Cost-Effectiveness of Three Poliovirus Immunization Schedules in Shanghai, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Definitions of AFP and VAPP
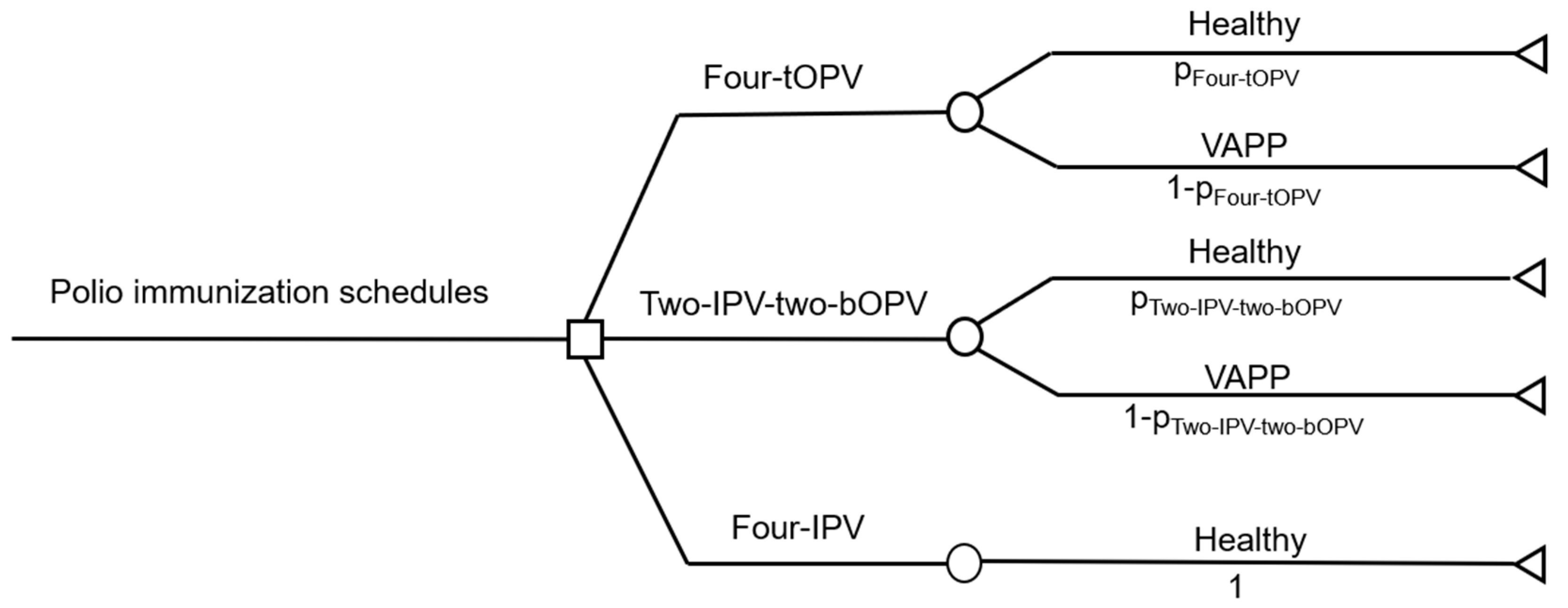
2.2. Construction of a Decision Tree Model
2.3. Measurement of Cost
2.3.1. Procurement of Vaccines
2.3.2. Storage of Vaccines
2.3.3. Compensation for VAPP Cases
2.3.4. Total Cost
2.4. Measurement of Effectiveness
2.4.1. Incidence of VAPP Cases
2.4.2. DALY Caused by VAPP Cases
2.5. Measurement of ICER
2.6. Determination of WTP
3. Results
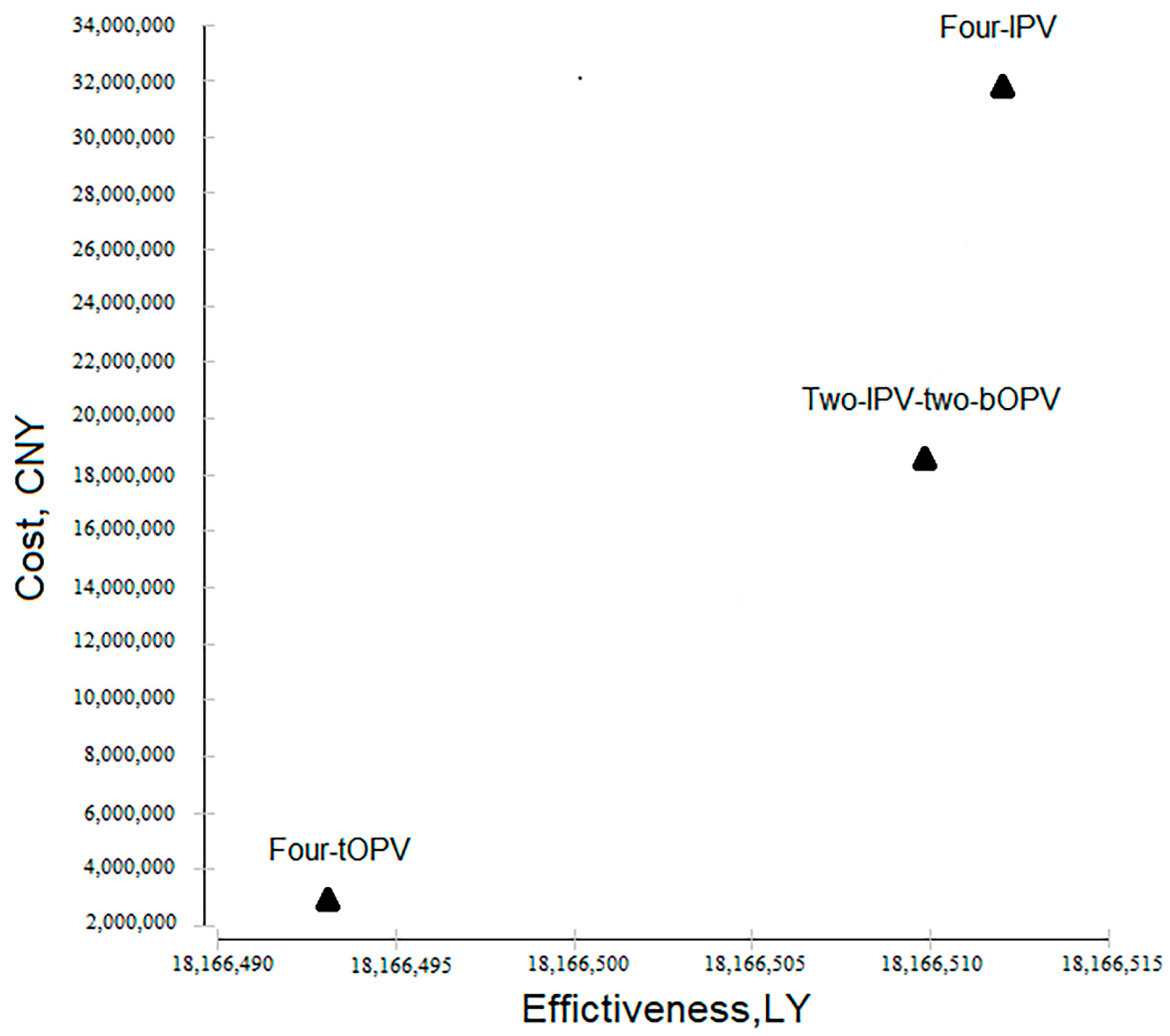
3.1. Cost-Effectiveness across Three Polio Immunization Schedules
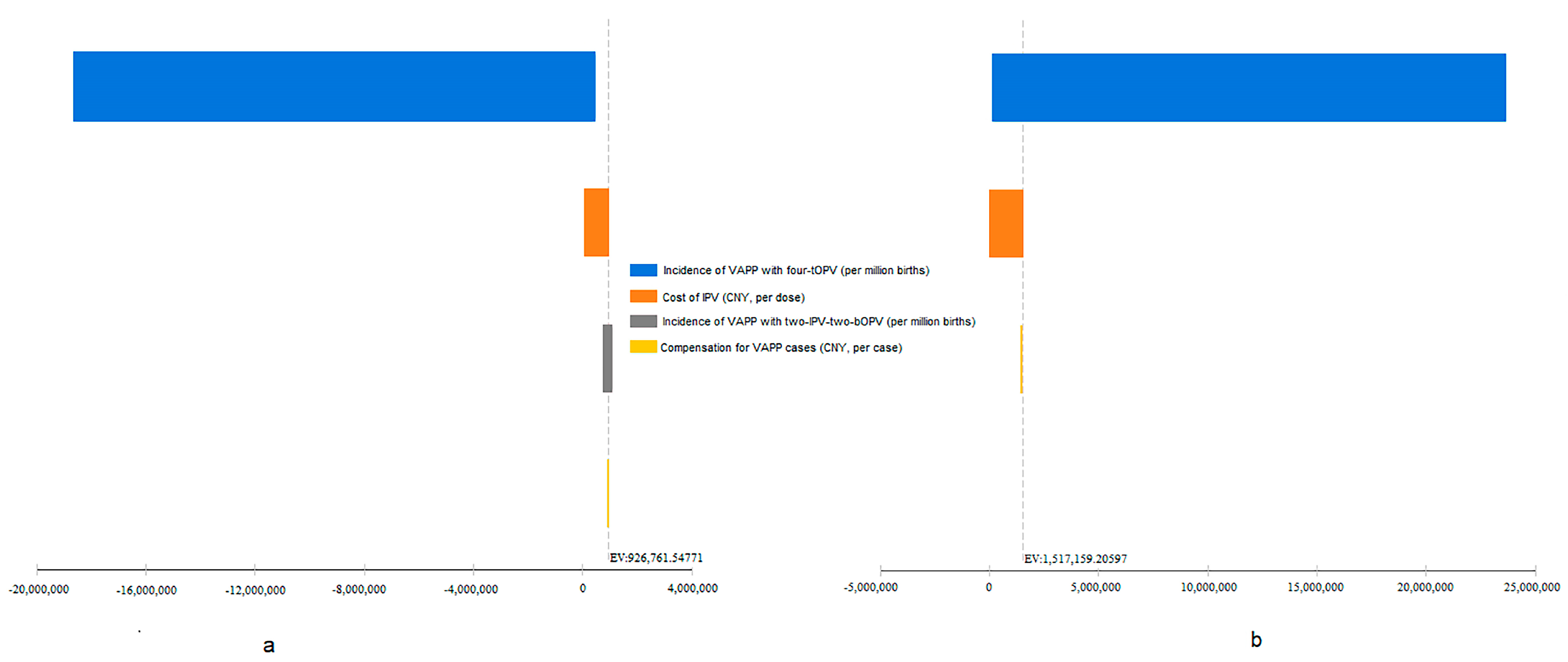
