Producing Vaccines against Enveloped Viruses in Plants: Making the Impossible, Difficult
Abstract
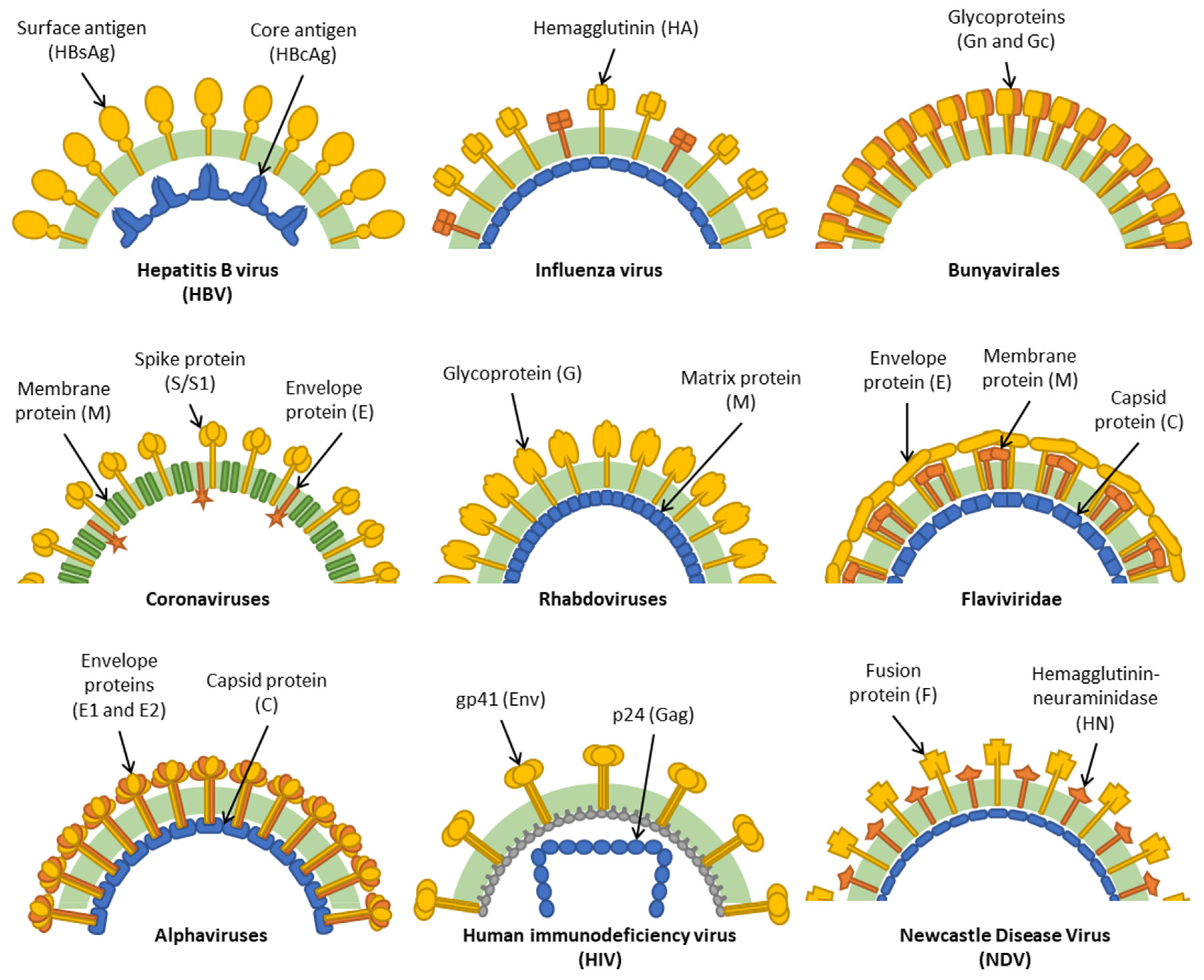
1. Introduction
2. Approaches
3. HBV
4. Influenza Viruses
5. Bunyavirales
6. Coronaviruses
7. Rhabdoviruses
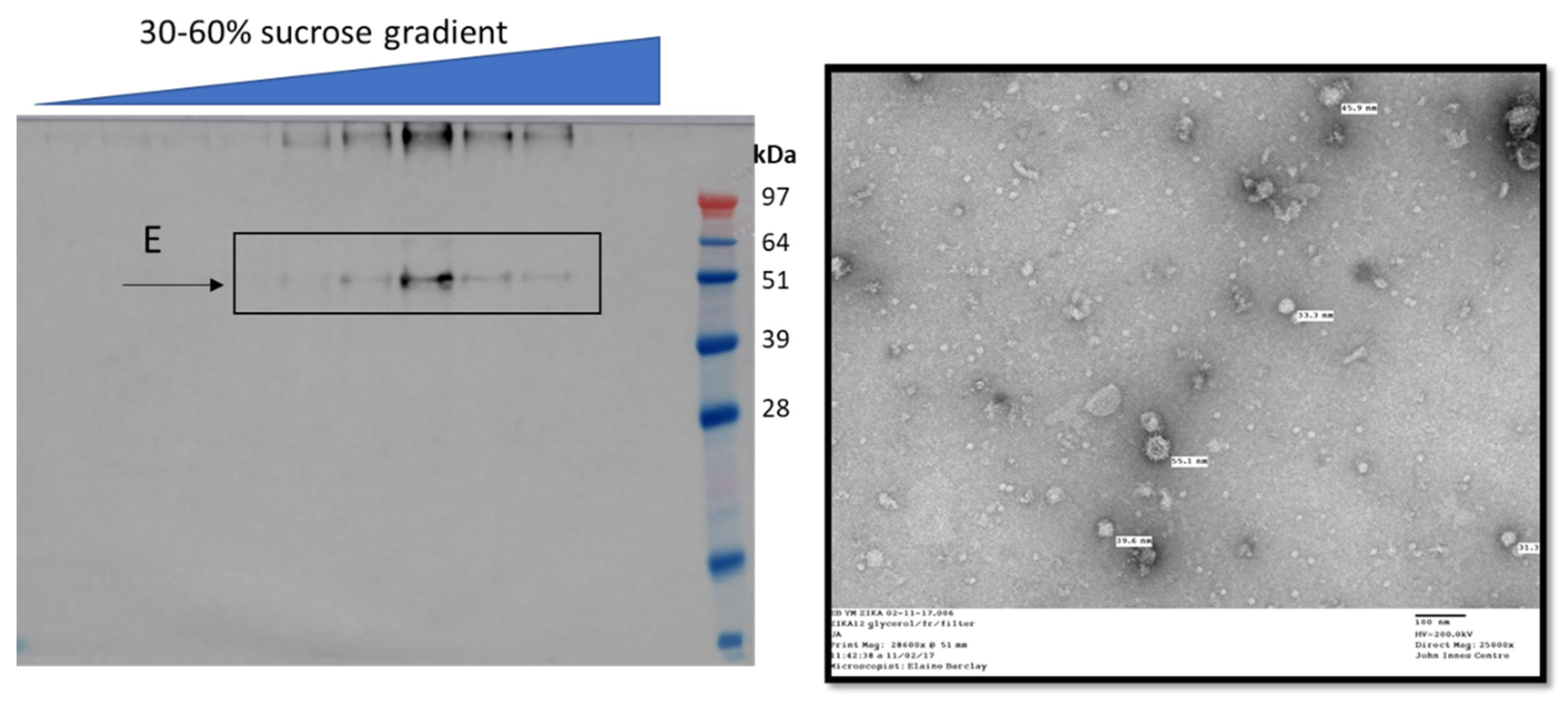
8. Flaviviridae
9. Alphaviruses
10. HIV
11. Newcastle Disease Virus
12. Other Virus Families
13. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Gomes, A.C.; Vogel, M.; Bachmann, M.F. Interaction of Viral Capsid-Derived Virus-Like Particles (VLPs) with the Innate Immune System. Vaccines 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Marsian, J.; Lomonossoff, G.P. Molecular pharming—VLPs made in plants. Curr. Opin. Biotechnol. 2016, 37, 201–206. [Google Scholar] [CrossRef]
- Thuenemann, E.C.; Lenzi, P.; Andrew, J.L.; Taliansky, M.; Bécares, M.; Zuñiga, S.; Enjuanes, L.; Zahmanova, G.G.; Minkov, I.N.; Matić, S.; et al. The use of transient expression systems for the rapid production of virus-like particles in plants. Curr. Pharm. Des. 2013, 19, 5564–5573. [Google Scholar] [CrossRef]
- Dalsgaard, K.; Uttenthal, A.; Jones, T.D.; Xu, F.; Merryweather, A.; Hamilton, W.D.; Langeveld, J.P.; Boshuizen, R.S.; Kamstrup, S.; Lomonossoff, G.P.; et al. Plant-derived vaccine protects target animals against a viral disease. Nat. Biotechnol. 1997, 15, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Thuenemann, E.C.; Meyers, A.E.; Verwey, J.; Rybicki, E.P.; Lomonossoff, G.P. A method for rapid production of heteromultimeric protein complexes in plants: Assembly of protective bluetongue virus-like particles. Plant Biotechnol. J. 2013, 11, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Marsian, J.; Fox, H.; Bahar, M.W.; Kotecha, A.; Fry, E.E.; Stuart, D.I.; Macadam, A.J.; Rowlands, D.J.; Lomonossoff, G.P. Plant-made polio type 3 stabilized VLPs—A candidate synthetic polio vaccine. Nat. Commun. 2017, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.S.; Lam, D.M.; Arntzen, C.J. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. USA 1992, 89, 11745–11749. [Google Scholar] [CrossRef]
- D’Aoust, M.A.; Lavoie, P.O.; Couture, M.M.; Trepanier, S.; Guay, J.M.; Dargis, M.; Mongrand, S.; Landry, N.; Ward, B.J.; Vezina, L.P. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol. J. 2008, 6, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E.P. Plant-based vaccines against viruses. Virol. J. 2014, 11, 205. [Google Scholar] [CrossRef]
- Lomonossoff, G.P.; Johnson, J.E. Use of macromolecular assemblies as expression systems for peptides and synthetic vaccines. Curr. Opin. Struct. Biol. 1996, 6, 176–182. [Google Scholar] [CrossRef]
- Lomonossoff, G.P. Virus particles and the uses of such particles in bio- and nanotechnology. In Recent Advances in Plant Virology; Caranta, C., Aranda, M.A., Tepfer, M., Lopez-Moya, J.J., Eds.; Caister Academic Press: Poole, UK, 2011. [Google Scholar] [CrossRef]
- Mardanova, E.S.; Kotlyarov, R.Y.; Kuprianov, V.V.; Stepanova, L.A.; Tsybalova, L.M.; Lomonosoff, G.P.; Ravin, N.V. Rapid high-yield expression of a candidate influenza vaccine based on the ectodomain of M2 protein linked to flagellin in plants using viral vectors. BMC Biotechnol. 2015, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Mardanova, E.S.; Kotlyarov, R.Y.; Kuprianov, V.V.; Stepanova, L.A.; Tsybalova, L.M.; Lomonossoff, G.P.; Ravin, N.V. High immunogenicity of plant-produced candidate influenza vaccine based on the M2e peptide fused to flagellin. Bioengineered 2016, 7, 28–32. [Google Scholar] [CrossRef]
- Thanavala, Y.; Yang, Y.F.; Lyons, P.; Mason, H.S.; Arntzen, C. Immunogenicity of transgenic plant-derived hepatitis B surface antigen. Proc. Natl. Acad. Sci. USA 1995, 92, 3358–3361. [Google Scholar] [CrossRef]
