Acute Transverse Myelitis Following COVID-19 Vaccination
Abstract
:1. Introduction
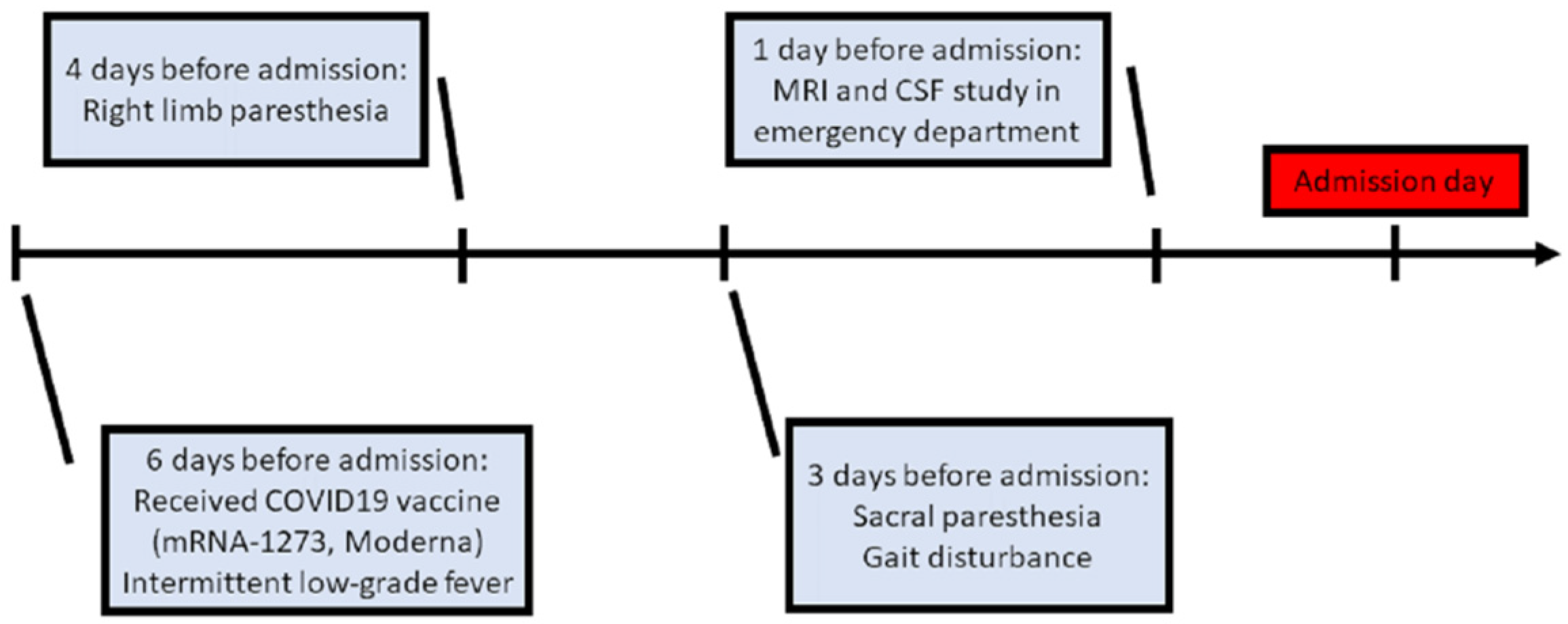
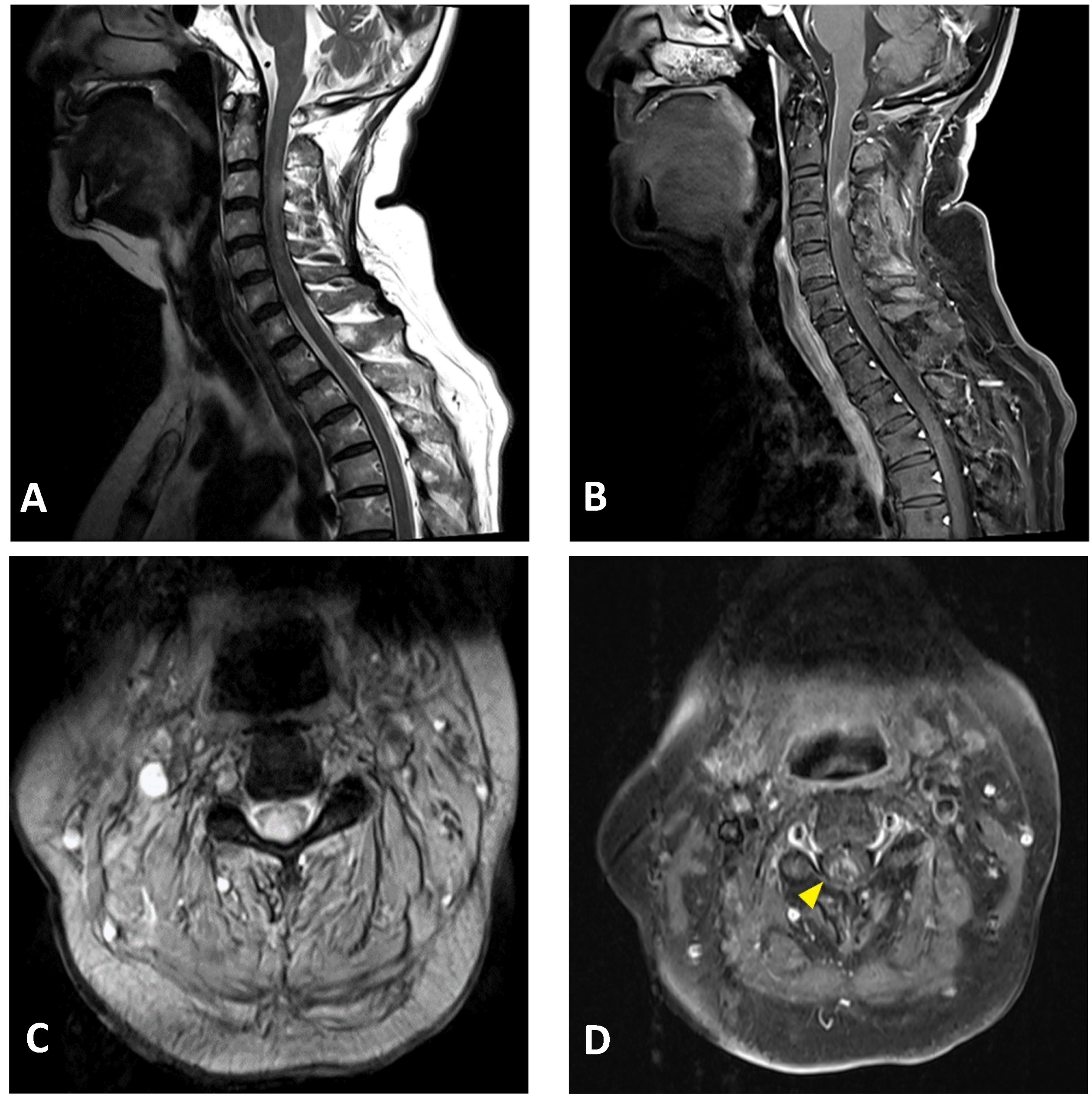
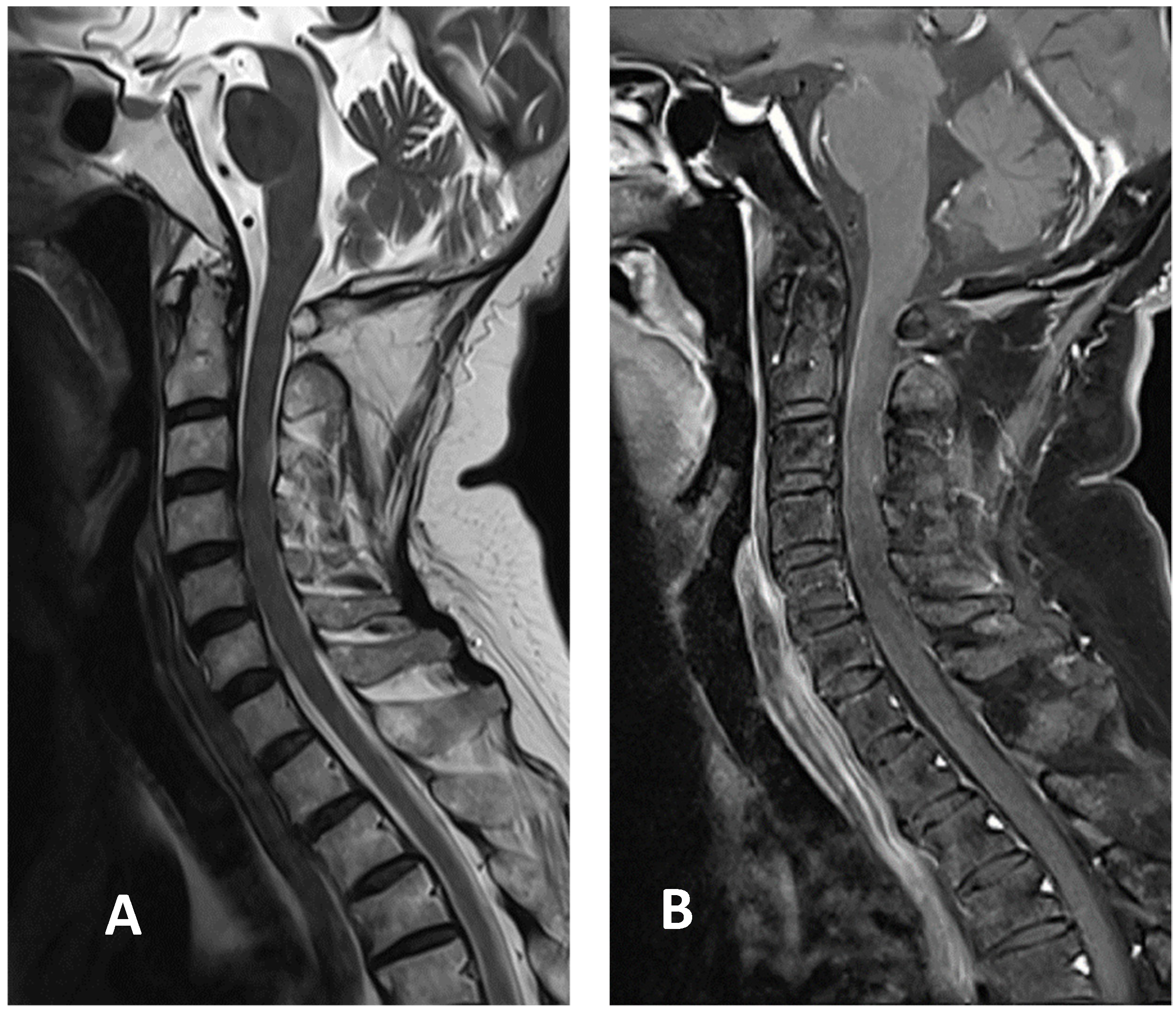
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitley, J.L.; Leite, M.I.; George, J.S.; Palace, J.A. The differential diagnosis of longitudinally extensive transverse myelitis. Mult. Scler. J. 2012, 18, 271–285. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Weinshenker, B.G. Acute disseminated encephalomyelitis, transverse myelitis, and neuromyelitis optica. Continuum (Minneapolis, Minn.) 2013, 19, 944–967. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, D.R.; Mandler, R.N.; Davis, L.E. Transverse myelitis. Retrospective analysis of 33 cases, with differentiation of cases associated with multiple sclerosis and parainfectious events. Arch. Neurol. 