Nervous and Muscular Adverse Events after COVID-19 Vaccination: A Systematic Review and Meta-Analysis of Clinical Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Searches Strategy
2.2. Study Selection Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
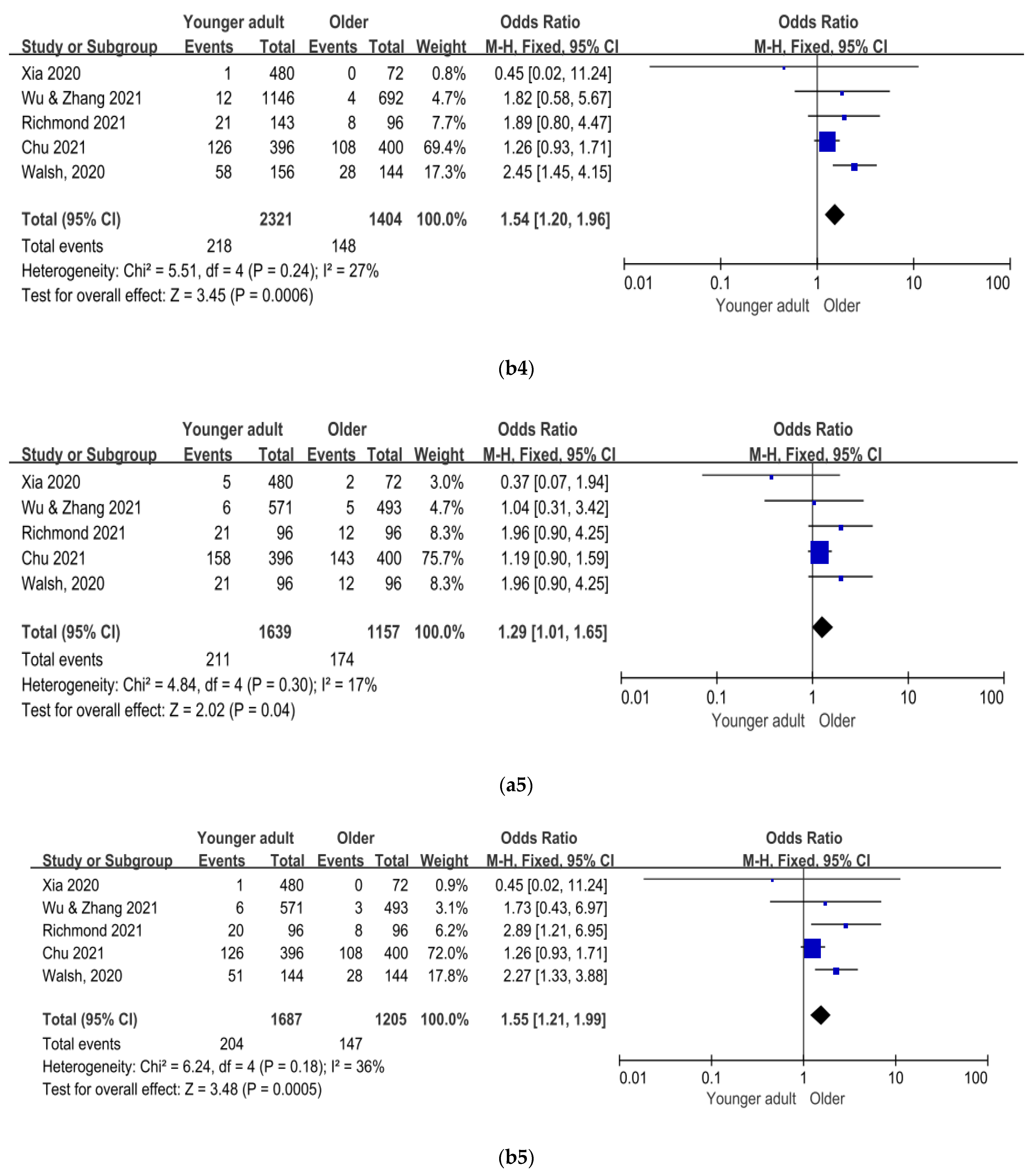
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Dashboard by the Center for Systems Science and Engineering at Johns Hopkins University. Available online: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (accessed on 4 August 2021).