- Kapusta, J.; Modelska, A.; Figlerowicz, M.; Pniewski, T.; Letellier, M.; Lisowa, O.; Yusibov, V.; Koprowski, H.; Plucienniczak, A.; Legocki, A.B. A plant-derived edible vaccine against hepatitis B virus. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1999, 13, 1796–1799. [Google Scholar] [CrossRef]
- Kapusta, J.; Modelska, A.; Pniewski, T.; Figlerowicz, M.; Jankowski, K.; Lisowa, O.; Plucienniczak, A.; Koprowski, H.; Legocki, A.B. Oral immunization of human with transgenic lettuce expressing hepatitis B surface antigen. Adv. Exp. Med. Biol. 2001, 495, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Richter, L.; Yang, Y.F.; Arntzen, C.J.; Mason, H.S.; Thanavala, Y. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc. Natl. Acad. Sci. USA 2001, 98, 11539–11544. [Google Scholar] [CrossRef]
- Thanavala, Y.; Mahoney, M.; Pal, S.; Scott, A.; Richter, L.; Natarajan, N.; Goodwin, P.; Arntzen, C.J.; Mason, H.S. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc. Natl. Acad. Sci. USA 2005, 102, 3378–3382. [Google Scholar] [CrossRef] [PubMed]
- Richter, L.J.; Thanavala, Y.; Arntzen, C.J.; Mason, H.S. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat. Biotechnol. 2000, 18, 1167–1171. [Google Scholar] [CrossRef]
- Kumar, G.B.; Ganapathi, T.R.; Revathi, C.J.; Srinivas, L.; Bapat, V.A. Expression of hepatitis B surface antigen in transgenic banana plants. Planta 2005, 222, 484–493. [Google Scholar] [CrossRef]
- Pniewski, T.; Kapusta, J.; Bociąg, P.; Wojciechowicz, J.; Kostrzak, A.; Gdula, M.; Fedorowicz-Strońska, O.; Wójcik, P.; Otta, H.; Samardakiewicz, S.; et al. Low-dose oral immunization with lyophilized tissue of herbicide-resistant lettuce expressing hepatitis B surface antigen for prototype plant-derived vaccine tablet formulation. J. Appl. Genet. 2011, 52, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Richter, L.; Arntzen, C.J.; Shuler, M.L.; Mason, H.S. Structural characterization of plant-derived hepatitis B surface antigen employed in oral immunization studies. Vaccine 2003, 21, 4011–4021. [Google Scholar] [CrossRef]
- Huang, Z.; LePore, K.; Elkin, G.; Thanavala, Y.; Mason, H.S. High-yield rapid production of hepatitis B surface antigen in plant leaf by a viral expression system. Plant Biotechnol. J. 2008, 6, 202–209. [Google Scholar] [CrossRef]
- Pniewski, T. Is an oral plant-based vaccine against hepatitis B virus possible? Curr. Pharm. Biotechnol. 2012, 13, 2692–2704. [Google Scholar] [CrossRef]
- Pniewski, T. The twenty-year story of a plant-based vaccine against hepatitis B: Stagnation or promising prospects? Int. J. Mol. Sci. 2013, 14, 1978–1998. [Google Scholar] [CrossRef] [PubMed]
- Joung, Y.H.; Park, S.H.; Moon, K.-B.; Jeon, J.-H.; Cho, H.-S.; Kim, H.-S. The Last Ten Years of Advancements in Plant-Derived Recombinant Vaccines against Hepatitis B. Int. J. Mol. Sci. 2016, 17, 1715. [Google Scholar] [CrossRef]
- Ho, J.K.-T.; Jeevan-Raj, B.; Netter, H.-J. Hepatitis B Virus (HBV) Subviral Particles as Protective Vaccines and Vaccine Platforms. Viruses 2020, 12, 126. [Google Scholar] [CrossRef]
- Mechtcheriakova, I.A.; Eldarov, M.A.; Nicholson, L.; Shanks, M.; Skryabin, K.G.; Lomonossoff, G.P. The use of viral vectors to produce hepatitis B virus core particles in plants. J. Virol. Methods 2006, 131, 10–15. [Google Scholar] [CrossRef]
- Huang, Z.; Santi, L.; LePore, K.; Kilbourne, J.; Arntzen, C.J.; Mason, H.S. Rapid, high-level production of hepatitis B core antigen in plant leaf and its immunogenicity in mice. Vaccine 2006, 24, 2506–2513. [Google Scholar] [CrossRef]
