TBE Vaccination Breakthrough Cases—Does Age Matter?
Abstract
:1. Introduction
2. Materials and Methods
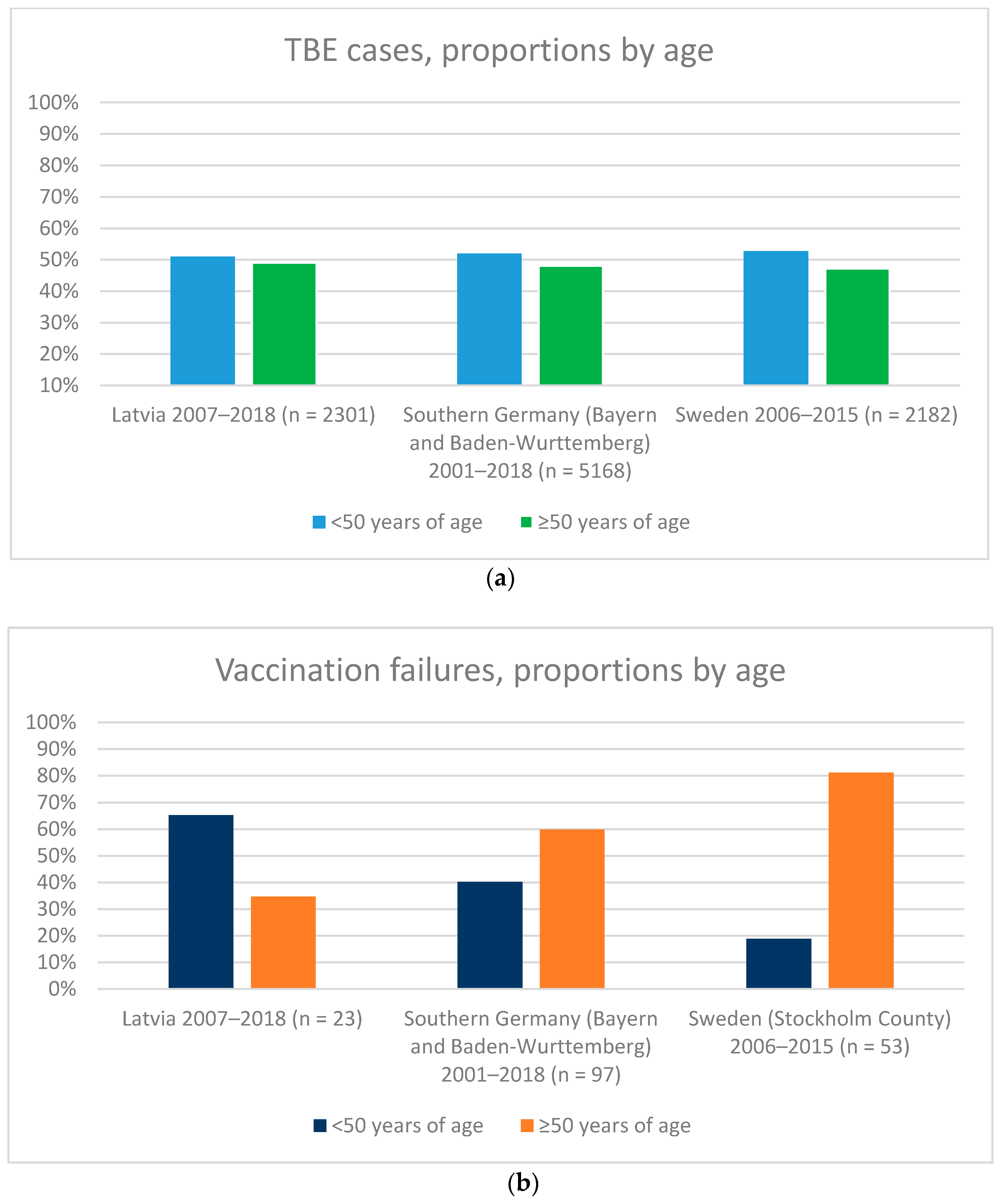
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobler, G.; Erber, W.; Bröker, M.; Schmitt, H.J. The TBE Book; Global Health Press: Singapore, 2019. [Google Scholar] [CrossRef]
- Hansson, K.E.; Rosdahl, A.; Insulander, M.; Vene, S.; Lindquist, L.; Gredmark-Russ, S.; Askling, H.H. Tick-borne Encephalitis Vaccine Failures: A 10-year Retrospective Study Supporting the Rationale for Adding an Extra Priming Dose in Individuals Starting at Age 50 Years. Clin. Infect. Dis. 2020, 70, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Engman, M.L.; Lindstrom, K.; Sallamba, M.; Hertz, C.; Sundberg, B.; Hansson, M.E.; Lindquist, L.; Orvell, C.; Lidefelt, K.J.; Sundin, M. One-year follow-up of tick-borne central nervous system infections in childhood. Pediatr. Infect. Dis. J. 2012, 31, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Sundin, M.; Hansson, M.E.; Engman, M.L.; Orvell, C.; Lindquist, L.; Wide, K.; Lidefelt, K.J. Pediatric tick-borne infections of the central nervous system in an endemic region of Sweden: A prospective evaluation of clinical manifestations. Eur. J. Pediatr. 2012, 171, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Erber, W.; Schmitt, H.J. Self-reported tick-borne encephalitis (TBE) vaccination coverage in Europe: Results from a cross-sectional study. Ticks Tick. Borne Dis. 2018, 9, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Askling, H.H.; Insulander, M.; Hergens, M.P.; Leval, A. Tick borne encephalitis (TBE)-vaccination coverage and analysis of variables associated with vaccination, Sweden. Vaccine 2015, 33, 4962–4968. [Google Scholar] [CrossRef] [PubMed]
- FSME-IMMUN. Summary of Products Characteristics. Available online: https://mri.cts-mrp.eu/human/downloads/AT_H_0126_002_FinalSPC.pdf (accessed on 8 July 2021).
- Wanke, K.H.L.; Mekker, A.; Steffen, R.; Stiasny, K.; Heinz, F.X.; Unger, B.; Karrer, U. Immunogenicity and safety of tick borne encephalitis vaccination in healthy elderly individuals. In Proceedings of the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), London, UK, 31 March–3 April 2012. [Google Scholar]
