sci. 2001, 80, 156–160. [Google Scholar] [CrossRef]
- Tajkarimi, M. Salmonella spp. Calif. Dep. Food Agric. 2007, 1–8. Available online: https://www.cdfa.ca.gov/ahfss/Animal_Health/PHR250/2007/25007Sal.pdf (accessed on 12 April 2021).
- Higginson, E.E.; Simon, R.; Tennant, S.M. Animal models for salmonellosis: Applications in vaccine research. Clin. Vaccine Immunol. 2016, 23, 746–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coburn, B.; Grassl, G.A.; Finlay, B.B. Salmonella, the host and disease: A brief review. Immunol. Cell Biol. 2007, 85, 112–118. [Google Scholar] [CrossRef]
- Buchmeier, N.A.; Heffron, F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect. Immun. 1991, 59, 2232–2238. [Google Scholar] [CrossRef] [Green Version]
- Rappl, C.; Deiwick, J.; Hensel, M. Acidic pH is required for the functional assembly of the type III secretion system encoded by Salmonella pathogenicity island 2. FEMS Microbiol. Lett. 2003, 226, 363–372. [Google Scholar] [CrossRef] [Green Version]
- House, D.; Bishop, A.; Parry, C.; Dougan, G.; Wain, J. Typhoid fever: Pathogenesis and disease. Curr. Opin. Infect. Dis. 2001, 14, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Gal-Mor, O.; Boyle, E.C.; Grassl, G.A. Same species, different diseases: How and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol. 2014, 5, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acheson, D.; Hohmann, E.L. Nontyphoidal Salmonellosis. Clin. Infect. Dis. 2001, 32, 263–269. [Google Scholar] [CrossRef]
- Feasey, N.A.; Dougan, G.; Kingsley, R.A.; Heyderman, R.S.; Gordon, M.A. Invasive non-typhoidal salmonella disease: An emerging and neglected tropical disease in Africa. Lancet 2012, 379, 2489–2499. [Google Scholar] [CrossRef]
- Mandal, B.K.; Brennand, J. Bacteraemia in salmonellosis: A 15 year retrospective study from a regional infectious diseases unit. Br. Med. J. 1988, 297, 1242–1243. [Google Scholar] [CrossRef] [Green Version]
- Kogut, M.H.; Chiang, H.I.; Swaggerty, C.L.; Pevzner, I.Y.; Zhou, H. Gene expression analysis of Toll-like receptor pathways in heterophils from genetic chicken lines that differ in their susceptibility to Salmonella enteritidis. Front. Genet. 2012, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kannan, L.; Liyanage, R.; Lay, J.O.; Rath, N.C. Evaluation of beta defensin 2 production by chicken heterophils using direct MALDI mass spectrometry. Mol. Immunol. 2009, 46, 3151–3156. [Google Scholar] [CrossRef]
- Kogut, M.H.; Rothwell, L.; Kaiser, P. Differential regulation of cytokine gene expression by avian heterophils during receptor-mediated phagocytosis of opsonized and nonopsonized Salmonella enteritidis. J. Interf. Cytokine Res. 2003, 23, 319–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kogut, M.H.; Rothwell, L.; Kaiser, P. Priming by recombinant chicken interleukin-2 induces selective expression of IL-8 and IL-18 mRNA in chicken heterophils during receptor-mediated phagocytosis of opsonized and nonopsonized Salmonella enterica serovar enteritidis. Mol. Immunol. 2003, 40, 603–610. [Google Scholar] [CrossRef] [Green Version]
- Genovese, K.J.; He, H.; Swaggerty, C.L.; Kogut, M.H. The avian heterophil. Dev. Comp. Immunol. 2013, 41, 334–340. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, A.; Tersteeg-Zijderveld, M.H.G.; Tjeerdsma-van Bokhoven, J.L.M.; Jansman, A.J.M.; Veldhuizen, E.J.A.; Haagsman, H.P. Chicken heterophils are recruited to the site of Salmonella infection and release antibacterial mature Cathelicidin-2 upon stimulation with LPS. Mol. Immunol. 2009, 46, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Iqbal, M.; He, H.; Philbin, V.; Kaiser, P.; Smith, A. Expression and function of Toll-like receptors in chicken heterophils. Dev. Comp. Immunol. 2005, 29, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Rothwell, L.; Kaiser, P. IFN-γ priming of chicken heterophils upregulates the expression of proinflammatory and Th1 cytokine mRNA following receptor-mediated phagocytosis of Salmonella enterica serovar enteritidis. J. Interf. Cytokine Res. 2005, 25, 73–81. [Google Scholar] [CrossRef]