- Tsuda, S.; Yoshioka, K.; Tanaka, T.; Iwata, A.; Yoshikawa, A.; Watanabe, Y.; Okada, Y. Application of the Human Hepatitis B Virus Core Antigen from Transgenic Tobacco Plants for Serological Diagnosis. Vox Sang. 1998, 74, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Pyrski, M.; Mieloch, A.A.; Plewiński, A.; Basińska-Barczak, A.; Gryciuk, A.; Bociąg, P.; Murias, M.; Rybka, J.D.; Pniewski, T. Parenteral-Oral Immunization with Plant-Derived HBcAg as a Potential Therapeutic Vaccine against Chronic Hepatitis B. Vaccines 2019, 7, 211. [Google Scholar] [CrossRef]
- Peyret, H.; Gehin, A.; Thuenemann, E.C.; Blond, D.; El Turabi, A.; Beales, L.; Clarke, D.; Gilbert, R.J.C.; Fry, E.E.; Stuart, D.I.; et al. Tandem Fusion of Hepatitis B Core Antigen Allows Assembly of Virus-Like Particles in Bacteria and Plants with Enhanced Capacity to Accommodate Foreign Proteins. PLoS ONE 2015, 10, e0120751. [Google Scholar] [CrossRef]
- Pang, E.L.; Peyret, H.; Ramirez, A.; Loh, H.S.; Lai, K.S.; Fang, C.M.; Rosenberg, W.M.; Lomonossoff, G.P. Epitope Presentation of Dengue Viral Envelope Glycoprotein Domain, I.I.I. on Hepatitis B Core Protein Virus-Like Particles Produced in Nicotiana benthamiana. Front. Plant Sci. 2019, 10, 455. [Google Scholar] [CrossRef]
- Zahmanova, G.; Mazalovska, M.; Takova, K.; Toneva, V.; Minkov, I.; Peyret, H.; Lomonossoff, G. Efficient Production of Chimeric Hepatitis B Virus-Like Particles Bearing an Epitope of Hepatitis, E. Virus Capsid by Transient Expression in Nicotiana benthamiana. Life 2021, 11, 64. [Google Scholar] [CrossRef]
- Meshcheriakova Iu, A.; El’darov, M.A.; Migunov, A.I.; Stepanova, L.A.; Repko, I.A.; Kiselev, O.I.; Lomonosov, D.P.; Skriabin, K.G. Cowpea mosaic virus chimeric particles bearing ectodomain of matrix protein 2 (M2E) of influenza A virus: Production and characteristics. Mol. Biol. 2009, 43, 741–750. [Google Scholar] [CrossRef]
- Ravin, N.V.; Kotlyarov, R.Y.; Mardanova, E.S.; Kuprianov, V.V.; Migunov, A.I.; Stepanova, L.A.; Tsybalova, L.M.; Kiselev, O.I.; Skryabin, K.G. Plant-produced recombinant influenza vaccine based on virus-like HBc particles carrying an extracellular domain of M2 protein. Biochem. Biokhimiia 2012, 77, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Mallajosyula, J.K.; Hiatt, E.; Hume, S.; Johnson, A.; Jeevan, T.; Chikwamba, R.; Pogue, G.P.; Bratcher, B.; Haydon, H.; Webby, R.J.; et al. Single-dose monomeric HA subunit vaccine generates full protection from influenza challenge. Hum. Vaccine Immunother. 2014, 10, 586–595. [Google Scholar] [CrossRef]
- Pham, N.B.; Ho, T.T.; Nguyen, G.T.; Le, T.T.; Le, N.T.; Chang, H.C.; Pham, M.D.; Conrad, U.; Chu, H.H. Nanodiamond enhances immune responses in mice against recombinant HA/H7N9 protein. J. Nanobiotechnol. 2017, 15, 69. [Google Scholar] [CrossRef][Green Version]
- Blokhina, E.A.; Mardanova, E.S.; Stepanova, L.A.; Tsybalova, L.M.; Ravin, N.V. Plant-Produced Recombinant Influenza A Virus Candidate Vaccine Based on Flagellin Linked to Conservative Fragments of M2 Protein and Hemagglutintin. Plants 2020, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Mett, V.; Musiychuk, K.; Bi, H.; Farrance, C.E.; Horsey, A.; Ugulava, N.; Shoji, Y.; de la Rosa, P.; Palmer, G.A.; Rabindran, S.; et al. A plant-produced influenza subunit vaccine protects ferrets against virus challenge. Influenza Other Respir. Viruses 2008, 2, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Shoji, Y.; Jones, R.M.; Mett, V.; Chichester, J.A.; Musiychuk, K.; Sun, X.; Tumpey, T.M.; Green, B.J.; Shamloul, M.; Norikane, J.; et al. A plant-produced H1N1 trimeric hemagglutinin protects mice from a lethal influenza virus challenge. Hum. Vaccine Immunother. 2013, 9, 553–560. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Phan, H.T.; Pohl, J.; Floss, D.M.; Rabenstein, F.; Veits, J.; Le, B.T.; Chu, H.H.; Hause, G.; Mettenleiter, T.; Conrad, U. ELPylated haemagglutinins produced in tobacco plants induce potentially neutralizing antibodies against H5N1 viruses in mice. Plant Biotechnol. J. 2013, 11, 582–593. [Google Scholar] [CrossRef]