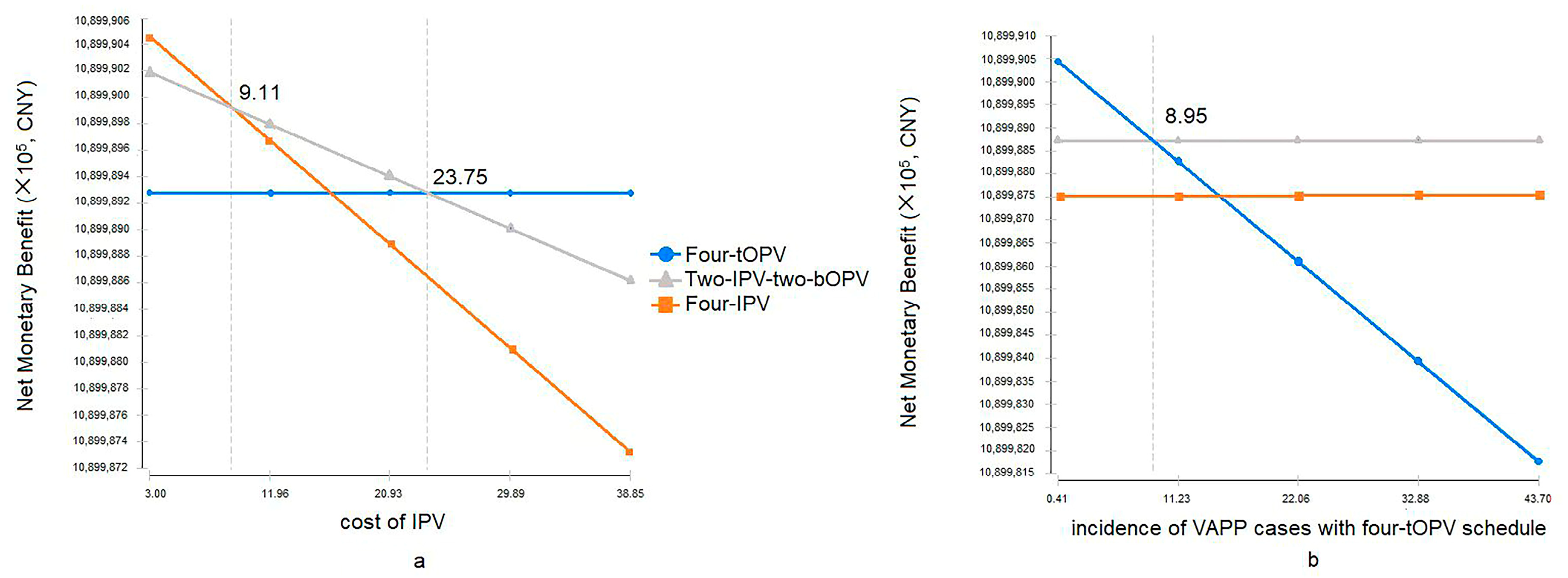
3.2. Sensitivity Analysis of Cost-Effectiveness
3.3. Cost-Benefit across Three Polio Immunization Schedules
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | Acute Flaccid Paralysis |
| bOPV | bivalent live attenuated Oral Polio Vaccines |
| CNY | Chinese Yuan |
| DALY | Disability-adjusted Life Years |
| EPI | Expanded Programme on Immunization |
| ICER | Incremental Cost-Effectiveness Ratio |
| IPV | Inactivated Polio Vaccines |
| LY | Life Year |
| NMB | Net Monetary Benefit |
| OPV | Live Attenuated Oral Polio Vaccines |
| PV | Poliovirus |
| SCDC | Shanghai Municipal Center for Disease Control and Prevention |
