Cost–Benefit Analysis of a Mass Vaccination Strategy to Control Brucellosis in Sheep and Goats in Northern Iraq
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Scope and Context
2.2. Economic Model
2.2.1. Data Sources and Model Input Parameters
2.2.2. Model Assumptions and Simplifications
2.2.3. Productivity and Reproduction Impacts
2.2.4. Strategy and Control of the Disease
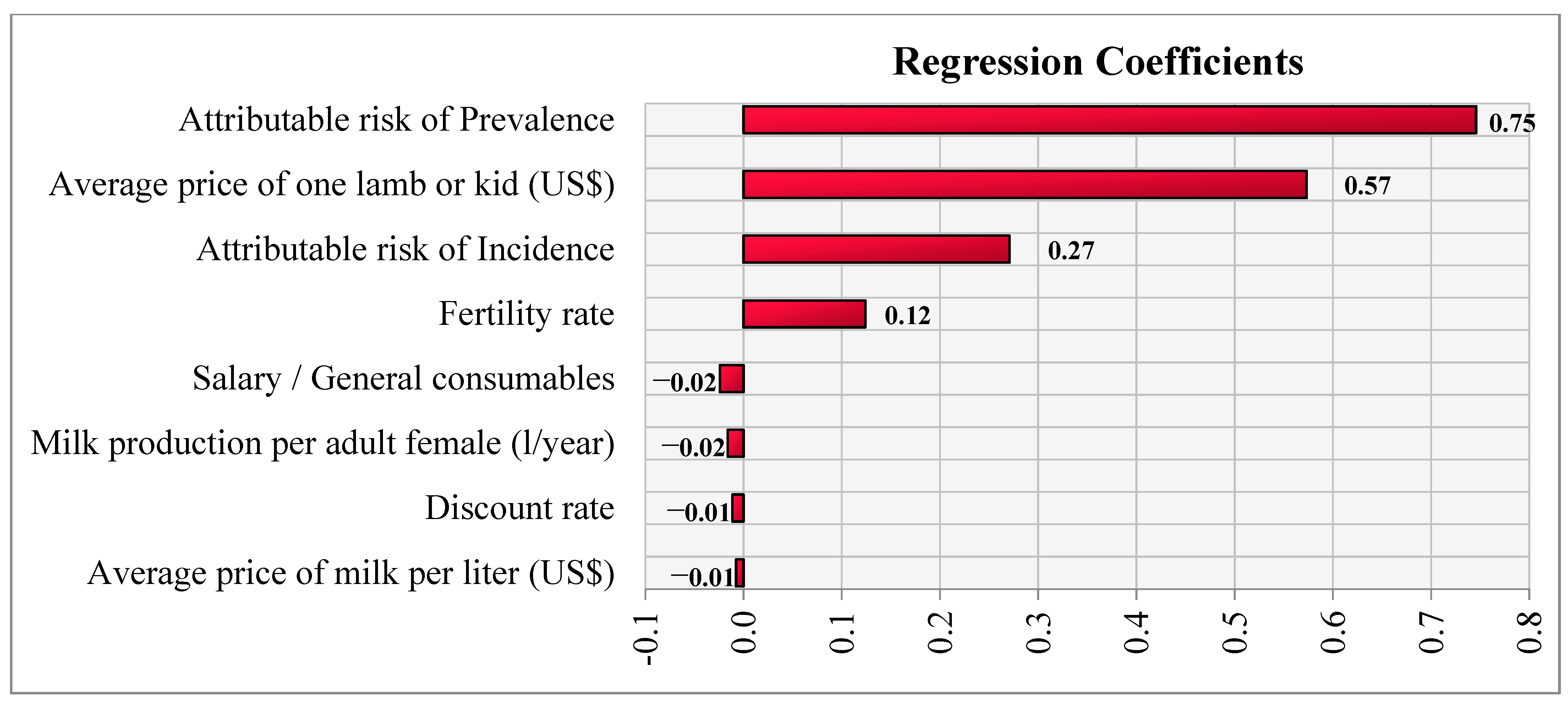
2.2.5. Sensitivity Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beauvais, W.; Musallam, I.; Guitian, J. Vaccination control programs for multiple livestock host species: An age-stratified, seasonal transmission model for brucellosis control in endemic settings. Parasit Vectors 2016, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- McDermott, J.; Grace, D.; Zinsstag, J. Economics of brucellosis impact and control in low-income countries. Rev. Off. Int. Epizoot. 2013, 32, 249–261. [Google Scholar] [CrossRef] [Green Version]
- Atluri, V.L.; Xavier, M.N.; De Jong, M.F.; Den Hartigh, A.B.; Tsolis, R.M. Interactions of the human pathogenic Brucella species with their hosts. Annu. Rev. Microbiol. 2011, 65, 523–541. [Google Scholar] [CrossRef]
- Samadi, A.; Ababneh, M.; Giadinis, N.; Lafi, S. Ovine and caprine brucellosis (Brucella melitensis) in aborted animals in Jordanian sheep and goat flocks. Vet. Med. Int. 2010, 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossetti, C.A.; Arenas-Gamboa, A.M.; Maurizio, E. Caprine brucellosis: A historically neglected disease with significant impact on public health. PLoS Negl. Trop. Dis. 2017, 11, e0005692. [Google Scholar] [CrossRef] [Green Version]
- Franc, K.; Krecek, R.; Häsler, B.; Arenas-Gamboa, A. Brucellosis remains a neglected disease in the developing world: A call for interdisciplinary action. BMC Public Health 2018, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, A.; Bakr, S.; Hameed, A.; Al Thamery, A.; Fartoci, M. Seroepidemiology of selected zoonotic infections in Basra region of Iraq. EMHJ-East. Mediterr. Health J. 2006, 12, 112–118. [Google Scholar]