- Williams, S.V.; Vusirikala, A.; Ladhani, S.N.; Fernandez, R.D.O.E.; Iyanger, N.; Aiano, F.; Stoker, K.; Gopal, R.G.; John, L.; Patel, B.; et al. An outbreak caused by the SARS-CoV-2 Delta (B.1.617.2) variant in a care home after partial vaccination with a single dose of the COVID-19 vaccine Vaxzevria, London, England, April 2021. EuroSurveillance 2021, 26, 2100626. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Chen, J.; Wen, Z.; Feng, F.; Zou, H.; Fu, C.; Chen, L.; Shu, Y.; Sun, C. An online survey of the attitude and willingness of Chinese adults to receive COVID-19 vaccination. Hum. Vaccines Immunother 2021, 17, 2279–2288. [Google Scholar] [CrossRef] [PubMed]
- Kourlaba, G.; Kourkouni, E.; Maistreli, S.; Tsopela, C.; Molocha, N.; Triantafyllou, C.; Koniordou, M.; Kopsidas, I.; Chorianopoulou, E.; Maroudi-Manta, S.; et al. Willingness of Greek general population to get a COVID-19 vaccine. Glob. Health Res. Policy 2021, 6, 3. [Google Scholar] [CrossRef]
- Shekhar, R.; Sheikh, A.B.; Upadhyay, S.; Singh, M.; Kottewar, S.; Mir, H.; Barrett, E.; Pal, S. COVID-19 vaccine acceptance among health care workers in the United States. Vaccines 2021, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- The First 22M Americans Have Been Vaccinated for COVID-19, and Initial Safety Data Shows Everything Is Going Well, CDC Says. Available online: https://www.usatoday.com/story/news/health/2021/01/28/covid-19-vaccines-cdc-safety-data-pfizer-moderna-coronavirus/4281434001/ (accessed on 25 June 2021).
- Coronavirus Vaccine—Weekly Summary of Yellow Card Reporting. Available online: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting (accessed on 25 June 2021).
- Singh, M.H.; Gupta, P.; Prabhu, V.; Garg, R.K.; Dandu, H.; Agarwal, V. COVID-19 vaccination-associated myelitis. QJM 2021. [Google Scholar] [CrossRef]
- Blauenfeldt, R.A.; Kristensen, S.R.; Ernstsen, S.L.; Kristensen, C.C.H.; Simonsen, C.Z.; Hvas, A.M. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J. Thromb Haemost 2021, 19, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- See, I.; Su, J.R.; Lale, A.; Woo, E.J.; Guh, A.Y.; Shimabukuro, T.T.; Streiff, M.B.; Rao, A.K.; Wheeler, A.P.; Beavers, S.F.; et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21. JAMA 2021, 325, 2448–2456. [Google Scholar] [CrossRef]
- 8430 Dead, 354,177 Injuries Following COVID-19 Experimental ‘Vaccines’ Reported in Europe. Available online: https://m.thebl.tv/world-news/8430-dead-354177-injuries-following-covid-19-experimental-vaccines-reported-in-europe.html (accessed on 25 June 2021).
- Moreira, F.R.R.; Bonfim, D.M.; Zauli, D.A.G.; Silva, J.P.; Lima, A.B.; Malta, F.S.V.; Ferreira, A.C.S.; Pardini, V.C.; Magalhães, W.C.S.; Queiroz, D.C.; et al. Epidemic spread of SARS-CoV-2 lineage B.1.1.7 in Brazil. Viruses 2021, 13, 984. [Google Scholar] [CrossRef]
- Thompson, C.N.; Hughes, S.; Ngai, S.; Baumgartner, J.; Wang, J.C.; McGibbon, E.; Devinney, K.; Luoma, E.; Bertolino, D.; Hwang, C.; et al. Rapid emergence and epidemiologic characteristics of the SARS-CoV-2 B.1.526 variant—New York City, New York, January 1–April 5, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 712–716. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- World Health Organization. Annex 9: Guidelines on Clinical Evaluation of Vaccines: Regulatory Expectations. Available online: https://www.who.int/biologicals/expert_committee/WHO_TRS_1004_web_Annex_9.pdf?ua=1 (accessed on 25 June 2021).