1993, 50, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.; Lewis, E.; Goddard, K.; Fireman, B.; Bakshi, N.; DeStefano, F.; Gee, J.; Tseng, H.F.; Naleway, A.L.; Klein, N.P. Acute Demyelinating Events Following Vaccines: A Case-Centered Analysis. Clin. Infect. Dis. 2016, 63, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Goss, A.L.; Samudralwar, R.D.; Das, R.R.; Nath, A. ANA Investigates: Neurological Complications of COVID-19 Vaccines. Ann. Neurol. 2021, 89, 856–857. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J.; Kohl, K.S.; Bilynsky, R.; Blumberg, D.; Cvetkovich, T.; Galama, J.; Gidudu, J.; Katikaneni, L.; Khuri-Bulos, N.; Oleske, J.; et al. Group. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): Case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2007, 25, 5771–5792. [Google Scholar] [CrossRef]
- Weinshenker, B.G.; Wingerchuk, D.M.; Vukusic, S.; Linbo, L.; Pittock, S.J.; Lucchinetti, C.F.; Lennon, V.A. Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann. Neurol. 2006, 59, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Gracia, F.; Torres, A.; Palacios, A.; Gracia, K.; Harris, D. Acute Transverse Myelitis (ATM): Clinical Review of 43 Patients With COVID-19-Associated ATM and 3 Post-Vaccination ATM Serious Adverse Events With the ChAdOx1 nCoV-19 Vaccine (AZD1222). Front. Immunol. 2021, 12, 653786. [Google Scholar] [CrossRef]
- Malhotra, H.S.; Gupta, P.; Prabhu, V.; Garg, R.K.; Dandu, H.; Agarwal, V. COVID-19 vaccination-associated myelitis. QJM. 2021, hcab069. [Google Scholar] [CrossRef]
- Pagenkopf, C.; Südmeyer, M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19. J. Neuroimmunol. 2021, 358, 577606. [Google Scholar] [CrossRef]
- Fitzsimmons, W.; Nance, C.S. Sudden Onset of Myelitis after COVID-19 Vaccination: An Under-Recognized Severe Rare Adverse Event. 5 May 2021. Available online: http://dx.doi.org/10.2139/ssrn.3841558 (accessed on 14 July 2021).