| sIPV | Sabin Strain Inactivated Poliovirus Vaccine |
| tOPV | trivalent Live Attenuated Oral Polio Vaccines |
| VAPP | Vaccine-associated Paralytic Poliomyelitis |
| VDPV | Vaccine-derived Poliovirus |
| WHO | World Health Organization |
| wIPV | wild-type Inactivated Polio Vaccines |
| WPV | Wild Poliovirus |
| WTP | Willingness to Pay |
Appendix A
References
- WHO. Polio vaccines: WHO position paper—March, 2016. Wkly. Epidemiol. Rec. 2016, 91, 145–168. [Google Scholar]
- Yu, W.-Z.; Wen, N.; Zhang, Y.; Wang, H.-B.; Fan, C.-X.; Zhu, S.-L.; Xu, W.-B.; Liang, X.-F.; Luo, H.-M.; Li, L. Poliomyelitis eradication in China: 1953–2012. J. Infect. Dis. 2014, 210, S268–S274. [Google Scholar] [CrossRef]
- Wu, W.; Wang, H.; Li, K.; Nuorti, J.P.; Liu, D.; Xu, D.; Ye, J.; Zheng, J.; Fan, C.; Wen, N.; et al. Recipient vaccine-associated paralytic poliomyelitis in China, 2010–2015. Vaccine 2018, 36, 1209–1213. [Google Scholar] [CrossRef]
- Wu, Z.M.; Hu, J.Y.; Xu, T.Q.; Li, X.Z.; Zhao, L.L. Epidemiological analysis of the vaccine-associated paralytic poliomyelitis in 1980–1998 in Shanghai. Chin. J. Vaccines Immun. 1999, 6, 6–8. [Google Scholar]
- World Health Organization. Poliomyelitis: WHO Response. 22 July 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/poliomyelitis (accessed on 25 June 2021).