- Phan, H.T.; Hause, B.; Hause, G.; Arcalis, E.; Stoger, E.; Maresch, D.; Altmann, F.; Joensuu, J.; Conrad, U. Influence of elastin-like polypeptide and hydrophobin on recombinant hemagglutinin accumulations in transgenic tobacco plants. PLoS ONE 2014, 9, e99347. [Google Scholar] [CrossRef]
- Phan, H.T.; Ho, T.T.; Chu, H.H.; Vu, T.H.; Gresch, U.; Conrad, U. Neutralizing immune responses induced by oligomeric H5N1-hemagglutinins from plants. Vet. Res. 2017, 48, 53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Phan, H.T.; Gresch, U.; Conrad, U. In vitro-Formulated Oligomers of Strep-Tagged Avian Influenza Haemagglutinin Produced in Plants Cause Neutralizing Immune Responses. Front. Bioeng. Biotechnol. 2018, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.T.; Pham, V.T.; Ho, T.T.; Pham, N.B.; Chu, H.H.; Vu, T.H.; Abdelwhab, E.M.; Scheibner, D.; Mettenleiter, T.C.; Hanh, T.X.; et al. Immunization with Plant-Derived Multimeric H5 Hemagglutinins Protect Chicken against Highly Pathogenic Avian Influenza Virus H5N1. Vaccines 2020, 8, 593. [Google Scholar] [CrossRef] [PubMed]
- Shoji, Y.; Chichester, J.A.; Bi, H.; Musiychuk, K.; de la Rosa, P.; Goldschmidt, L.; Horsey, A.; Ugulava, N.; Palmer, G.A.; Mett, V.; et al. Plant-expressed HA as a seasonal influenza vaccine candidate. Vaccine 2008, 26, 2930–2934. [Google Scholar] [CrossRef] [PubMed]
- Shoji, Y.; Bi, H.; Musiychuk, K.; Rhee, A.; Horsey, A.; Roy, G.; Green, B.; Shamloul, M.; Farrance, C.E.; Taggart, B.; et al. Plant-derived hemagglutinin protects ferrets against challenge infection with the A/Indonesia/05/05 strain of avian influenza. Vaccine 2009, 27, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Shoji, Y.; Chichester, J.A.; Jones, M.; Manceva, S.D.; Damon, E.; Mett, V.; Musiychuk, K.; Bi, H.; Farrance, C.; Shamloul, M.; et al. Plant-based rapid production of recombinant subunit hemagglutinin vaccines targeting H1N1 and H5N1 influenza. Hum. Vaccines 2011, 7 (Suppl. 1), 41–50. [Google Scholar] [CrossRef]
- Mortimer, E.; Maclean, J.M.; Mbewana, S.; Buys, A.; Williamson, A.L.; Hitzeroth, I.I.; Rybicki, E.P. Setting up a platform for plant-based influenza virus vaccine production in South Africa. BMC Biotechnol. 2012, 12, 14. [Google Scholar] [CrossRef]
- Neuhaus, V.; Chichester, J.A.; Ebensen, T.; Schwarz, K.; Hartman, C.E.; Shoji, Y.; Guzmán, C.A.; Yusibov, V.; Sewald, K.; Braun, A. A new adjuvanted nanoparticle-based H1N1 influenza vaccine induced antigen-specific local mucosal and systemic immune responses after administration into the lung. Vaccine 2014, 32, 3216–3222. [Google Scholar] [CrossRef]
- Ceballo, Y.; Tiel, K.; López, A.; Cabrera, G.; Pérez, M.; Ramos, O.; Rosabal, Y.; Montero, C.; Menassa, R.; Depicker, A.; et al. High accumulation in tobacco seeds of hemagglutinin antigen from avian (H5N1) influenza. Transgenic Res. 2017, 26, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Nahampun, H.N.; Bosworth, B.; Cunnick, J.; Mogler, M.; Wang, K. Expression of H3N2 nucleoprotein in maize seeds and immunogenicity in mice. Plant Cell Rep. 2015, 34, 969–980. [Google Scholar] [CrossRef]
- Chichester, J.A.; Jones, R.M.; Green, B.J.; Stow, M.; Miao, F.; Moonsammy, G.; Streatfield, S.J.; Yusibov, V. Safety and immunogenicity of a plant-produced recombinant hemagglutinin-based influenza vaccine (HAI-05) derived from A/Indonesia/05/2005 (H5N1) influenza virus: A phase 1 randomized, double-blind, placebo-controlled, dose-escalation study in healthy adults. Viruses 2012, 4, 3227–3244. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.F.; Guerrero, M.L.; Moon, J.E.; Waterman, P.; Nielsen, R.K.; Jefferson, S.; Gross, F.L.; Hancock, K.; Katz, J.M.; Yusibov, V. Safety and immunogenicity of a plant-produced recombinant monomer hemagglutinin-based influenza vaccine derived from influenza A (H1N1)pdm09 virus: A Phase 1 dose-escalation study in healthy adults. Vaccine 2014, 32, 2251–2259. [Google Scholar] [CrossRef]