- Higgs, R.; Cormican, P.; Cahalane, S.; Allan, B.; Lloyd, A.T.; Meade, K.; James, T.; Lynn, D.J.; Babiuk, L.A.; O’Farrelly, C. Induction of a novel chicken Toll-like receptor following Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2006, 74, 1692–1698. [Google Scholar] [CrossRef] [Green Version]
- Nerren, J.R.; Kogut, M.H. The selective Dectin-1 agonist, curdlan, induces an oxidative burst response in chicken heterophils and peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 2009, 127, 162–166. [Google Scholar] [CrossRef]
- Swaggerty, C.L.; Pevzner, I.Y.; Lowry, V.K.; Farnell, M.B.; Kogut, M.H. Functional comparison of heterophils isolated from commercial broiler chickens. Avian Pathol. 2003, 32, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Redmond, S.B.; Chuammitri, P.; Andreasen, C.B.; Palić, D.; Lamont, S.J. Chicken heterophils from commercially selected and non-selected genetic lines express cytokines differently after in vitro exposure to Salmonella enteritidis. Vet. Immunol. Immunopathol. 2009, 132, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Swaggerty, C.L.; Ferro, P.J.; Pevzner, I.Y.; Kogut, M.H. Heterophils are associated with resistance to systemic Salmonella enteritidis infections in genetically distinct chicken lines. FEMS Immunol. Med. Microbiol. 2005, 43, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Berndt, A.; Methner, U. Gamma/delta T cell response of chickens after oral administration of attenuated and non-attenuated Salmonella typhimurium strains. Vet. Immunol. Immunopathol. 2001, 78, 143–161. [Google Scholar] [CrossRef]
- Berndt, A.; Pieper, J.; Methner, U. Circulating γδ T cells in response to Salmonella enterica serovar enteritidis exposure in chickens. Infect. Immun. 2006, 74, 3967–3978. [Google Scholar] [CrossRef] [Green Version]
- Withanage, G.S.K.; Sasai, K.; Fukata, T.; Miyamoto, T.; Baba, E.; Lillehoj, H.S. T lymphocytes, B lymphocytes, and macrophages in the ovaries and oviducts of laying hens experimentally infected with salmonella enteritidis. Vet. Immunol. Immunopathol. 1998, 66, 173–184. [Google Scholar] [CrossRef]
- Withanage, G.S.K.; Sasai, K.; Fukata, T.; Miyamoto, T.; Lillehoj, H.S.; Baba, E. Increased lymphocyte subpopulations and macrophages in the ovaries and oviducts of laying hens infected with Salmonella enterica serovar Enteritidis. Avian Pathol. 2003, 32, 583–590. [Google Scholar] [CrossRef]
- Sasai, K.; Yoshimura, K.; Lillehoj, H.S.; Withanage, G.S.K.; Fukata, T.; Baba, E.; Arakawa, A. Analysis of splenic and thymic lymphocyte subpopulations in chickens infected with Salmonella enteritidis. Vet. Immunol. Immunopathol. 1997, 59, 359–367. [Google Scholar] [CrossRef]
- Berndt, A.; Methner, U. B cell macrophage response in chicks after oral administration of Salmonella typhimurium strains. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 235–246. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Kogut, M.H.; Arsenault, R.J.; Swaggerty, C.L.; Cole, K.; Reddish, J.M.; Selvaraj, R.K. Effect of Salmonella infection on cecal tonsil regulatory T cell properties in chickens. Poult. Sci. 2015, 94, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Rahim, S.S.; Khan, N.; Boddupalli, C.S.; Hasnain, S.E.; Mukhopadhyay, S. Interleukin-10 (IL-10) mediated suppression of IL-12 production in RAW 264.7 cells involves c-rel transcription factor. Immunology 2005, 114, 313–321. [Google Scholar] [CrossRef]
- Crhanova, M.; Hradecka, H.; Faldynova, M.; Matulova, M.; Havlickova, H.; Sisak, F.; Rychlik, I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect. Immun. 2011, 79, 2755–2763. [Google Scholar] [CrossRef] [Green Version]
- Withanage, G.S.K.; Wigley, P.; Kaiser, P.; Mastroeni, P.; Brooks, H.; Powers, C.; Beal, R.; Barrow, P.; Maskell, D.; McConnell, I. Cytokine and chemokine responses associated with clearance of a primary Salmonella enterica serovar typhimurium infection in the chicken and in protective immunity to rechallenge. Infect. Immun. 2005, 73, 5173–5182. [Google Scholar] [CrossRef] [Green Version]
- Cheeseman, J.H.; Harris, D.L.H.; Thacker, E.; Wannemuehler, M.J. Avian Immunology, Immunogenetics, and Host Immune Response to Salmonella Enterica Serovar Enteritidis Infection in Chickens; Iowa State University: Ames, IA, USA, 2007; pp. 1–154. [Google Scholar]