- Shah, S.; Patel, J.M.; Alchaki, A.; Eddin, M.F.; Souayah, N. Development of Transverse Myelitis after Vaccination, A CDC/FDA Vaccine Adverse Event Reporting System (VAERS) Study, 1985–2017. (P5.099). Neurology 2018, 90 (Suppl. 15), P5.099. [Google Scholar]
- Zanoni, G.; Nguyen, T.M.D.; Destefani, E.; Masala, L.; Nardelli, E.; Tridente, G. Transverse myelitis after vaccination. Eur. J. Neurol. 2002, 9, 696–697. [Google Scholar] [CrossRef] [PubMed]
- Agmon-Levin, N.; Kivity, S.; Szyper-Kravitz, M.; Shoenfeld, Y. Transverse myelitis and vaccines: A multi-analysis. Lupus 2009, 18, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Tincani, A.; Kivity, S.; Arango, M.-T.; Shoenfeld, Y. Transverse Myelitis Activation Post-H1N1 Immunization: A Case of Adjuvant Induction? Isr. Med. Assoc. J. 2015, 17, 120–122. [Google Scholar]
- McDonald, I.; Murray, S.M.; Reynolds, C.J.; Altmann, D.M.; Boyton, R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines 2021, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.; Taibi, R.; Pavone, P.; Marino, L.; Falsaperla, R. Neurotropism of SARS-CoV 2 and others coronavirus in children: Mechanisms and clinical manifestations. EJMO 2021, 5, 91–93. [Google Scholar] [CrossRef]
- Nemoto, W.; Yamagata, R.; Nakagawasai, O.; Nakagawa, K.; Hung, W.Y.; Fujita, M.; Tadano, T.; Tan-No, K. Effect of spinal angiotensin-converting enzyme 2 activation on the formalin-induced nociceptive response in mice. Eur. J. Pharmacol. 2020, 5, 172950. [Google Scholar] [CrossRef]
- Goldish, D.; Massagli, T.L. Subacute Progressive Myelopathy: Transverse Myelitis or Subacute Combined Degeneration? A Case Report. PM&R 2018, 10, 320–324. [Google Scholar] [CrossRef]
- Matsuura, H.; Nakamura, T. Inverted V sign: Subacute combined degeneration of the spinal cord. QJM: Int. J. Med. 2018, 111, 65–66. [Google Scholar] [CrossRef] [Green Version]
- Narra, R.; Mandapalli, A.; Jukuri, N.; Guddanti, P. "Inverted V sign" in Sub-Acute Combined Degeneration of Cord. J. Clin. Diagn. Res. 2015, 9, TJ01. [Google Scholar] [CrossRef] [PubMed]
- Daroff, R. Structural Imaging Using Magnetic Resonance Imaging and Computed Tomography. In Bradley and Daroff’s Neurology in Clinical Practice, 7th ed.; Joseph, J., John, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 496–546. [Google Scholar]
- Devi, A.; Singh, K.K.; Gupta, S.; Bhutani, N.; Agarwal, P. A descriptive study of clinical and radiological profile of longitudinal extensive myelitis in a tertiary hospital in Rajasthan, India. Clin. Neurol. Neurosurg. 2019, 181, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, V.S.; Bedenko, A.S.; Totolyan, N.A.; Skoromets, A.A. Longitudinally extensive transverse myelitis associated with intrathecal synthesis of oligoclonal immunoglobulin G and with vitamin B12 deficiency. Neurol. Neuropsychiatry Psychosomatic. 2016, 8, 71–75. [Google Scholar] [CrossRef]