- Landry, N.; Ward, B.J.; Trepanier, S.; Montomoli, E.; Dargis, M.; Lapini, G.; Vezina, L.P. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS ONE 2010, 5, e15559. [Google Scholar] [CrossRef]
- Pillet, S.; Aubin, É.; Trépanier, S.; Poulin, J.F.; Yassine-Diab, B.; Ter Meulen, J.; Ward, B.J.; Landry, N. Humoral and cell-mediated immune responses to H5N1 plant-made virus-like particle vaccine are differentially impacted by alum and GLA-SE adjuvants in a Phase 2 clinical trial. NPJ Vaccines 2018, 3, 3. [Google Scholar] [CrossRef]
- Pillet, S.; Racine, T.; Nfon, C.; Di Lenardo, T.Z.; Babiuk, S.; Ward, B.J.; Kobinger, G.P.; Landry, N. Plant-derived H7 VLP vaccine elicits protective immune response against H7N9 influenza virus in mice and ferrets. Vaccine 2015, 33, 6282–6289. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Aubin, É.; Trépanier, S.; Bussière, D.; Dargis, M.; Poulin, J.F.; Yassine-Diab, B.; Ward, B.J.; Landry, N. A plant-derived quadrivalent virus like particle influenza vaccine induces cross-reactive antibody and T cell response in healthy adults. Clin. Immunol. 2016, 168, 72–87. [Google Scholar] [CrossRef]
- Pillet, S.; Couillard, J.; Trépanier, S.; Poulin, J.F.; Yassine-Diab, B.; Guy, B.; Ward, B.J.; Landry, N. Immunogenicity and safety of a quadrivalent plant-derived virus like particle influenza vaccine candidate-Two randomized Phase II clinical trials in 18 to 49 and ≥50 years old adults. PLoS ONE 2019, 14, e0216533. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.J.; Makarkov, A.; Séguin, A.; Pillet, S.; Trépanier, S.; Dhaliwall, J.; Libman, M.D.; Vesikari, T.; Landry, N. Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18–64 years) and older adults (≥65 years): Two multicentre, randomised phase 3 trials. Lancet 2020, 396, 1491–1503. [Google Scholar] [CrossRef]
- Smith, T.; O’Kennedy, M.M.; Wandrag, D.B.R.; Adeyemi, M.; Abolnik, C. Efficacy of a plant-produced virus-like particle vaccine in chickens challenged with Influenza A H6N2 virus. Plant Biotechnol. J. 2020, 18, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Mbewana, S.; Meyers, A.E.; Rybicki, E.P. Chimaeric Rift Valley Fever Virus-Like Particle Vaccine Candidate Production in Nicotiana benthamiana. Biotechnol. J. 2019, 14, 1800238. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, S.M.; Salmanian, A.H.; Chinikar, S.; Zakeri, S. Mice orally immunized with a transgenic plant expressing the glycoprotein of Crimean-Congo hemorrhagic fever virus. Clin. Vaccine Immunol. CVI 2011, 18, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Kalbina, I.; Lagerqvist, N.; Moiane, B.; Ahlm, C.; Andersson, S.; Strid, Å.; Falk, K.I. Arabidopsis thaliana plants expressing Rift Valley fever virus antigens: Mice exhibit systemic immune responses as the result of oral administration of the transgenic plants. Protein Expr. Purif. 2016, 127, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Capell, T.; Twyman, R.M.; Armario-Najera, V.; Ma, J.K.; Schillberg, S.; Christou, P. Potential Applications of Plant Biotechnology against SARS-CoV-2. Trends Plant Sci. 2020, 25, 635–643. [Google Scholar] [CrossRef]