- Berndt, A.; Wilhelm, A.; Jugert, C.; Pieper, J.; Sachse, K.; Methner, U. Chicken cecum immune response to Salmonella enterica serovars of different levels of invasiveness. Infect. Immun. 2007, 75, 5993–6007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheeseman, J.H.; Kaiser, M.G.; Ciraci, C.; Kaiser, P.; Lamont, S.J. Breed effect on early cytokine mRNA expression in spleen and cecum of chickens with and without Salmonella enteritidis infection. Dev. Comp. Immunol. 2007, 31, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Chaussé, A.M.; Grépinet, O.; Bottreau, E.; Robert, V.; Hennequet-Antier, C.; Lalmanach, A.C.; Lecardonnel, J.Ô.; Beaumont, C.; Velge, P. Susceptibility to Salmonella carrier-state: A possible Th2 response in susceptible chicks. Vet. Immunol. Immunopathol. 2014, 159, 16–28. [Google Scholar] [CrossRef]
- Männe, C.; Takaya, A.; Yamasaki, Y.; Mursell, M.; Hojyo, S.; Wu, T.Y.; Sarkander, J.; McGrath, M.A.; Cornelis, R.; Hahne, S.; et al. Salmonella SiiE prevents an efficient humoral immune memory by interfering with IgG+ plasma cell persistence in the bone marrow. Proc. Natl. Acad. Sci. USA 2019, 116, 7425–7430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, J.W.; Holt, P.S. Response to Salmonella enteritidis infection by the immunocompromised avian host. Poult. Sci. 1995, 74, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Beal, R.K.; Powers, C.; Davison, T.F.; Barrow, P.A.; Smith, A.L. Clearance of enteric Salmonella enterica serovar typhimurium in chickens is independent of B-cell function. Infect. Immun. 2006, 74, 1442–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas Abul, K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 8th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015; ISBN 8535259724. [Google Scholar]
- Diehl, S.; Anguita, J.; Hoffmeyer, A.; Zapton, T.; Ihle, J.N.; Fikrig, E.; Rincón, M. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity 2000, 13, 805–815. [Google Scholar] [CrossRef] [Green Version]
- Kiyono, H.; Pearay Ogra, J.M. Mucosal Vaccines; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Gong, J.; Zhang, J.; Xu, M.; Zhu, C.; Yu, Y.; Liu, X.; Kelly, P.; Xu, B.; Wang, C. Prevalence and Fimbrial Genotype Distribution of Poultry Salmonella Isolates in China (2006 to 2012). Appl. Environ. Microbiol. 2014, 80, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Revolledo, L.; Ferreira, A.J.P. Current perspectives in avian salmonellosis: Vaccines and immune mechanisms of protection. J. Appl. Poult. Res. 2012, 21, 418–431. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, R. Intestinal health, the key to productivity: The case of organic acids. In Proceedings of the XXVII Convencion American Association of Avian Pathologists-Western Poultry Diseases Conference, St-Hyacinthe, QC, Canada, 8–10 January 2002; Available online: https://www.researchgate.net/publication/313391701_Intestinal_health_the_key_to_productivity_The_case_of_organic_acids (accessed on 16 July 2019).
- Herath, M.; Hosie, S.; Bornstein, J.C.; Franks, A.E.; Hill-Yardin, E.L. The Role of the Gastrointestinal Mucus System in Intestinal Homeostasis: Implications for Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 248. [Google Scholar] [CrossRef]
- Hallstrom, K.; McCormick, B.A. Salmonella interaction with and passage through the intestinal mucosa: Through the lens of the organism. Front. Microbiol. 2011, 2, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldara, M.; Friedlander, R.S.; Kavanaugh, N.L.; Aizenberg, J.; Foster, K.R.; Ribbeck, K. Mucin biopolymers prevent bacterial aggregation by retaining cells in the free-swimming state. Curr. Biol. 2012, 22, 2325–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Kim, K.; Ryu, J.H.; Lee, H. Chitosan-catechol: A polymer with long-lasting mucoadhesive properties. Biomaterials 2015, 52, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Prajakta, K.; Khobragade, P.K.P. Chitosan: A Mucoadhesive Polymer. Available online: https://www.wjpps.com/Wjpps_controller/abstract_id/2983 (accessed on 14 January 2019).