- Chandler, R.E. Optimizing safety surveillance for COVID-19 vaccines. Nat. Rev. Immunol. 2020, 20, 451–452. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: Interim analysis of 2 randomized clinical trials. JAMA 2020, 324, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 803–812. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Raches, E.; Mohan, K.V.; Harsh, J.; Sai, P.; Siddharth, R.; Vamshi, S.; Brunda, G.; Gajanan, S.; Pragya, Y.; Priya, A.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 637–646. [Google Scholar]
- Raches, E.; Siddharth, R.; Harsh, J.; Vamshi, S.; Brunda, G.; Sai, P.; Dipankar, D.; Dugyala, R.; Usha, P.; Gajanan, S.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: Interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect. Dis. 2021, 21, 950–961. [Google Scholar]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Zhu, F.; Guan, X.; Li, Y.; Huang, J.; Jiang, T.; Hou, L.; Li, J.; Yang, B.; Wang, L.; Wang, W.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. New Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef]
- Richmond, P.; Hatchuel, L.; Dong, M.; Ma, B.; Hu, B.; Smolenov, I.; Li, P.; Liang, P.; Han, H.; Liang, J.; et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: A phase 1, randomised, double-blind, placebo-controlled trial. Lancet 2021, 397, 682–694. [Google Scholar] [CrossRef]
- Chappell, K.J.; Mordant, F.L.; Li, Z.; Wijesundara, D.K.; Ellenberg, P.; Lackenby, J.A.; Cheung, S.T.M.; Modhiran, N.; Avumegah, M.S.; Henderson, C.L.; et al. Safety and immunogenicity of an MF59-adjuvanted spike glycoprotein-clamp vaccine for SARS-CoV-2: A randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2021. In Press. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef]