- Jansen, W.; Linard, C.; Noll, M.; Noeckler, K.; Al Dahouk, S. Brucella-positive raw milk cheese sold on the inner European market: A public health threat due to illegal import? Food Control. 2019, 100, 130–137. [Google Scholar] [CrossRef]
- Khan, M.Z.; Zahoor, M. An overview of brucellosis in cattle and humans, and its serological and molecular diagnosis in control strategies. Trop. Med. Infect. Dis. 2018, 3, 65. [Google Scholar] [CrossRef] [Green Version]
- Dean, A.S.; Crump, L.; Greter, H.; Schelling, E.; Zinsstag, J. Global burden of human brucellosis: A systematic review of disease frequency. PLoS Negl. Trop. Dis. 2012, 6, e1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seleem, M.N.; Boyle, S.M.; Sriranganathan, N. Brucellosis: A re-emerging zoonosis. Vet. Microbiol. 2010, 140, 392–398. [Google Scholar] [CrossRef]
- Alshwany, E.A.A. The Epidemiology of Brucellosis in Sheep, Goats and Humans in the Iraqi Kurdistan Region. Ph.D. Thesis, Murdoch University, Perth, Australia, 2019. [Google Scholar]
- Alhamada, A.; Habib, I.; Barnes, A.; Robertson, I. Risk factors associated with brucella seropositivity in sheep and goats in Duhok Province, Iraq. Vet. Sci. 2017, 4, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bechtol, D.; Carpenter, L.; Mosites, E.; Smalley, D.; Dunn, J. Brucella melitensis infection following military duty in Iraq. Zoonoses Public Health 2011, 58, 489–492. [Google Scholar] [CrossRef]
- Zundel, E.; Verger, J.; Grayon, M.; Michel, R. Conjunctival vaccination of pregnant ewes and goats with Brucella melitensis Rev 1 vaccine: Safety and serological responses. Ann. Rech. Vet. 1992, 23, 177–188. [Google Scholar]
- Zangana, I.K.; Ali, B.A.; Naqid, I.A. Distribution of ectoparasites infested sheep and goats in Duhok province, north Iraq. Bas. J. Vet. Res. 2013, 12, 54–64. [Google Scholar]
- AlHamada, A.; Habib, I.; Bruce, M.; Barnes, A.; Robertson, I.D. Seroconversion to Brucella spp. and Toxoplasma gondii in Sheep and Goats in Dohuk Province, Iraq and its association with pregnancy loss. Animals 2021, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- Unicef (The United Nations Children′s Fund). Head Office, New York, NY, USA. 2016. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/UN064587_Costs_of_EDU.pdf (accessed on 15 July 2021).