- Renu, S.; Markazi, A.D.; Dhakal, S.; Lakshmanappa, Y.S.; Shanmugasundaram, R.; Selvaraj, R.K.; Renukaradhya, G.J. Oral deliverable mucoadhesive Chitosan-Salmonella subunit nanovaccine for layer chickens. Int. J. Nanomed. 2020, 15, 761. [Google Scholar] [CrossRef] [Green Version]
- Sauls, R.S. Histology, M Cell; Updated 3 July 2020; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Renu, S.; Markazi, D.; Yashavanth, S.; Gourapura, R.; Senapati, S.; Ramesh, K.; Gourapura, J. Surface engineered polyanhydride-based oral Salmonella subunit nanovaccine for poultry. Int. J. Nanomed. 2018, 13, 8195–8215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janeway, C. Immunobiology, 5th ed.; W.B. Saunders Co.: Philadelphia, PA, USA, 2012; ISBN 9781455707072. [Google Scholar]
- Melief, C.J.M. Regulation of cytotoxic T lymphocyte responses by dendritic cells: Peaceful coexistence of cross-priming and direct priming? Eur. J. Immunol. 2003, 33, 2645–2654. [Google Scholar] [CrossRef]
- Pasquale, A.; Preiss, S.; Silva, F.; Garçon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef] [Green Version]
- Neutra, N.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef]
- Berthelot-Hérault, F.; Mompart, F.; Zygmunt, M.S.; Dubray, G.; Duchet-Suchaux, M. Antibody responses in the serum and gut of chicken lines differing in cecal carriage of Salmonella enteritidis. Vet. Immunol. Immunopathol. 2003, 96, 43–52. [Google Scholar] [CrossRef]
- Forbes, S.J.; Eschmann, M.; Mantis, N.J. Inhibition of Salmonella enterica serovar typhimurium motility and entry into epithelial cells by a protective antilipopolysaccharide monoclonal immunoglobulin a antibody. Infect. Immun. 2008, 76, 4137–4144. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jin, L.; Chen, T.; Pirozzi, C.J. The Effects of Secretory IgA in the Mucosal Immune System. Biomed Res. Int. 2020, 2020, 2032057. [Google Scholar] [CrossRef]
- Food and Drug Administration. Prevention of Salmonella enteritidis in shell eggs during production, storage, and transportation. Final rule. Fed. Regist. 2009, 74, 33029–33101. [Google Scholar]
- Marangon, S.; Busani, L. The use of vaccination in poultry production. Rev. Sci. et Tech-Off. Int. des Epizoot. 2006, 26, 265. [Google Scholar] [CrossRef] [Green Version]
- Stewart-Brown, B. Merck Veterinary Manual:Vaccination Programs in Poultry; Merck & Co., Inc.: Kenilworth, NJ, USA, 2019. [Google Scholar]
- Zoetis. POULVAC® ST. Available online: https://www2.zoetisus.com/products/poultry/poulvac-st (accessed on 9 March 2021).
- Zoetis. Effective Salmonella Management: POULVAC® SE. Available online: https://www.zoetisus.com/products/poultry/poulvac-se.aspx (accessed on 9 March 2021).
- Zoetis. POULVAC® SE-ND-IB. Available online: https://www2.zoetisus.com/products/poultry/poulvac-se-nd-ib#:~:text=Poulvac%20%C2%AE%20SE%2DND%2DIB%20is%20an%20inactivated%20multivalent%20vaccine,Newcastle%20disease%20and%20infectious%20bronchitis. (accessed on 9 March 2021).
- Paul-Ehrlich-Institut. Poultry: Salmovac SE. Available online: https://www.pei.de/EN/medicinal-products/veterinary/poultry/poultry-node.html?cms_gts=167062_list%253Dheader_text_sort%252Bdesc (accessed on 9 March 2021).