- Chu, L.; McPhee, R.; Huang, W.; Bennett, H.; Pajon, R.; Nestorova, B.; Leav, B.; mRNA-1273 Study Group. A preliminary report of a randomized controlled phase 2 trial of the safety and immunogenicity of mRNA-1273 SARS-CoV-2 vaccine. Vaccine 2021, 39, 2791–2799. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. New Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatulin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Zhu, F.; Li, Y.; Guan, X.; Hou, L.; Wang, W.; Li, J.; Wu, S.; Wang, B.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA vaccine against SARS-CoV-2—Preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Kaabi, A.I.N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: A randomized clinical trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Baden, L.R.; Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Kim, J.H.; Marks, F.; Clemens, J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat. Med. 2021, 27, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Razizadeh, M.H.; Turner, D.L.; Turner, R.J. Efficacy and safety of COVID-19 vaccines: A systematic review and meta-analysis of randomized clinical trials. Vaccines 2021, 9, 467. [Google Scholar] [CrossRef]
- Chen, M.; Yuan, Y.; Zhou, Y.; Deng, Z.; Zhao, J.; Feng, F.; Zou, H.; Sun, C. Safety of SARS-CoV-2 vaccines: A systematic review and meta-analysis of randomized controlled trials. Infect. Dis. Poverty 2021, 10, 94. [Google Scholar] [CrossRef]
- Pushparajah, D.; Jimenez, S.; Wong, S.; Alattas, H.; Nafissi, N.; Slavcev, R.A. Advances in gene-based vaccine platforms to address the COVID-19 pandemic. Adv. Drug Deliv. Rev. 2021, 170, 113–141. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Ai, P.; Liu, Y.; Ai, Z.; Wang, Y.; Cao, W.; Xia, X.; Zheng, J.C. Safety, tolerability, and immunogenicity of COVID-19 vaccines: A systematic review and meta-analysis. medRxiv 2020. [Google Scholar] [CrossRef]