- Nielsen, K.; Gall, D.; Smith, P.; Balsevicius, S.; Garrido, F.; Ferrer, M.D.; Biancifiori, F.; Dajer, A.; Luna, E.; Samartino, L. Comparison of serological tests for the detection of ovine and caprine antibody to Brucella melitensis. Rev. Sci. Tech. 2004, 23, 979–987. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Scientific opinion on performance of brucellosis diagnostic methods for bovines, sheep, and goats. EFSA J. 2006, 432, 1–44. [Google Scholar]
- Iniguez, L. Characterization of Small Ruminant Breeds in West Asia and North Africa. Vol 2: North Africa; International Center for Agricultural Research in Dry Areas (ICARDA): Aleppo, Syria, 2005. [Google Scholar]
- Marsh, W. The economics of animal health in farmed livestock at the herd level. Rev. Sci. Tech. Off. Int. Des. Epizoot. 1999, 18, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, Y.; Ridler, A.; Guitian, F. Assessment and simulation of the implementation of brucellosis control programme in an endemic area of the Middle East. Epidemiol. Infect. 2009, 137, 1436–1448. [Google Scholar] [CrossRef]
- Pacinovski, N.; Dzabirski, V.; Porcu, K.; Cilev, G.; Joshevska, E.; Petrovic, M.P.; Antunovic, Z. Factors influencing productive traits of Awassi crossbreeds in Macedonia. J. Anim. Sci. Biotechnol. 2016, 32, 145–161. [Google Scholar] [CrossRef]
- Galal, S.; Gürsoy, O.; Shaat, I. Awassi sheep as a genetic resource and efforts for their genetic improvement—A review. Small Rumin. Res. 2008, 79, 99–108. [Google Scholar] [CrossRef]
- Benkirane, A.; El Idrissi, A.; Doumbia, A.; de Balogh, K. Innocuity and immune response to Brucella melitensis Rev. 1 vaccine in camels (Camelus dromedarius). Open J. Vet. Med. 2014, 4, 96–102. [Google Scholar]
- Alves, A.J.S.; Rocha, F.; Amaku, M.; Ferreira, F.; Telles, E.O.; Grisi Filho, J.H.d.H.; Neto, J.F.; Zylbersztajn, D.; Dias, R.A. Economic analysis of vaccination to control bovine brucellosis in the States of Sao Paulo and Mato Grosso, Brazil. Prev. Vet. Med. 2015, 118, 351–358. [Google Scholar] [CrossRef]
- Bamaiyi, P.H.; Hassan, L.; Khairani-Bejo, S.; ZainalAbidin, M. The economic impact attributable to brucellosis among goat farms in Peninsula Malaysia and cost benefit analysis. Res. Opin. Anim. Vet. Sci. 2015, 5, 57–64. [Google Scholar]
- Sulima, M.; Venkataraman, K. Economic losses due to Brucella melitensis infection in sheep and goats. Tamilnadu J. Vet. Anim. Sci. 2010, 6, 191–192. [Google Scholar]
- Roth, F.; Zinsstag, J.; Orkhon, D.; Chimed-Ochir, G.; Hutton, G.; Cosivi, O.; Carrin, G.; Otte, J. Human health benefits from livestock vaccination for brucellosis: Case study. Bull. World Health Organ. 2003, 81, 867–876. [Google Scholar] [PubMed]
- Scharp, D.; Al Khalaf, S.S.; Al Muhanna, M.; Cheema, R.; Godana, W. Use of mass vaccination with a reduced dose of REV 1 vaccine for Brucella melitensis control in a population of small ruminants. Trop. Anim. Health Prod. 1999, 31, 135–141. [Google Scholar] [CrossRef]
- Corbel, M.J. Brucellosis in Humans and Animals; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Blasco, J.M. Control and eradication strategies for Brucella melitensis infection in sheep and goats. Prilozi 2010, 31, 145–165. [Google Scholar] [PubMed]
- Blasco, J.M.; Molina-Flores, B. Control and eradication of Brucella melitensis infection in sheep and goats. Vet. Clin. Food Anim. Pract. 2011, 27, 95–104. [Google Scholar] [CrossRef]