- Paul-Ehrlich-Institut. Poultry: Zoosaloral H. Available online: https://www.pei.de/EN/medicinal-products/veterinary/poultry/poultry-node.html?cms_gts=167062_list%253Dheader_text_sort%252Bdesc (accessed on 9 March 2021).
- CEVA. Vaccines: LAYERMUNE® SE. Available online: https://www.thepoultrysite.com/focus/ceva/ceva-layermune-se-025ml-for-the-immunisation-of-chickens-against-salmonella-enteritidis-from-ceva-sante-animale (accessed on 9 March 2021).
- CEVA. Vaccines: Cevac Corymune® Range: Broad Spectrum Infectious Coryza and Salmonella Enteritidis (SE) Vaccine. Available online: https://www.ceva-me.com/Products/Poultry/Vaccines (accessed on 9 March 2021).
- ELANCO. AviPro Megan Vac 1. Available online: https://assets-us-01.kc-usercontent.com/2de3931d-61f2-00a3-6635-b7b29fac0245/4c3c7639-58a2-4794-a0d5-1666aa791039/MeganVac_1_in_broilers_Detailer__2_.pdf (accessed on 9 March 2021).
- ELANCO. AviPro Megan Egg (Canada). Available online: https://assets-us-01.kc-usercontent.com/2de3931d-61f2-00a3-6635-b7b29fac0245/a5834a86-9a0c-46af-81d0-e89a735b32bb/Megan_Egg_in_Layers_Detailer.pdf (accessed on 9 March 2021).
- ELANCO. AviPro 329 ND-IB2-SE4. Available online: https://www.elanco.us/poultry-vaccines#:~:text=AviPro%C2%AE%20329%20ND%2DIB2,organs%2C%20including%20the%20reproductive%20tract. (accessed on 9 March 2021).
- Hoft, D.F.; Brusic, V.; Sakala, I.G. Optimizing vaccine development. Cell. Microbiol. 2011, 13, 934–942. [Google Scholar] [CrossRef]
- Acevedo-Villanueva, K.; Renu, S.; Gourapura, R.; Selvaraj, R. Efficacy of a nanoparticle vaccine administered in-ovo against Salmonella in broilers. PLoS ONE 2021, 16, e0247938. [Google Scholar] [CrossRef]
- Renu, S.; Han, Y.; Dhakal, S.; Lakshmanappa, Y.S.; Ghimire, S.; Feliciano-Ruiz, N.; Senapati, S.; Narasimhan, B.; Selvaraj, R.; Renukaradhya, G.J. Chitosan-adjuvanted Salmonella subunit nanoparticle vaccine for poultry delivered through drinking water and feed. Carbohydr. Polym. 2020, 243, 116434. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Villanueva, K.Y.; Lester, B.; Renu, S.; Han, Y.; Shanmugasundaram, R.; Gourapura, R.; Selvaraj, R. Efficacy of chitosan-based nanoparticle vaccine administered to broiler birds challenged with Salmonella. PLoS ONE 2020, 15, 1–17. [Google Scholar] [CrossRef]
- Han, Y.; Renu, S.; Patil, V.; Schrock, J.; Feliciano-Ruiz, N.; Selvaraj, R.; Renukaradhya, G.J. Immune Response to Salmonella Enteritidis Infection in Broilers Immunized Orally With Chitosan-Based Salmonella Subunit Nanoparticle Vaccine. Front. Immunol. 2020, 11, 935. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Renu, S.; Patil, V.; Schrock, J.; Feliciano-Ruiz, N.; Selvaraj, R.; Renukaradhya, G.J. Mannose-modified chitosan-nanoparticle-based salmonella subunit oralvaccine-induced immune response and efficacy in a challenge trial in broilers. Vaccines 2020, 8, 299. [Google Scholar] [CrossRef]
- Han, Y.; Renu, S.; Schrock, J.; Acevedo-Villanuev, K.Y.; Lester, B.; Selvaraj, R.K.; Renukaradhya, G.J. Temporal dynamics of innate and adaptive immune responses in broiler birds to oral delivered chitosan nanoparticle-based Salmonella subunit antigens. Vet. Immunol. Immunopathol. 2020, 228, 110111. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Sebastià, E.; Sitjà, M.; Tamayo, I.; Irache, J.M.; Gamazo, C. Protection conferred by drinking water administration of a nanoparticle-based vaccine against salmonella enteritidis in hens. Vaccines 2021, 9, 216. [Google Scholar] [CrossRef]
- Akerele, G.; Ramadan, N.; Renu, S.; Renukaradhya, G.J.; Shanmugasundaram, R.; Selvaraj, R.K. In vitro characterization and immunogenicity of chitosan nanoparticles loaded with native and inactivated extracellular proteins from a field strain of Clostridium perfringens associated with necrotic enteritis. Vet. Immunol. Immunopathol. 2020, 224, 110059. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-based nanomaterials and applications for vaccines and drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, S.E.; Lickteig, D.J.; Plunkett, K.N.; Ryerse, J.S.; Konjufca, V. The uptake of soluble and particulate antigens by epithelial cells in the mouse small intestine. PLoS ONE 2014, 9, 86656. [Google Scholar] [CrossRef]