- Mamedov, T.; Yuksel, D.; Ilgın, M.; Gürbüzaslan, İ.; Gulec, B.; Mammadova, G.; Say, D.; Hasanova, G. Engineering, production and characterization of Spike and Nucleocapsid structural proteins of SARS–CoV-2 in Nicotiana benthamiana as vaccine candidates against COVID-19. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ward, B.J.; Gobeil, P.; Séguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.-Y.; Couture, M.; D’Aoust, M.-A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021, 27, 1071–1078. [Google Scholar] [CrossRef]
- Li, H.Y.; Ramalingam, S.; Chye, M.L. Accumulation of recombinant SARS-CoV spike protein in plant cytosol and chloroplasts indicate potential for development of plant-derived oral vaccines. Exp. Biol. Med. 2006, 231, 1346–1352. [Google Scholar] [CrossRef]
- Zheng, N.; Xia, R.; Yang, C.; Yin, B.; Li, Y.; Duan, C.; Liang, L.; Guo, H.; Xie, Q. Boosted expression of the SARS-CoV nucleocapsid protein in tobacco and its immunogenicity in mice. Vaccine 2009, 27, 5001–5007. [Google Scholar] [CrossRef]
- Demurtas, O.C.; Massa, S.; Illiano, E.; De Martinis, D.; Chan, P.K.; Di Bonito, P.; Franconi, R. Antigen Production in Plant to Tackle Infectious Diseases Flare Up: The Case of SARS. Front. Plant Sci. 2016, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Gómez, N.; Carrillo, C.; Salinas, J.; Parra, F.; Borca, M.V.; Escribano, J.M. Expression of Immunogenic Glycoprotein S Polypeptides from Transmissible Gastroenteritis Coronavirus in Transgenic Plants. Virology 1998, 249, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Gómez, N.; Wigdorovitz, A.; Castañón, S.; Gil, F.; Ordás, R.; Borca, M.V.; Escribano, J.M. Oral immunogenicity of the plant derived spike protein from swine-transmissible gastroenteritis coronavirus. Arch. Virol. 2000, 145, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Huy, N.X.; Kim, Y.S.; Jun, S.C.; Jin, Z.; Park, S.M.; Yang, M.S.; Kim, T.G. Production of a heat-labile enterotoxin B subunit-porcine epidemic diarrhea virus-neutralizing epitope fusion protein in transgenic lettuce (Lactuca sativa). Biotechnol. Bioprocess Eng. BBE 2009, 14, 731–737. [Google Scholar] [CrossRef]
- Huy, N.X.; Yang, M.S.; Kim, T.G. Expression of a cholera toxin B subunit-neutralizing epitope of the porcine epidemic diarrhea virus fusion gene in transgenic lettuce (Lactuca sativa L.). Mol. Biotechnol. 2011, 48, 201–209. [Google Scholar] [CrossRef]
- Huy, N.X.; Kim, S.H.; Yang, M.S.; Kim, T.G. Immunogenicity of a neutralizing epitope from porcine epidemic diarrhea virus: M cell targeting ligand fusion protein expressed in transgenic rice calli. Plant Cell Rep. 2012, 31, 1933–1942. [Google Scholar] [CrossRef]
- Huy, N.-X.; Tien, N.-Q.-D.; Kim, M.-Y.; Kim, T.-G.; Jang, Y.-S.; Yang, M.-S. Immunogenicity of an S1D epitope from porcine epidemic diarrhea virus and cholera toxin B subunit fusion protein transiently expressed in infiltrated Nicotiana benthamiana leaves. Plant Cell Tissue Organ Cult. 2016, 127, 369–380. [Google Scholar] [CrossRef]
- Tien, N.Q.; Huy, N.X.; Kim, M.Y. Improved expression of porcine epidemic diarrhea antigen by fusion with cholera toxin B subunit and chloroplast transformation in Nicotiana tabacum. Plant Cell Tissue Organ Cult. 2019, 137, 213–223. [Google Scholar] [CrossRef]
- Ho, T.T.; Nguyen, G.T.; Pham, N.B.; Le, V.P.; Trinh, T.B.N.; Vu, T.H.; Phan, H.T.; Conrad, U.; Chu, H.H. Plant-Derived Trimeric CO-26K-Equivalent Epitope Induced Neutralizing Antibodies Against Porcine Epidemic Diarrhea Virus. Front. Immunol. 2020, 11, 2152. [Google Scholar] [CrossRef]
- Egelkrout, E.; Hayden, C.; Fake, G.; Keener, T.; Arruda, P.; Saltzman, R.; Walker, J.; Howard, J. Oral delivery of maize-produced porcine epidemic diarrhea virus spike protein elicits neutralizing antibodies in pigs. Plant Cell Tissue Organ Cult. 2020, 1–8. [Google Scholar] [CrossRef]