- Wu, Q.; Dudley, M.Z.; Chen, X.; Bai, X.; Dong, K.; Zhuang, T.; Salmon, D.; Yu, H. Evaluation of the safety profile of COVID-19 vaccines: A rapid review. BMC Med. 2021, 19, 173. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Ledgerwood, J.E.; Pierson, T.C.; Hubka, S.A.; Desai, N.; Rucker, S.; Gordon, I.J.; Enama, M.E.; Nelson, S.; Nason, M.; Gu, W.; et al. A west nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J. Infect. Dis. 2011, 203, 1396–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciabattini, A.; Garagnani, P.; Santoro, F.; Rappuoli, R.; Franceschi, C.; Medaglini, D. Shelter from the cytokine storm: Pitfalls and prospects in the development of SARS-CoV-2 vaccines for an elderly population. Semin. Immunopathol. 2020, 42, 619–634. [Google Scholar] [CrossRef]
- Hu, J.; Jolkkonen, J.; Zhao, C. Neurotropism of SARS-CoV-2 and its neuropathological alterations: Similarities with other coronaviruses. Neurosci. Neurobehav. Rev. 2020, 119, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Fumery, T.; Baudar, C.; Ossemann, M.; London, F. Longitudinally extensivetransverse myelitis following acute COVID-19 infection. Mult. Scler. Relat. Disord. 2021, 48, 102723. [Google Scholar] [CrossRef] [PubMed]
- Tobaiqy, M.; Elkout, H.; MacLure, K. Analysis of thrombotic adverse reactions of COVID-19 AstraZeneca vaccine reported to EudraVigilance Database. Vaccines 2021, 9, 393. [Google Scholar] [CrossRef]
- Dias, L.; Soares-Dos-Reis, R.; Meira, J.; Ferrão, D.; Soares, P.R.; Pastor, A.; Gama, G.; Fonseca, L.; Fagundes, V.; Carvalho, M. Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. Stroke Cereb. Dis. 2021, 30, 105906. [Google Scholar] [CrossRef]
- Fan, Y.; Yu, J.; Chen, H.; Zhang, J.; Duan, J.; Mo, D.; Zhu, W.; Wang, B.; Ouyang, F.; Chen, Y.; et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: Executive summary and 2019 update of clinical management of cerebral venous sinus thrombosis. Stroke Vasc. Neurol. 2020, 5, 152–158. [Google Scholar] [CrossRef] [PubMed]
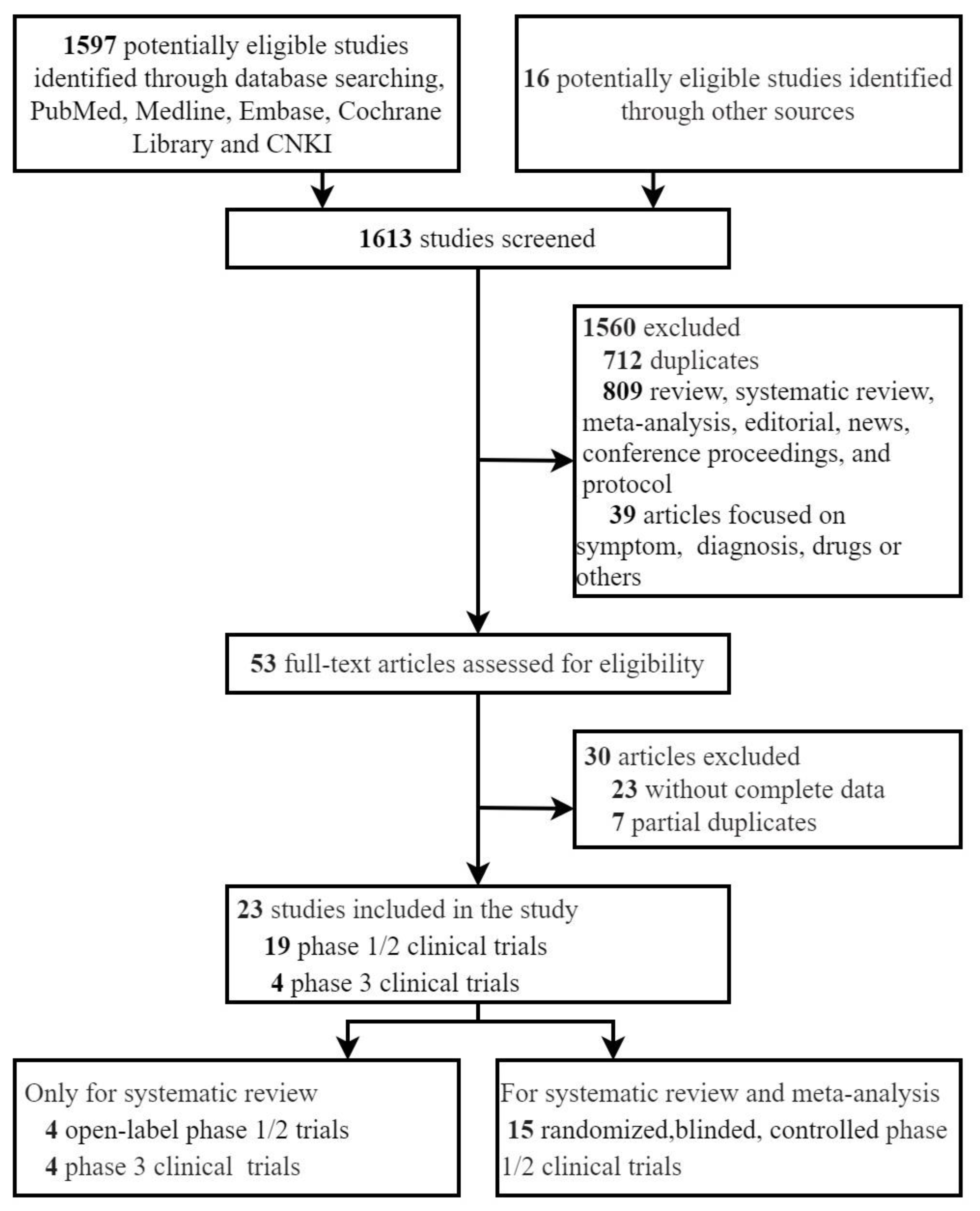
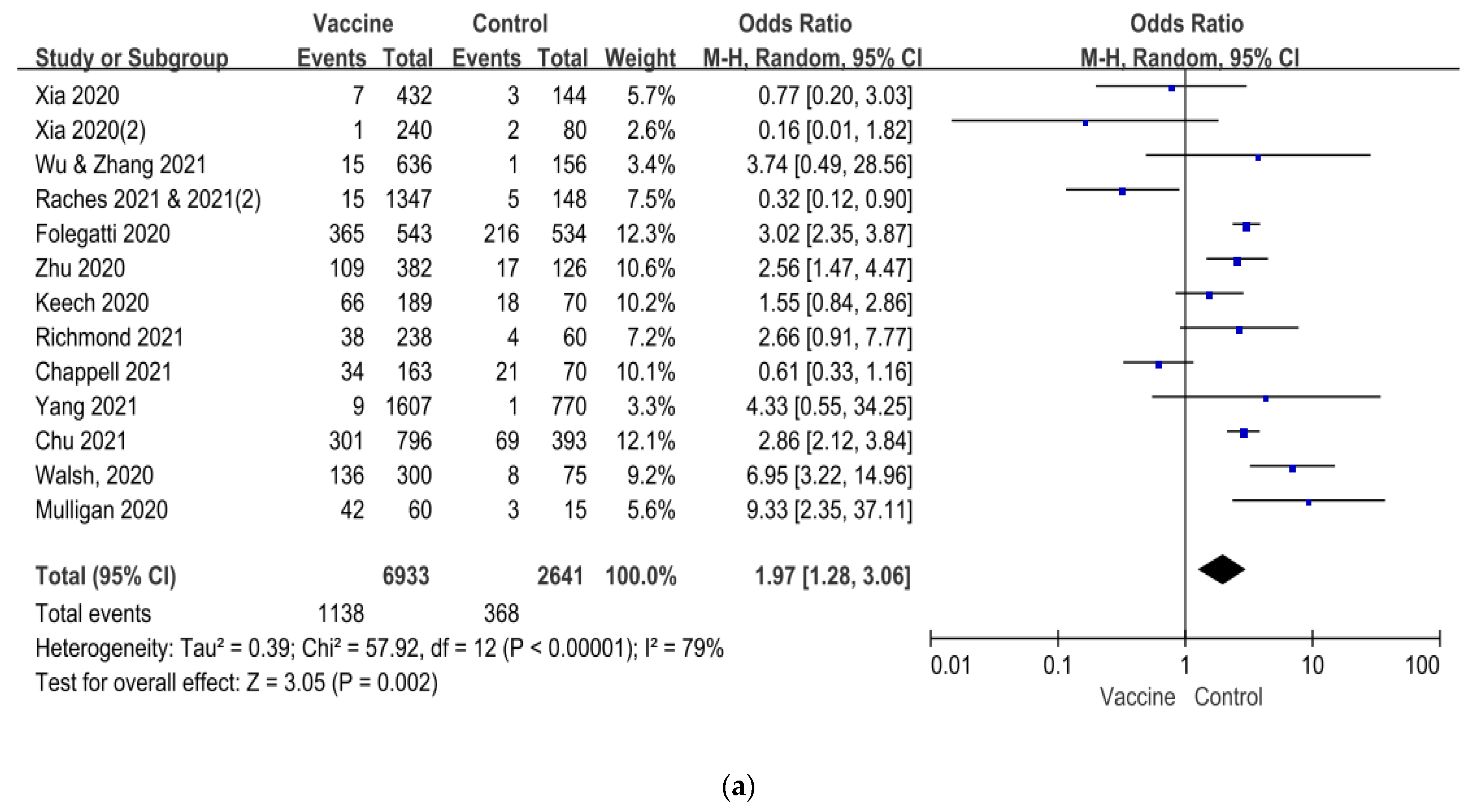
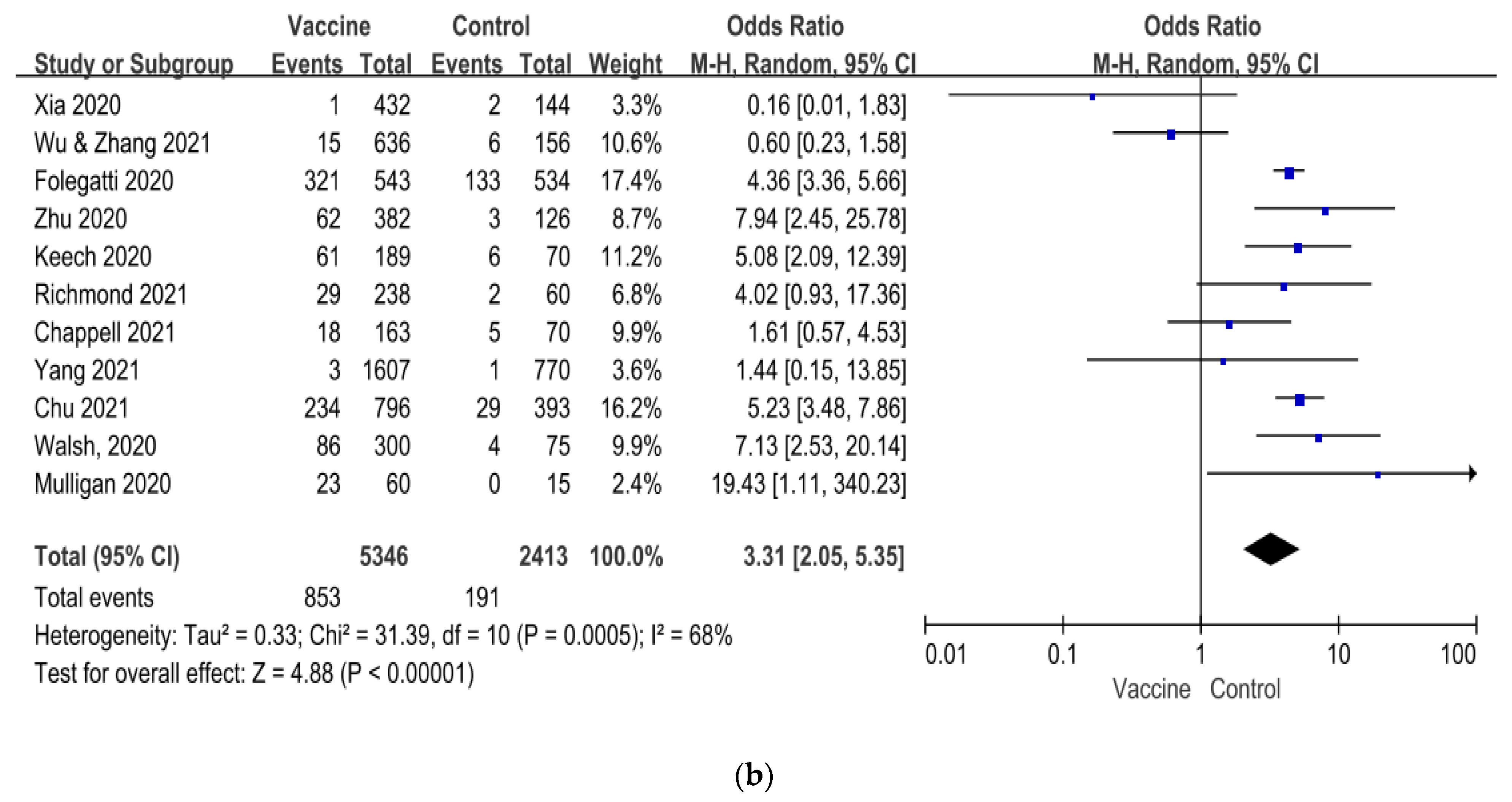
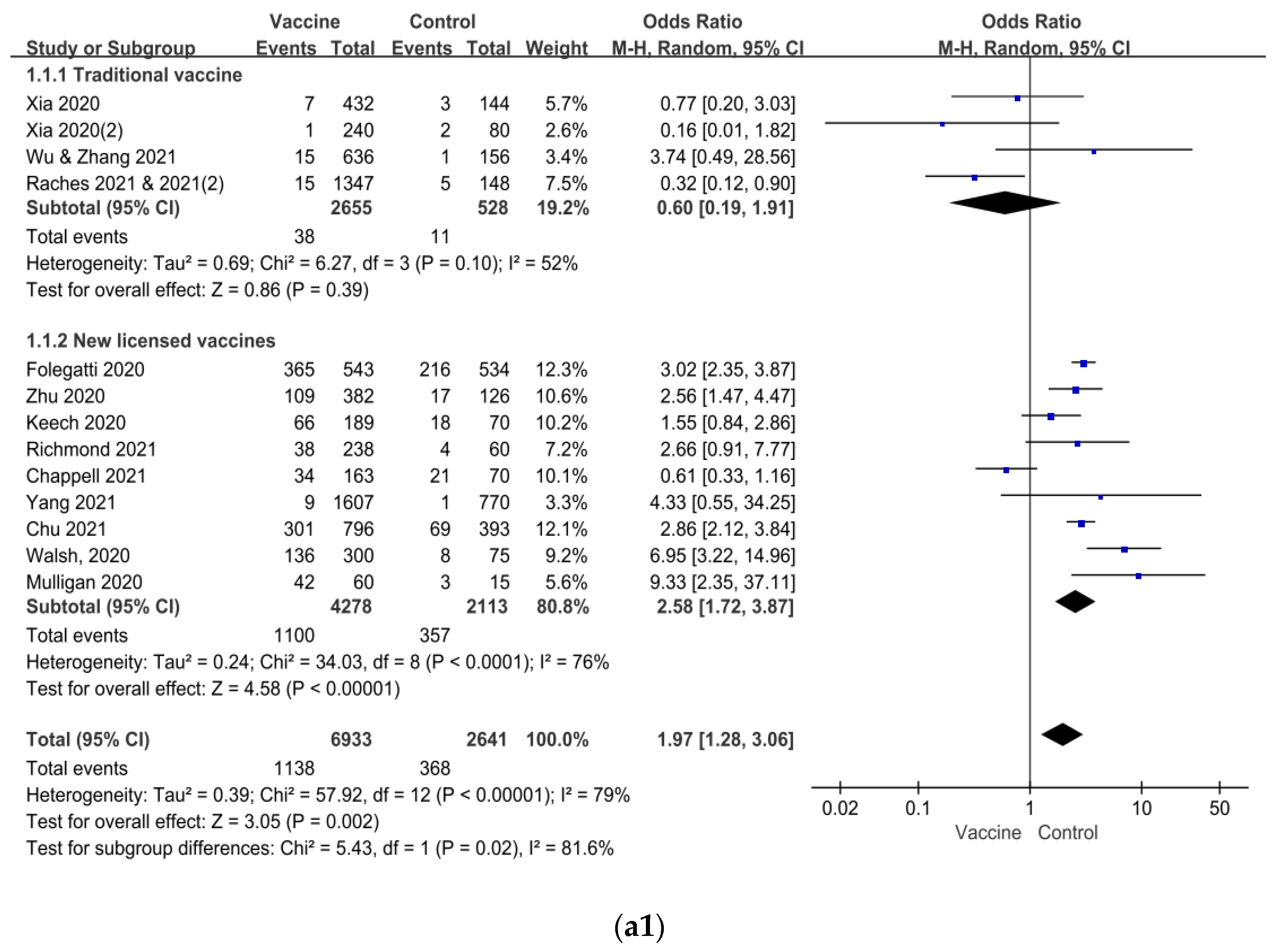