- Jackson, A.W.; Fulton, D.A. Making polymeric nanoparticles stimuli-responsive with dynamic covalent bonds. Polym. Chem. 2013, 4, 31–45. [Google Scholar] [CrossRef]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Wang, R.; Nie, G. Applications of nanomaterials as vaccine adjuvants. Hum. Vaccines Immunother. 2014, 10, 2761–2774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, R.P.; Lee, H.; Luzuriaga, M.A.; Brohlin, O.R.; Gassensmith, J.J. Protein-Polymer Delivery: Chemistry from the Cold Chain to the Clinic. Bioconjug. Chem. 2018, 29, 2867–2883. [Google Scholar] [CrossRef] [PubMed]
- Khobragade, P.K.; Puranik, P.K.; Road, V. Chitosan: A Mucoadhesive. Polymer 2015, 4, 1829–1847. [Google Scholar]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dormont, F.; Rouquette, M.; Mahatsekake, C.; Gobeaux, F.; Peramo, A.; Brusini, R.; Calet, S.; Testard, F.; Lepetre-Mouelhi, S.; Desmaële, D.; et al. Translation of nanomedicines from lab to industrial scale synthesis: The case of squalene-adenosine nanoparticles. J. Control. Release 2019, 307, 302–314. [Google Scholar] [CrossRef] [PubMed]
- McNeill, E.; Crabtree, M.J.; Sahgal, N.; Patel, J.; Chuaiphichai, S.; Iqbal, A.J.; Hale, A.B.; Greaves, D.R.; Channon, K.M. Regulation of iNOS function and cellular redox state by macrophage Gch1 reveals specific requirements for tetrahydrobiopterin in NRF2 activation. Free Radic. Biol. Med. 2015, 79, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Abramson, S.B.; Amin, A.R.; Clancy, R.M.; Attur, M. The role of nitric oxide in tissue destruction. Best Pract. Res. Clin. Rheumatol. 2001, 15, 831–845. [Google Scholar] [CrossRef]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Tang, X.; Thankappan, S.K.; Lee, P.; Fard, S.E.; Harmon, M.D.; Tran, K.; Yu, X. Polymeric Biomaterials in Tissue Engineering and Regenerative Medicine; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780123969835. [Google Scholar]
- Arakawa, C.K.; Badeau, B.A.; Zheng, Y.; DeForest, C.A. Multicellular Vascularized Engineered Tissues through User-Programmable Biomaterial Photodegradation. Adv. Mater. 2017, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kohrs, N.J.; Liyanage, T.; Venkatesan, N.; Najarzadeh, A.; Puleo, D.A. Drug Delivery Systems and Controlled Release; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 1–3, ISBN 9780128051443. [Google Scholar]
- Karolewicz, B. A review of polymers as multifunctional excipients in drug dosage form technology. Saudi Pharm. J. 2016, 24, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Karandikar, S.; Mirani, A.; Waybhase, V.; Patravale, V.B.; Patankar, S. Nanovaccines for Oral Delivery-Formulation Strategies and Challenges; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780323477215. [Google Scholar]
- Salman, H.H.; Irache, J.M.; Gamazo, C. Immunoadjuvant capacity of flagellin and mannosamine-coated poly(anhydride) nanoparticles in oral vaccination. Vaccine 2009, 27, 4784–4790. [Google Scholar] [CrossRef]
- Tamayo, I.; Irache, J.M.; Mansilla, C.; Ochoa-Repáraz, J.; Lasarte, J.J.; Gamazo, C. Poly(anhydride) nanoparticles act as active Th1 adjuvants through toll-like receptor exploitation. Clin. Vaccine Immunol. 2010, 17, 1356–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojer, P.; Neutsch, L.; Gabor, F.; Irache, J.M.; López De Cerain, A. Cytotoxicity and cell interaction studies of bioadhesive poly(anhydride) nanoparticles for oral antigen/drug delivery. J. Biomed. Nanotechnol. 2013, 9, 1891–1903. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.; Irache, J.M.; Tamayo, I.; Walz, A.; DelVecchio, V.G.; Gamazo, C. Protective immunity of biodegradable nanoparticle-based vaccine against an experimental challenge with Salmonella Enteritidis in mice. Vaccine 2007, 25, 4410–4419. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Chellaram, C.; Murugaboopathi, G.; John, A.A.; Sivakumar, R.; Ganesan, S.; Krithika, S.; Priya, G. Significance of Nanotechnology in Food Industry. APCBEE Procedia 2014, 8, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Hill, E.K.; Li, J. Current and future prospects for nanotechnology in animal production. J. Anim. Sci. Biotechnol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gracia Lux, C.; Joshi-Barr, S.; Nguyen, T.; Mahmoud, E.; Schopf, E.; Fomina, N.; Almutairi, A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J. Am. Chem. Soc. 2012, 134, 15758–15764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe nanoparticles: Are we there yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Systemic review of biodegradable nanomaterials in nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [Green Version]


| Live Vaccines | Killed or Inactivated Vaccines |
|---|---|
| Live vaccine strain can revert to its virulent form and spread to the environment and to humans | Vaccine antigens do not revert to virulence. No multiplication after administration |
| Live vaccine strain can interfere with the salmonellosis monitoring programs | No danger of vaccine contamination |
| Known to elicit both cell-mediated and humoral immune responses | Known to elicit a lower cell-mediated immunity |
| Adjuvants in live vaccines are not common | Adjuvants in killed vaccines are often needed |
| Known to elicit both cell-mediated and humoral immune responses and rarely require a booster | Known to elicit a shorter length of protection, so they are more likely to require boosters to create long-term immunity |
| Vaccines for breeders, broilers, and layers can be administered by spray or via feed or water | Vaccines for broilers and layers are administered intramuscularly, which can decrease the quality of the tissue, hence the value of the final product |
| Company/Vaccine | Live | Killed | Bird | Administration Route |
|---|---|---|---|---|
| Zoetis/POULVAC® ST | X | Broilers/layers | Spray | |
| Zoetis/POULVAC® SE | X | Broilers/layers | Injection | |
| Zoetis/POULVAC® SE-ND-IB | X | Broilers/layers | Injection | |
| IDT Bio/SALMOVAC® SE | X | Broilers/layers | Oral | |
| IDT Bio/ZOOSALORAL H | X | Breeders/layers | Oral | |
| CEVA/LAYERMUNE® SE | X | Breeders/layers | Injection | |
| CEVA/CORYMUNE ® RANGE | X | Breeders/layers | Injection | |
| ELANCO/AviPro® Megan® Vac 1 | X | * Young chickens | Spray | |
| ELANCO/AviPro® Megan® Egg | X | Layers/turkeys | Spray | |
| ELANCO/AviPro® 329 ND-IB2-SE4 | X | Breeders/layers | Injection |
| Publication | Journal | Year | Citation |
|---|---|---|---|
| Protection Conferred by Drinking Water Administration of a Nanoparticle-Based Vaccine against Salmonella Enteritidis in Hens | Vaccines | 2021 | [108] |
| Efficacy of a Nanoparticle Vaccine Administered In Ovo Against Salmonella in Broilers | PLOS ONE | 2021 | [102] |
| Chitosan-Adjuvanted Salmonella Subunit Nanoparticle Vaccine for Poultry Delivered Through Drinking Water and Feed | Carbohydrate Polymers | 2020 | [103] |
| Efficacy of Chitosan-Based Nanoparticle Vaccine Administered to Broiler Birds Challenged with Salmonella | PLOS ONE | 2020 | [104] |
| Immune Response to Salmonella Enteritidis Infection in Broilers Immunized Orally With Chitosan-Based Salmonella Subunit Nanoparticle Vaccine | Frontiers in Immunology | 2020 | [105] |
| Oral Deliverable Mucoadhesive Chitosan-Salmonella Subunit Nanovaccine for Layer Chickens | International Journal of Nanomedicine | 2020 | [78] |
| Mannose-Modified Chitosan-Nanoparticle-Based Salmonella Subunit Oral Vaccine-Induced Immune Response and Efficacy in a Challenge Trial in Broilers | Vaccines | 2020 | [106] |