- Konior, R.; Brzostek, J.; Poellabauer, E.M.; Jiang, Q.; Harper, L.; Erber, W. Seropersistence of TBE virus antibodies 10 years after first booster vaccination and response to a second booster vaccination with FSME-IMMUN 0.5mL in adults. Vaccine 2017, 35, 3607–3613. [Google Scholar] [CrossRef] [PubMed]
- Schosser, R.; Reichert, A.; Mansmann, U.; Unger, B.; Heininger, U.; Kaiser, R. Irregular tick-borne encephalitis vaccination schedules: The effect of a single catch-up vaccination with FSME-IMMUN. A prospective non-interventional study. Vaccine 2014, 32, 2375–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinz, F.X.; Stiasny, K.; Holzmann, H.; Grgic-Vitek, M.; Kriz, B.; Essl, A.; Kundi, M. Vaccination and tick-borne encephalitis, central Europe. Emerg Infect. Dis 2013, 19, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Baroutsou, V.; Zens, K.D.; Sinniger, P.; Fehr, J.; Lang, P. Analysis of Tick-borne Encephalitis vaccination coverage and compliance in adults in Switzerland, 2018. Vaccine 2020, 38, 7825–7833. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitt, H.-J.; Dobler, G.; Zavadska, D.; Freimane, Z.; Fousteris, D.; Erber, W.; Jodar, L.; Palmborg, A. TBE Vaccination Breakthrough Cases—Does Age Matter? Vaccines 2021, 9, 932. https://doi.org/10.3390/vaccines9080932
Schmitt H-J, Dobler G, Zavadska D, Freimane Z, Fousteris D, Erber W, Jodar L, Palmborg A. TBE Vaccination Breakthrough Cases—Does Age Matter? Vaccines. 2021; 9(8):932. https://doi.org/10.3390/vaccines9080932
Chicago/Turabian StyleSchmitt, Heinz-J., Gerhard Dobler, Dace Zavadska, Zane Freimane, Dimitrios Fousteris, Wilhelm Erber, Luis Jodar, and Andreas Palmborg. 2021. "TBE Vaccination Breakthrough Cases—Does Age Matter?" Vaccines 9, no. 8: 932. https://doi.org/10.3390/vaccines9080932
APA StyleSchmitt, H.-J., Dobler, G., Zavadska, D., Freimane, Z., Fousteris, D., Erber, W., Jodar, L., & Palmborg, A. (2021). TBE Vaccination Breakthrough Cases—Does Age Matter? Vaccines, 9(8), 932. https://doi.org/10.3390/vaccines9080932