- Hsu, C.H.; Kader, M.; Mahamud, A.; Bullard, K.; Jorba, J.; Agbor, J.; Safi, M.M.; Jafari, H.S.; Ehrhardt, D. Progress toward poliomyelitis eradication—Pakistan, January 2018–September 2019. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68, 1029–1033. [Google Scholar] [CrossRef]
- Pan, J.; Wang, Y.; Cao, L.; Wang, Y.; Zhao, Q.; Tang, S.; Gong, W.; Guo, L.; Liu, Z.; Wen, Z.; et al. Impact of immunization programs on 11 childhood vaccine-preventable diseases in China: 1950–2018. Innovation 2021, 2. [Google Scholar] [CrossRef]
- Lopalco, P.L. Wild and vaccine-derived poliovirus circulation, and implications for polio eradication. Epidemiol. Infect. 2016, 145, 413–419. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Sun, X.D.; Liu, M.Y.; Li, C.S.; Ren, J.; Hu, J.Y.; Yang, J.P.; Liu, J.C.; Li, Z.; Yang, Y.Y.; et al. Immunogenicity analysis of sequential inoculation of different strains of poliomyelitis vaccines in Shanghai. Chin. J. Prev. Med. 2019, 53, 513–518. [Google Scholar]
- Liu, M.Y.; Huang, Z.Y.; Lu, J.; Yang, J.P.; Yang, Y.Y. Polio antibody levels in infants during 2015–2016 in Shanghai before and after polio vaccination strategy adjustment. Shanghai J. Prev. Med. 2020, 32, 627–630. [Google Scholar]
- Shanghai Municipal Health Commission. Notice on the Adjustment of the Immunization Program of Polio Vaccine and Measles-Containing Vaccine in the City; Shanghai Municipal Health Commission: Shanghai, China, 2020. Available online: http://wsjkw.sh.gov.cn/jbfk2/20200724/784eaeeaf7ce4ebd8e2528cdd6d35280.html (accessed on 14 May 2021).
- Tebbens, R.J.D.; Pallansch, M.A.; Cochi, S.L.; Wassilak, S.G.; Linkins, J.; Sutter, R.W.; Aylward, R.B.; Thompson, K. Economic analysis of the global polio eradication initiative. Vaccine 2010, 29, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M. Economics of polio vaccination in the post-eradication era: Should OPV-using countries adopt IPV? Vaccine 2008, 26, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M.; Tebbens, R.J.D.; Pallansch, M.A.; Kew, O.M.; Sutter, R.W.; Aylward, R.B.; Watkins, M.; Gary, H.E.; Alexander, J.; Jafari, H.; et al. The risks, costs, and benefits of possible future global policies for managing polioviruses. Am. J. Public Health 2008, 98, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Alvis, N.; de la Hoz, F.; Narváez, J. Economic impact of introducing the injectable inactivated polio vaccine in Colombia. Rev. Panam. Salud. Publica 2010, 27, 352–359. [Google Scholar] [CrossRef][Green Version]
- Li, R.P. Strategic Analysis and Cost Estimation on OPV Switching to Sabin-IPV at the Stage of the Endgame of Polio Eradication in China; Shandong University: Jinan, China, 2007. [Google Scholar]
- Griffiths, U.K.; Botham, L.; Schoub, B.D. The cost-effectiveness of alternative polio immunization policies in South Africa. Vaccine 2006, 24, 5670–5678. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.W.; Isaacs, D.; Burgess, M. Cost-effectiveness analysis of changing from live oral poliovirus vaccine to inactivated poliovirus vaccine in Australia. Aust. N. Zealand J. Public Health 2001, 25, 411–416. [Google Scholar] [CrossRef]
- Miller, M.A. Cost-effectiveness of incorporating inactivated poliovirus vaccine into the routine childhood immunization schedule. JAMA 1996, 276, 967. [Google Scholar] [CrossRef]
- Tebbens, R.J.D.; Pallansch, M.A.; Cochi, S.L.; Wassilak, S.G.; Thompson, K.M. An economic analysis of poliovirus risk management policy options for 2013–2052. BMC Infect. Dis. 2015, 15, 1–21. [Google Scholar] [CrossRef]
- Yang, L.X. Incremental Cost Study of Inactivated Poliovirus Vaccine into National Immunization Program; Chinese Center for Disease Control and Prevention: Beijing, China, 2015.