| Author/ Year of Published | Vaccine Platform | Sample Size of Study | Age of Subjects (Year) | Dosage of Vaccine | Number of Vaccination | Control | Current Status of Clinical Trial |
|---|---|---|---|---|---|---|---|
| Randomized, blinded, controlled phase 1/2 clinical trials | |||||||
| Xia 2020 [18] | Inactivated vaccine | Phase 1: 96, Phase 2: 448 | 18~80 | 2 µg, 4 µg, 8 µg | 1 or 2 | Saline containing aluminium hydroxide | Phase 3 ongoing |
| Xia 2020(2) [19] | Inactivated vaccine | Phase 1: 96, Phase2: 224 | 18~59 | 2.5 µg, 5 µg, 10 µg | 2 or 3 | Aluminum hydroxide (alum) adjuvant | Phase 3 ongoing |
| Wu 2021 [20] and Zhang 2021 [21] | Inactivated vaccine | 18~59:744 ≥60: 422 | 18~59, ≥60 | 1.5 µg, 3 µg, 6 µg | 2 | Aluminium hydroxide diluent solution | Phase 3 ongoing |
| Raches 2021 [22] and Raches 2021(2) [23] | Inactivated vaccine | 375 | 18~55 | 3 µg, 6 µg | 2 | Sterile solution and adjuvants | Phase 2 ongoing |
| Folegatti 2020 [24] | Replication-incompetent vectors vaccine | 1077 | 18~55 | 5 × 1010 viral particles/mL | 1 or 2 | MenACWY vaccine | Suspended in some regions |
| Zhu 2020 [25] | Replication-incompetent vectors vaccine | 508 | ≥18 | 1 × 1011 or 5 × 1010 viral particles/mL | 1 | Vaccine excipients | Phase 3 ongoing |
| Keech 2020 [26] | Recombinant protein vaccine | 131 | 18~59 | 5 µg, 25 µg | 1 or 2 | Saline | Phase 3 in preparation |
| Richmond 2021 [27] | Recombinant protein vaccine | 151 | 18~54, 55~75 | 3 µg, 9 µg, 30 µg | 2 | Saline | Phase 2 ongoing |
| Chappell 2021 [28] | Recombinant protein vaccine | 120 | 18~55 | 5 µg, 15 µg, 45 µg | 1 or 2 | Saline | Phase 2 ongoing |
| Yang 2021 [29] | Recombinant protein vaccine | Phase 1: 50, Phase2: 900 | 18~59 | 25 µg, 50 µg | 2 or 3 | Aluminium hydroxide in buffer | Phase 3 ongoing |
| Chu 2021 [30] | mRNA vaccine | 600 | 18~55, ≥55 | 50 µg, 100 µg | 2 | Saline | Phase 3 ongoing |