- Montiel, D.O.; Bruce, M.; Frankena, K.; Udo, H.; van der Zijpp, A.; Rushton, J. Financial analysis of brucellosis control for small-scale goat farming in the Bajío region, Mexico. Prev. Vet. Med. 2015, 118, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.B.; Kostoulas, P.; Gill, J.P.; Dhand, N.K. Cost-benefit analysis of intervention policies for prevention and control of brucellosis in India. PLoS Negl. Trop. Dis. 2018, 12, e0006488. [Google Scholar] [CrossRef] [PubMed]
- Minas, A.; Minas, M.; Stournara, A.; Tselepidis, S. The “effects” of Rev-1 vaccination of sheep and goats on human brucellosis in Greece. Prev. Vet. Med. 2004, 64, 41–47. [Google Scholar] [CrossRef]
- Perrett, L.; Brew, S.; Stack, J.; MacMillan, A.; Bashiruddin, J. Experimental assessment of the pathogenicity of Brucella strains from marine mammals for pregnant sheep. Small Rumin. Res. 2004, 51, 221–228. [Google Scholar] [CrossRef]
- Blasco, J. A review of the use of B. melitensis Rev 1 vaccine in adult sheep and goats. Prev. Vet. Med. 1997, 31, 275–283. [Google Scholar] [CrossRef]
- Saeedzadeh, A.; Sharifiyazdi, H.; Firouzi, R. Molecular characterization of Brucella melitensis Rev. 1 strain in aborted sheep and goats in Iran. Comp. Clin. Pathol. 2013, 22, 409–412. [Google Scholar] [CrossRef]
- Al-Majali, A.M.; Talafha, A.Q.; Ababneh, M.M.; Ababneh, M.M. Seroprevalence and risk factors for bovine brucellosis in Jordan. J. Vet. Sci. 2009, 10, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Montiel, D.O. Keeping Goats or Going North? Enhancing Livelihoods of Smallholder Goat Farmers through Brucellosis Control in Mexico. Ph.D. Thesis, Wageningen UR, Wageningen, The Netherlands, 2014. [Google Scholar]

| Parameters | Description | Value | Reference/Source |
|---|---|---|---|
| Annual milk production | litre per adult female (ewe/doe) per year | Pert distribution (Min = 60; Mode = 109; Max = 134) | Pacinovski, et al. [24] |
| Fertility rate | Per year, for adult female % | Pert distribution (Min = 0.76; Mode = 0.85; Max = 0.95) | Galal, et al. [25] |
| Average price of milk * | Per kg | Pert distribution (Min = 0.6317; Mode = 0.8421; Max = 1.0528) | Questionnaire data |
| Average price of a lamb or kid | Per a lamb or kid | Pert distribution (Min = 25; Mode = 50; Max = 75) | Questionnaire data |
| Percentage of adult females | Out of the total population | 70.7% | Questionnaire data |
| Percentage of lambs/kids | Out of the total population | 26% | Questionnaire data |
| Attributable risk | For new (incidence) cases | Pert distribution (Min = 0.05; Mode = 0.25; Max = 0.45) | AlHamada, et al. [17] |
| Attributable risk | For existing (prevalence) cases | Pert distribution (Min = 0; Mode = 0.18; Max = 0.34) | AlHamada, et al. [17] |
| TP | True prevalence (TP) = | 9.22% | Calculated |
| AP | Apparent prevalence (AP) | 8.33% | Alhamada, et al. [13] |
| Se | Sensitivity (series testing) | 90.22% | EFSA [20] and Nielsen, et al. [19] |
| Sp | Specificity (series testing) | 99.99% | EFSA [20] and Nielsen, et al. [19] |
| s | Proportion of susceptible animals [t = 0] | 0.46 | Calculation (s = 1 − TP − Pr) |
| Pr | Proportion of protected animals [t = 0] | 0.45 | Calculation (Pr = Vc × Ve) |
| Vc | Vaccination coverage | 60% | Directorate of Dohuk Veterinary Hospital |
| Ve | Vaccine efficacy | 75% | Benkirane, et al. [26] |
| N | Number of adult female sheep and goats in Dohuk Governorate | 706,800 | Total number of animals × percentage of adult females |
| P | Number of protected females in Dohuk Governorate (year 0) | 299,115 | Calculated (P = (N × Pr)) |
| Re | Effective reproduction number | 1 | assumption of endemic equilibrium |