- Thompson, K.M.; Tebbens, R.J.D. Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Anal. 2006, 26, 1423–1440. [Google Scholar] [CrossRef]
- Sartori, A.M.C.; Vicentine, M.P.; Gryninger, L.C.F.; Soarez, P.; Novaes, H. Polio inactivated vaccine costs into routine childhood immunization in Brazil. Rev. Saude Public 2015, 49, 1–10. [Google Scholar] [CrossRef][Green Version]
- Khan, M.; Sharma, S.; Tripathi, B.; Alvarez, F.P. Budget impact of polio immunization strategy for India: Introduction of one dose of inactivated poliomyelitis vaccine and reductions in supplemental polio immunization. Public Health 2017, 142, 31–38. [Google Scholar] [CrossRef]
- Domingues, C.M.A.S.; de Fátima Pereira, S.; Cunha Marreiros, A.C.; Menezes, N.; Flannery, B. Introduction of sequential inactivated polio vaccine-oral polio vaccine schedule for routine infant immunization in Brazil’s National Immunization Program. J. Infect. Dis. 2014, 210 (Suppl. S1), S143–S151. [Google Scholar] [CrossRef] [PubMed]
- Nanteza, M.B.; Kisakye, A.; Ota, M.O.; Gumede, N.; Bwogi, J. The expanded program on immunization laboratory team—Uganda Virus Research Institute (EPI LAB—UVRI) Vaccine associated paralytic poliomyelitis cases from children presenting with acute flaccid paralysis in Uganda. J. Med. Virol. 2015, 87, 2163–2167. [Google Scholar] [CrossRef] [PubMed]
- Diop, O.M.; Burns, C.C.; Wassilak, S.G.; Kew, O.M. Update on vaccine-derived polioviruses—Worldwide, July 2012–December 2013. MMWR. Morb. Mortal. Wkly. Rep. 2014, 63, 242. [Google Scholar] [PubMed]
- WS 294-2016 Poliomyelitis Diagnosis. 2016. Available online: http://www.nhc.gov.cn/wjw/s9491/201606/55fca4a0e4b0458bba1-659fda26c22e6.shtml (accessed on 14 May 2021).
- Shanghai Bureau of Statistics National Investigation Corps, Shanghai Bureau of Statistics. 2017 Shanghai Statistical Yearbook; China Statistics Press: Shanghai, China, 2017.
- TreeAge. Tornado diagrams. In TreeAge Pro 2017 User’s Manual; TreeAge Software, Inc.: Williamstown, MA, USA, 2017; pp. 256–260. [Google Scholar]
- Le, V.; Zhong, L.; Narsipur, N.; Hays, E.; Tran, D.K.; Rosario, K.; Wilson, L. Cost-effectiveness of ribociclib plus endocrine therapy versus placebo plus endocrine therapy in HR-positive, HER2-negative breast cancer. J. Manag. Care Speéc. Pharm. 2021, 27, 327–338. [Google Scholar] [CrossRef]
- Arakawa, I.; Momoeda, M.; Osuga, Y.; Ota, I.; Koga, K. Cost-effectiveness of the recommended medical intervention for the treatment of dysmenorrhea and endometriosis in Japan. Cost Eff. Resour. Alloc. 2018, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Practical Regulations for Vaccination in Shanghai (2017 Edition). 2017. Available online: http://wsjkw.sh.gov.cn/mygh-zcwj/20191012/0012-65397.html (accessed on 7 July 2021).
- Duan, M.J.; Cao, L.; Cao, L.S. Wastage factor estimation of bivalent polio attenuated live vaccine in China. Chin. J. Vaccines Immun. 2017, 23, 134–137. [Google Scholar]
- Shanghai Compensation Measures for Vaccination Related Abnormal Reactions (for Trial Implementation). 2017. Available online: http://wsjkw.sh.gov.cn/mygh-zcwj/20191012/0012-65399.html (accessed on 25 June 2021).
- World Bank. Country Income Groups. Available online: http://chartsbin.com/view/2438 (accessed on 25 June 2021).
- Mao, Z.H.; Hu, D.W. Medical Economics 2004; China Statistics Press: Beijing, China, 2004. [Google Scholar]
- Circulating Vaccine-derived Poliovirus (cVDPV). Available online: https://polioeradication.org/wp-content/uploads/2020/12/20201118_cVDPV2_Global_Update_EN.pdf (accessed on 21 September 2021).