| Walsh 2020 [31] | mRNA vaccine | 195 | 18~55, 65~85 | 10 µg, 20 µg, 30 µg, 100 µg | 1 or 2 | Saline | Phase 3 ongoing |
| Mulligan 2020 [32] | mRNA vaccine | 45 | 18~55 | 10 µg, 30 µg, 100 µg | 1 or 2 | Saline | Phase 3 ongoing |
| Open-label phase 1/2 clinical trials | |||||||
| Logunov 2020 [33] | Replication-incompetent vectors vaccine | 76 | 18~60 | Vac 0.5 mL, Vac-Lyo 1.0 mL | 1 or 2 | No, open-label | Phase 3 ongoing |
| Zhu 2020(2) [34] | Replication-incompetent vectors vaccine | 108 | 18~60 | 5 × 1011 or 1 × 1011 or 5 × 1010 viral particles/mL | 1 | No, open-label | Phase 3 ongoing |
| Jackson 2020 [35] and Anderson 2020 [36] | mRNA vaccine | 85 | 18~55, 56~70, ≥71 | 25 µg, 100 µg, 250 µg | 2 | No, open-label | Phase 3 ongoing |
| Phase 3 clinical trials * | |||||||
| Kaabi 2021 [37] | Inactivated vaccine | 40,411 | ≥18 | 5 µg, 4 µg | 2 | Alum adjuvant and saline | Ongoing |
| Voysey 2020 [38] | Replication-incompetent vectors vaccine | 23,843 | ≥18 | (3.5–6.5) × 1010 or 2.2 × 1010 viral particles/mL | 2 | MenACWY vaccine | Suspended in some regions |
| Logunov 2021 [39] | Replication-incompetent vectors vaccine | 21,977 | ≥18 | 0.5 mL | 2 | Vaccine buffer | Ongoing |
| Baden 2020 [40] | mRNA vaccine | 30,420 | ≥18 | 100 µg | 2 | Saline | Ongoing |
| Inactivated Vaccine | Replication-Incompetent Vectors Vaccine | mRNA Vaccine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kaabi 2021 [37] | Voysey 2020 [38] | Logunov 2021 [39] | Baden 2020 [40] | ||||||||
| Vaccine | Control n = 13,453 | Vaccine n = 12,021 | Control n = 11,724 | Vaccine | Control | Vaccine n = 15,185 | Control n = 15,166 | ||||
| WIV04 n = 13,464 | HB02 n = 13,471 | At Least One Dose n = 16,427 | Two Dose n = 9258 | At Least One Dose n = 5435 | Two Dose n = 3038 | ||||||
| Systemic neurological symptoms * | 0 | 1 | 0 | 9 | 10 | 1 | NA | 0 | NA | 14 | 19 |
| Confusional state, drowsiness | NA | NA | NA | NA | NA | NA | 1 | NA | 0 | 2 | 0 |