| R0 | Basic reproduction numberR0 = Re ÷ s | 2.18 | Hegazy, et al. [23] |
| D | Duration of overall managed breeding before culling | Five years | Calculation (1 ÷ u) |
| U | Replacement sheep per year (Cull rate) | 20% | Director of the vet. Services in Dohuk city |
| beta | Transmission coefficient | 6.18 × 10−7 | Calculated beta = Re ÷ (N × D × s) |
| Rev. 1 vaccine | Price per dose | US$0.10 | Directorate of Dohuk Veterinary in Dohuk city |
| Years | Future Benefits | Future Costs | Future Value | PV of Benefits | PV of Costs | NPV |
|---|---|---|---|---|---|---|
| 1 | $0 | $191,200 | −$191,200 | $0 | $179,728 | −$179,728 |
| 2 | $176,770 | $191,200 | −$14,430 | $156,194 | $168,944 | −$12,750 |
| 3 | $347,854 | $191,200 | $156,654 | $288,922 | $158,808 | $130,114 |
| 4 | $499,649 | $191,200 | $308,449 | $390,101 | $149,279 | $240,821 |
| 5 | $632,108 | $191,200 | $440,908 | $463,907 | $140,322 | $323,584 |
| 6 | $747,260 | $191,200 | $556,060 | $515,512 | $131,903 | $383,609 |
| 7 | $847,307 | $191,200 | $656,107 | $549,460 | $123,989 | $425,471 |
| 8 | $934,257 | $191,200 | $743,057 | $569,494 | $116,550 | $452,944 |
| 9 | $1,009,865 | $191,200 | $818,665 | $578,647 | $109,557 | $469,091 |
| 10 | $1,075,649 | $191,200 | $884,449 | $579,361 | $102,983 | $476,377 |
| 11 | $1,132,914 | $191,200 | $941,714 | $573,592 | $96,804 | $476,788 |
| 12 | $1,182,789 | $191,200 | $991,589 | $562,913 | $90,996 | $471,917 |
| 13 | $1,226,245 | $191,200 | $1,035,045 | $548,579 | $85,536 | $463,043 |
| 14 | $1,264,123 | $191,200 | $1,072,923 | $531,593 | $80,404 | $451,189 |
| 15 | $1,297,150 | $191,200 | $1,105,950 | $512,753 | $75,580 | $437,173 |
| 16 | $1,325,955 | $191,200 | $1,134,755 | $492,691 | $71,045 | $421,646 |
| 17 | $1,351,084 | $191,200 | $1,159,884 | $471,906 | $66,782 | $405,124 |
| 18 | $1,373,011 | $191,200 | $1,181,811 | $450,791 | $62,775 | $388,016 |
| 19 | $1,392,148 | $191,200 | $1,200,948 | $429,650 | $59,009 | $370,641 |
| 20 | $1,408,853 | $191,200 | $1,217,653 | $408,717 | $55,468 | $353,249 |
| Total | $19,224,992 | $3,824,000 | $15,400,992 | $9,074,783 | $2,126,463 | $6,948,320 |
| Benefits (Median and 95% CI) | |
|---|---|
| PV Benefits | US$ 13,813,524 (95% CI: −12,964,774–40,290,145) |
| PV Costs | US$ 3,241,685 (95% CI: 2,971,912–3,515,547) |
| NPV | US$ 10,564,828 (95% CI: −16,203,454–37,049,245) |
| BCR | 4.25 (95% CI: −2.71–11.22) |
| IRR | 91.38% (95% CI: 11.7–190.6%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Hamada, A.; Bruce, M.; Barnes, A.; Habib, I.; D. Robertson, I. Cost–Benefit Analysis of a Mass Vaccination Strategy to Control Brucellosis in Sheep and Goats in Northern Iraq. Vaccines 2021, 9, 878. https://doi.org/10.3390/vaccines9080878
Al Hamada A, Bruce M, Barnes A, Habib I, D. Robertson I. Cost–Benefit Analysis of a Mass Vaccination Strategy to Control Brucellosis in Sheep and Goats in Northern Iraq. Vaccines. 2021; 9(8):878. https://doi.org/10.3390/vaccines9080878
Chicago/Turabian StyleAl Hamada, Ali, Mieghan Bruce, Anne Barnes, Ihab Habib, and Ian D. Robertson. 2021. "Cost–Benefit Analysis of a Mass Vaccination Strategy to Control Brucellosis in Sheep and Goats in Northern Iraq" Vaccines 9, no. 8: 878. https://doi.org/10.3390/vaccines9080878
APA StyleAl Hamada, A., Bruce, M., Barnes, A., Habib, I., & D. Robertson, I. (2021). Cost–Benefit Analysis of a Mass Vaccination Strategy to Control Brucellosis in Sheep and Goats in Northern Iraq. Vaccines, 9(8), 878. https://doi.org/10.3390/vaccines9080878