- Platt, L.R.; Estívariz, C.F.; Sutter, R.W. Vaccine-associated paralytic poliomyelitis: A review of the epidemiology and estimation of the global burden. J. Infect. Dis. 2014, 210, S380–S389. [Google Scholar] [CrossRef]
- Sutter, R.W.; Kew, O.M.; Cochi, S.L. Poliovirus vaccine-live. Vaccines 2008, 631–685. [Google Scholar] [CrossRef]
- Ivanova, O.E.; Eremeeva, T.P.; Morozova, N.S.; Shakaryan, A.K.; Korotkova, E.A.; Kozlovskaya, L.I.; Baykova, O.Y.; Krasota, A.Y.; Gmyl, A.P. Vaccine-associated paralytic poliomyelitis in the Russian Federation in 1998–2014. Int. J. Infect. Dis. 2018, 76, 64–69. [Google Scholar] [CrossRef]
- Alexander, L.N. Vaccine policy changes and epidemiology of poliomyelitis in the United States. JAMA 2004, 292, 1696–1701. [Google Scholar] [CrossRef]
- World Health Organization Department Immunization. Principles and Considerations for Adding a Vaccine to a National Immuniza-tion Programme: From Decision to Implementation and Monitoring; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Huang, Z.; Hu, J.; Sun, X.; Yang, J. Analysis of poliomyelitis eradication and recommendations for immunization strategies. J. Microbes Infect. 2020, 15, 337–344. [Google Scholar]
- WHO. Polio vaccines: WHO position paper, March 2016—Recommendations. Vaccine 2017, 35, 1197–1199. [Google Scholar] [CrossRef] [PubMed]
- Nasir, U.N.; Bandyopadhyay, A.S.; Montagnani, F.; Akite, J.E.; Mungu, E.B.; Uche, I.; Ismaila, A.M. Polio elimination in Nigeria: A review. Hum. Vaccines Immunother. 2015, 12, 658–663. [Google Scholar] [CrossRef]
- Shanghai Bureau of Statistics. Shanghai Statistical Bulletin of National Economic and Social Development, 2011. Statistical Science and Practice; Shanghai Bureau of Statistics: Shanghai, China, 2013; Volume 3, pp. 7–14.
- Shanghai Bureau of Statistics. Shanghai Statistical Bulletin of National Economic and Social Development, 2012. Statistical Science and Practice; Shanghai Bureau of Statistics: Shanghai, China, 2013; Volume 3, pp. 16–23.
- Shanghai Bureau of Statistics. Shanghai Statistical Bulletin of National Economic and Social Development, 2013. Statistical Science and Practice; Shanghai Bureau of Statistics: Shanghai, China, 2014; Volume 3, pp. 14–24.
- Shanghai Bureau of Statistics. Shanghai Statistical Bulletin of National Economic and Social Development, 2014. Statistical Science and Practice; Shanghai Bureau of Statistics: Shanghai, China, 2015; Volume 3, pp. 20–29.
- Shanghai Bureau of Statistics. Shanghai Statistical Bulletin of National Economic and Social Development, 2015. Statistical Science and Practice; Shanghai Bureau of Statistics: Shanghai, China, 2016; Volume 3, pp. 19–28.