| Seizure/tonic convulsion | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0 | 2 |
| Cranial nerve lesions † | 51 | 58 | 62 | 6 | 10 | NA | 6 | NA | 1 | NA | NA |
| Cerebrovascular events ‡ | NA | NA | NA | 2 | 4 | 3 | NA | 2 | NA | 5 | 1 |
| Spinal cord events § | 0 | 2 | 0 | 4 | 2 | 0 | NA | 1 | NA | NA | NA |
| Motor disorder ¶ | NA | NA | NA | 1 | 3 | 1 | NA | 0 | NA | 3 | 3 |
| Sensory disorder || | NA | NA | NA | 60 | 63 | NA | 1 | NA | 1 | 2 | 0 |
| Neuralgia, neuritis | NA | NA | NA | 4 | 1 | NA | NA | NA | NA | NA | NA |
| Muscle spasms/ facial spasm | NA | NA | NA | 1 | 0 | NA | NA | NA | NA | 0 | 2 |
| Autonomic nerve dysfunction ** | NA | NA | NA | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 |
| Total, No. (%) | 51 (0.4) | 61 (0.5) | 62 (0.5) | 88 (0.7) | 93 (0.8) | 6 (<0.1) | 9 (<0.1) | 3 (<0.1) | 2 (<0.1) | 26 (0.2) | 28 (0.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Cai, Y.; Chen, Y.; Williams, A.P.; Gao, Y.; Zeng, J. Nervous and Muscular Adverse Events after COVID-19 Vaccination: A Systematic Review and Meta-Analysis of Clinical Trials. Vaccines 2021, 9, 939. https://doi.org/10.3390/vaccines9080939
Chen J, Cai Y, Chen Y, Williams AP, Gao Y, Zeng J. Nervous and Muscular Adverse Events after COVID-19 Vaccination: A Systematic Review and Meta-Analysis of Clinical Trials. Vaccines. 2021; 9(8):939. https://doi.org/10.3390/vaccines9080939
Chicago/Turabian StyleChen, Jiaxin, Yuangui Cai, Yicong Chen, Anthony P. Williams, Yifang Gao, and Jinsheng Zeng. 2021. "Nervous and Muscular Adverse Events after COVID-19 Vaccination: A Systematic Review and Meta-Analysis of Clinical Trials" Vaccines 9, no. 8: 939. https://doi.org/10.3390/vaccines9080939
APA StyleChen, J., Cai, Y., Chen, Y., Williams, A. P., Gao, Y., & Zeng, J. (2021). Nervous and Muscular Adverse Events after COVID-19 Vaccination: A Systematic Review and Meta-Analysis of Clinical Trials. Vaccines, 9(8), 939. https://doi.org/10.3390/vaccines9080939