- Miller, A.; Korem, M.; Almog, R.; Galboiz, Y. Vitamin B12, demyelination, remyelination and repair in multiple sclerosis. J Neurol. Sci. 2005, 233, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Graven, K.; Joseph, N.I.; Johnson, J.; Fulton, S.; Hostoffer, R.; Abboud, H. Case Report: Postvaccination Anti-Myelin Oligodendrocyte Glycoprotein Neuromyelitis Optica Spectrum Disorder: A Case Report and Literature Review of Postvaccination Demyelination. Int. J. MS Care 2020, 22, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Jarius, S.; in cooperation with the Neuromyelitis Optica Study Group (NEMOS); Ruprecht, K.; Kleiter, I.; Borisow, N.; Asgari, N.; Pitarokoili, K.; Pache, F.; Stich, O.; Beume, L.-A.; et al. MOG-IgG in NMO and related disorders: A multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J. Neuroinflammation 2016, 13, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameter [Unit] | Result | Reference Value |
|---|---|---|
| WBC [109/L] | 4.60 | 4.50–11.00 |
| Hgb [g/dL] | 12.0 | 12.00–16.0 |
| Platelet [109/L] | 296 | 150–400 |
| MCV [fL] | 113.7 | 80.0–96.0 |
| WBC CSF [/µL] | 15 | |
| RBC CSF [µL] | 73 | |
| Lymphocyte CSF [%] | 27 | 28–96 |
| Neutrophil CSF [%] | 73 | |
| Lactate CSF [mmol/L] | 2.7 | <2.8 |
| Protein CSF [mg/dL] | 57.2 | 15.0–45.0 |
| Glucose CSF [mg/dL] | 71 | 40–70 |
| Glucose serum [mg/dL] | 117 | |
| ESR [mm] | 27 | 0–20 |
| CRP [mg/dL] | 0.06 | <0.50 |
| Anti-HIV EIA [COI] | 0.11 | <0.9 |
| TPPA CSF | Negative | Negative |
| RPR/VDRL CSF | Non-reactive | Non-reactive |
| Electrophoresis CSF | No oligoclonal band | |
| Vitamin B12 [pg/mL] | 131 | Deficient < 211 |
| RF [IU/mL] | <10.0 | <14.0 |
| ANA | 1:40(−) | |
| TSH [µIU/mL] | 0.18 | 0.270–4.20 |
| Free T4 [ng/dL] | 1.57 | 0.93–1.70 |
| A-beta2GPI IgG [U/mL] | <1.4 | <20.0 U/mL |
| Anti-cardiolipin IgG [GPL-U/mL] | <1.6 | <20.0 |
| ENA anti-SSA [AI] | <0.2 | <1.0 |
| ENA anti-SSA 52 [AI] | <0.2 | <1.0 |
| ENA anti-SSA 60 [AI] | <0.2 | <1.0 |
| ENA anti-SSB [AI] | <0.2 | <1.0 |
| cANCA | 10× (Negative) | |
| pANCA | 10× (Negative) | |
| Atypical pANCA | 10× (Negative) | |
| Anti-dsDNA [IU/mL] | <1.0 | Negative < 4 |
| TSH receptor Ab [IU/L] | <0.10 | Negative < 0.1 |
| Aquaporin 4 antibody | Negative | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.-J.; Tseng, H.-P.; Lin, C.-L.; Shiu, J.-S.; Lee, M.-H.; Liu, C.-H. Acute Transverse Myelitis Following COVID-19 Vaccination. Vaccines 2021, 9, 1008. https://doi.org/10.3390/vaccines9091008
Gao J-J, Tseng H-P, Lin C-L, Shiu J-S, Lee M-H, Liu C-H. Acute Transverse Myelitis Following COVID-19 Vaccination. Vaccines. 2021; 9(9):1008. https://doi.org/10.3390/vaccines9091008
Chicago/Turabian StyleGao, Jhih-Jian, Hung-Pin Tseng, Chun-Liang Lin, Jr-Shiang Shiu, Ming-Hsun Lee, and Ching-Hsiung Liu. 2021. "Acute Transverse Myelitis Following COVID-19 Vaccination" Vaccines 9, no. 9: 1008. https://doi.org/10.3390/vaccines9091008
APA StyleGao, J.-J., Tseng, H.-P., Lin, C.-L., Shiu, J.-S., Lee, M.-H., & Liu, C.-H. (2021). Acute Transverse Myelitis Following COVID-19 Vaccination. Vaccines, 9(9), 1008. https://doi.org/10.3390/vaccines9091008