| Temporal Dynamics of Innate and Adaptive Immune Responses in Broiler Birds to Oral-Delivered Chitosan Nanoparticle-Based Salmonella Subunit Antigens | Veterinary Immunology and Immunopathology | 2020 | [107] |
| Surface Engineered Polyanhydride-Based Oral Salmonella Subunit Nanovaccine for Poultry | International Journal of Nanomedicine | 2018 | [80] |
| Antigen Delivery System | Findings |
|---|---|
| A Salmonella subunit Chitosan nanoparticle vaccine synthesized to contain S. enteritidis OMPs and flagellin protein combined with a flagellin surface coating | Biocompatible with chickens, average size optimal for DCs uptake, and stable at highly acidic pH environment over a long period of time |
| Can adhere to mucosal surface and are uptaken by ileal PPs and lamina propria immune cells | |
| Can induce significantly higher antigen specific mucosal IgA production | |
| Have also shown to significantly increased levels of antigen-specific IgY | |
| Can significantly enhance the rapid proliferation of OMPs and flagellin-specific lymphocytes | |
| Can increase significant levels of iNOS, TLR-1, TLR-2, TLR-3, TLR-4, TLR-5, TLR-7, TLR-15, TLR-21 and IL-1β, IL-4, IL-10, IFN-γ, and TGF-β mRNA expression in immunized birds | |
| Can significantly decrease Salmonella colonization in broilers and layers when administered using either an individual oral gavage, via water, feed, or through in ovo delivery | |
| Numerically reduced the S. heidelberg loads in the liver and spleen of vaccinated broilers | |
| Mannose modification of the CNP can reduce the S. enteritidis cecal load | |
| A Salmonella subunit PVM/MA nanoparticle vaccine synthesized to contain S. enteritidis OMPs and flagellin protein combined with a flagellin surface coating | Biocompatible with chickens |
| Average size optimal for DCs uptake | |
| Stable over a range of acidic and alkaline environments for 3 h | |
| Mucoadhesive and immunogenic compared to unloaded proteins and non-immunized controls | |
| Enhanced levels of mucosal IgA, TLR-4 and CD8+/CD4+ ratio in the cecal tonsils of immunized birds | |
| Cecal colonization by a homologous challenge was reduced in 33% of vaccinated birds | |
| A Salmonella PVM/MA nanoparticle vaccine that is synthesized to contain a heat extract fraction of the cell surface of S. enteritidis | Biocompatible with chickens |
| Average size optimal for DCs uptake | |
| High stability in tap water and acidic and basic pH | |
| Can significantly reduce the excretion of S. enteritidis | |
| Numerically reduced the percentages of S. enteritidis in cecum, liver, and spleen of the immunized hens | |
| Possible mechanism is chiefly promoting an early proinflammatory Th1 cell response and late anti-inflammatory Th2 response |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acevedo-Villanueva, K.Y.; Akerele, G.O.; Al Hakeem, W.G.; Renu, S.; Shanmugasundaram, R.; Selvaraj, R.K. A Novel Approach against Salmonella: A Review of Polymeric Nanoparticle Vaccines for Broilers and Layers. Vaccines 2021, 9, 1041. https://doi.org/10.3390/vaccines9091041
Acevedo-Villanueva KY, Akerele GO, Al Hakeem WG, Renu S, Shanmugasundaram R, Selvaraj RK. A Novel Approach against Salmonella: A Review of Polymeric Nanoparticle Vaccines for Broilers and Layers. Vaccines. 2021; 9(9):1041. https://doi.org/10.3390/vaccines9091041
Chicago/Turabian StyleAcevedo-Villanueva, Keila Y., Gabriel O. Akerele, Walid Ghazi Al Hakeem, Sankar Renu, Revathi Shanmugasundaram, and Ramesh K. Selvaraj. 2021. "A Novel Approach against Salmonella: A Review of Polymeric Nanoparticle Vaccines for Broilers and Layers" Vaccines 9, no. 9: 1041. https://doi.org/10.3390/vaccines9091041
APA StyleAcevedo-Villanueva, K. Y., Akerele, G. O., Al Hakeem, W. G., Renu, S., Shanmugasundaram, R., & Selvaraj, R. K. (2021). A Novel Approach against Salmonella: A Review of Polymeric Nanoparticle Vaccines for Broilers and Layers. Vaccines, 9(9), 1041. https://doi.org/10.3390/vaccines9091041