- Zhang, L.F.; Yu, Z.W.; Wen, N.; Luo, H.M. Occurrence and Epidemiological Characteristics of Vaccine-associated Paralytic Poliomyelitis. Chin. J. Vaccines. Immun. 2015, 21, 101–107. [Google Scholar]
- Wattigney, W.A.; Mootrey, G.T.; Braun, M.M.; Chen, R.T. Surveillance for poliovirus vaccine adverse events, 1991 to 1998: Impact of a sequential vaccination schedule of inactivated poliovirus vaccine followed by oral poliovirus vaccine. Pediatrics 2001, 107, e83. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Baseline Value | Minimum Value | Maximum Value | Source of Data |
|---|---|---|---|---|
| Cost | ||||
| Government procurement price of vaccines (CNY/vial) | ||||
| tOPV (for 10-dose) | 9.70 | 9.70 | 9.70 | China Government Procurement Network |
| bOPV (for 10-dose) | 27.20 | 27.20 | 27.20 | China Government Procurement Network |
| sIPV (for 1-dose) | 35.00 | 35.00 | 35.00 | China Government Procurement Network |
| wIPV (for 1-dose) | 39.00 | 39.00 | 39.00 | China Government Procurement Network |
| Coefficient of loss | ||||
| 10-dose per vial | 1.80 | 1.80 | 2.50 | Practical Regulations for Vaccination in Shanghai (2017 edition) [33]; SCDC |
| 1-dose per vial | 1.00 | 1.00 | 1.05 | Practical Regulations for Vaccination in Shanghai (2017 edition) [33]; SCDC |
| Procurement ratio of sIPV:wIPV | 2:1 | 1:1 | 2:1 | SCDC |
| Procurement price of the low-temperature refrigerator (CNY) | 15,000 | 10,000 | 20,000 | SCDC |
| Depreciation of low-temperature refrigerator (years) | 9 | 8 | 10 | SCDC |
| Compensation for VAPP cases (CNY, per case) | 748,005 | 748,005 | 1,250,000 | SCDC; Shanghai Compensation Measures for Vaccination Related Adverse Events (trial edition) [35]; Yang LS [21] |
| Effectiveness | ||||
| Number of local resident births in Shanghai in 2016 (per 10,000) | 21.84 | 21.84 | 21.84 | Shanghai Statistical Yearbook 2017 [29] |
| Incidence of VAPP (per 1 million births) | ||||
| Four-tOPV schedule | 6.20 | 0.41 | 43.70 | SCDC; WHO study [39] |
| Two-IPV-two-bOPV schedule | 0.70 | 0.00 | 1.56 | SCDC; WHO position paper [1]; Sutter et al. [40]; Ivanova et al. [41] |
| Four-IPV schedule | 0 | 0 | 0 | |
| DALY caused by VAPP | 14 | 13 | 14 | Duintjer et al. [12], and Thompson et al. [14] |
| Four-tOPV Schedule | Two-IPV-Two-bOPV Schedule | Four-IPV Schedule | |
|---|---|---|---|
| Total cost (CNY 10,000 per year) | 297.98 | 1856.42 | 3174.08 |
| Cost of vaccines (CNY 10,000 per year) | 152.53 | 1800.90 | 3174.08 |
| Maintenance of low-temperature refrigerator (CNY 10,000 per year) | 44.17 | 44.17 | 0 |
| Compensation for VAPP cases (CNY 10,000 per year) | 101.29 | 11.36 | 0 |
| Incremental cost (CNY 10,000 per year) | reference | 1558.44 | 2876.10 |
| Number of VAPP cases prevented (per year) | reference | 1.20 | 1.35 |
| DALY averted (per year) | reference | 16.83 | 18.96 |
| ICERVAPP (CNY 10,000 per VAPP case) | reference | 1296.26 | 2124.02 |
| ICERDALY (CNY 10,000 per DALY) | reference | 92.59 | 151.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.; Maimaiti, H.; Sun, X.; Huang, Z.; Liu, J.; Yang, J.; Li, Z.; Bai, Q.; Lu, Y. Cost-Effectiveness of Three Poliovirus Immunization Schedules in Shanghai, China. Vaccines 2021, 9, 1062. https://doi.org/10.3390/vaccines9101062
Ren J, Maimaiti H, Sun X, Huang Z, Liu J, Yang J, Li Z, Bai Q, Lu Y. Cost-Effectiveness of Three Poliovirus Immunization Schedules in Shanghai, China. Vaccines. 2021; 9(10):1062. https://doi.org/10.3390/vaccines9101062
Chicago/Turabian StyleRen, Jia, Hairenguli Maimaiti, Xiaodong Sun, Zhuoying Huang, Jiechen Liu, Jianping Yang, Zhi Li, Qingrui Bai, and Yihan Lu. 2021. "Cost-Effectiveness of Three Poliovirus Immunization Schedules in Shanghai, China" Vaccines 9, no. 10: 1062. https://doi.org/10.3390/vaccines9101062
APA StyleRen, J., Maimaiti, H., Sun, X., Huang, Z., Liu, J., Yang, J., Li, Z., Bai, Q., & Lu, Y. (2021). Cost-Effectiveness of Three Poliovirus Immunization Schedules in Shanghai, China. Vaccines, 9(10), 1062. https://doi.org/10.3390/vaccines9101062