- Walker, P.J.; Blasdell, K.R.; Calisher, C.H.; Dietzgen, R.G.; Kondo, H.; Kurath, G.; Longdon, B.; Stone, D.M.; Tesh, R.B.; Tordo, N.; et al. ICTV Virus Taxonomy Profile: Rhabdoviridae. J. Gen. Virol. 2018, 99, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Belot, L.; Albertini, A.; Gaudin, Y. Structural and cellular biology of rhabdovirus entry. Adv. Virus Res. 2019, 104, 147–183. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Z. Significantly Improved Recovery of Recombinant Sonchus Yellow Net Rhabdovirus by Expressing the Negative-Strand Genomic RNA. Viruses 2020, 12, 1459. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, X.; Qian, S.; Zhou, X.; Sun, K.; Chen, X.; Zhou, X.; Jackson, A.O.; Li, Z. Rescue of a Plant Negative-Strand RNA Virus from Cloned cDNA: Insights into Enveloped Plant Virus Movement and Morphogenesis. PLoS Pathog. 2015, 11, e1005223. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.O.; Wagner, J.D.O. Procedures for plant rhabdovirus purification, polyribosome isolation, and replicase extraction. In Plant Virology Protocols: From Virus Isolation to Transgenic Resistance; Foster, G.D., Taylor, S.C., Eds.; Humana Press: Totowa, NJ, USA, 1998; pp. 77–97. [Google Scholar] [CrossRef]
- Ibrahim, A.E.C.; Reljic, R.; Drake Pascal, M.W.; Ma, J.K.C. Rational design and expression of a recombinant plant rhabdovirus glycoprotein for production of immunoreactive murine anti-sera. Protein Expr. Purif. 2020, 175, 105691. [Google Scholar] [CrossRef]
- Dietzschold, B.; Gore, M.; Marchadier, D.; Niu, H.S.; Bunschoten, H.M.; Otvos, L., Jr.; Wunner, W.H.; Ertl, H.C.; Osterhaus, A.D.; Koprowski, H. Structural and immunological characterization of a linear virus-neutralizing epitope of the rabies virus glycoprotein and its possible use in a synthetic vaccine. J. Virol. 1990, 64, 3804–3809. [Google Scholar] [CrossRef]
- Perea Arango, I.; Loza Rubio, E.; Rojas Anaya, E.; Olivera Flores, T.; Gonzalez de la Vara, L.; Gómez Lim, M.A. Expression of the rabies virus nucleoprotein in plants at high-levels and evaluation of immune responses in mice. Plant Cell Rep. 2008, 27, 677–685. [Google Scholar] [CrossRef]
- Yusibov, V.; Hooper, D.C.; Spitsin, S.V.; Fleysh, N.; Kean, R.B.; Mikheeva, T.; Deka, D.; Karasev, A.; Cox, S.; Randall, J.; et al. Expression in plants and immunogenicity of plant virus-based experimental rabies vaccine. Vaccine 2002, 20, 3155–3164. [Google Scholar] [CrossRef]
- Modelska, A.; Dietzschold, B.; Sleysh, N.; Fu, Z.F.; Steplewski, K.; Hooper, D.C.; Koprowski, H.; Yusibov, V. Immunization against rabies with plant-derived antigen. Proc. Natl. Acad. Sci. USA 1998, 95, 2481–2485. [Google Scholar] [CrossRef] [PubMed]
- Yusibov, V.; Modelska, A.; Steplewski, K.; Agadjanyan, M.; Weiner, D.; Hooper, D.C.; Koprowski, H. Antigens produced in plants by infection with chimeric plant viruses immunize against rabies virus and HIV-1. Proc. Natl. Acad. Sci. USA 1997, 94, 5784–5788. [Google Scholar] [CrossRef]
- Wunner, W.H.; Dietzschold, B.; Smith, C.L.; Lafon, M.; Golub, E. Antigenic variants of CVS rabies virus with altered glycosylation sites. Virology 1985, 140, 1–12. [Google Scholar] [CrossRef]
- Maki, J.; Guiot, A.L.; Aubert, M.; Brochier, B.; Cliquet, F.; Hanlon, C.A.; King, R.; Oertli, E.H.; Rupprecht, C.E.; Schumacher, C.; et al. Oral vaccination of wildlife using a vaccinia-rabies-glycoprotein recombinant virus vaccine (RABORAL V-RG®): A global review. Vet. Res. 2017, 48, 57. [Google Scholar] [CrossRef]
- Loza-Rubio, E.; Rojas-Anaya, E.; López, J.; Olivera-Flores, M.T.; Gómez-Lim, M.; Tapia-Pérez, G. Induction of a protective immune response to rabies virus in sheep after oral immunization with transgenic maize, expressing the rabies virus glycoprotein. Vaccine 2012, 30, 5551–5556. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, P.B.; Hammond, J.; Dienelt, M.M.; Hooper, D.C.; Fu, Z.F.; Dietzschold, B.; Koprowski, H.; Michaels, F.H. Expression of the rabies virus glycoprotein in transgenic tomatoes. Biotechnology 1995, 13, 1484–1487. [Google Scholar] [CrossRef]
- Singh, A.; Srivastava, S.; Chouksey, A.; Panwar, B.S.; Verma, P.C.; Roy, S.; Singh, P.K.; Saxena, G.; Tuli, R. Expression of rabies glycoprotein and ricin toxin B chain (RGP-RTB) fusion protein in tomato hairy roots: A step towards oral vaccination for rabies. Mol. Biotechnol. 2015, 57, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Yang, D.K.; Nah, J.J.; Song, J.Y.; Cho, I.S. Comparison of the protective efficacy between single and combination of recombinant adenoviruses expressing complete and truncated glycoprotein, and nucleoprotein of the pathogenic street rabies virus in mice. Virol. J. 2017, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Lamphear, B.J.; Streatfield, S.J.; Jilka, J.M.; Brooks, C.A.; Barker, D.K.; Turner, D.D.; Delaney, D.E.; Garcia, M.; Wiggins, B.; Woodard, S.L.; et al. Delivery of subunit vaccines in maize seed. J. Control. Release 2002, 85, 169–180. [Google Scholar] [CrossRef]
- Kibenge, F.S. Emerging viruses in aquaculture. Curr. Opin. Virol. 2019, 34, 97–103. [Google Scholar] [CrossRef]
- Clarke, J.L.; Waheed, M.T.; Lössl, A.G.; Martinussen, I.; Daniell, H. How can plant genetic engineering contribute to cost-effective fish vaccine development for promoting sustainable aquaculture? Plant Mol. Biol. 2013, 83, 33–40. [Google Scholar] [CrossRef]
- Lecocq-Xhonneux, F.; Thiry, M.; Dheur, I.; Rossius, M.; Vanderheijden, N.; Martial, J.; de Kinkelin, P. A recombinant viral haemorrhagic septicaemia virus glycoprotein expressed in insect cells induces protective immunity in rainbow trout. J. Gen. Virol. 1994, 75 Pt 7, 1579–1587. [Google Scholar] [CrossRef]
- Encinas, P.; Gomez-Sebastian, S.; Nunez, M.C.; Gomez-Casado, E.; Escribano, J.M.; Estepa, A.; Coll, J. Antibody recognition of the glycoprotein g of viral haemorrhagic septicemia virus (VHSV) purified in large amounts from insect larvae. BMC Res. Notes 2011, 4, 210. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.; Estepa, A.; Coll, J.M. Purification of the glycoprotein G from viral haemorrhagic septicaemia virus, a fish rhabdovirus, by lectin affinity chromatography. J. Virol. Methods 1998, 76, 1–8. [Google Scholar] [CrossRef]
- Biacchesi, S.; Béarzotti, M.; Bouguyon, E.; Brémont, M. Heterologous exchanges of the glycoprotein and the matrix protein in a Novirhabdovirus. J. Virol. 2002, 76, 2881–2889. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lorenzen, N.; Olesen, N.J.; Jørgensen, P.E.V.; Etzerodt, M.; Holtet, T.L.; Thøgersen, H.C. Molecular cloning and expression in Escherichia coli of the glycoprotein gene of VHS virus, and immunization of rainbow trout with the recombinant protein. J. Gen. Virol. 1993, 74, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.Q.; Kim, T.J.; Kim, T.G. Viral hemorrhagic septicemia virus glycoprotein production in tobacco. Protein Expr. Purif. 2017, 133, 170–176. [Google Scholar] [CrossRef]
- D’Aoust, M.-A.; Lavoie, P.-O.; Vezina, L.-P.; Couture, M. Rabies Virus Like Particle Production in Plants. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012171104 (accessed on 9 July 2021).
- Nemchinov, L.G.; Liang, T.J.; Rifaat, M.M.; Mazyad, H.M.; Hadidi, A.; Keith, J.M. Development of a plant-derived subunit vaccine candidate against hepatitis C virus. Arch. Virol. 2000, 2012, 2557–2573. [Google Scholar] [CrossRef]
- Attar, A.E.; Shamloul, A.; Shalaby, A.; Riad, B.; Saad, A.; Mazyad, H.; Keith, J. Expression of chimeric HCV peptide in transgenic tobacco plants infected with recombinant alfalfa mosaic virus for development of a plant-derived vaccine against HCV. Afr. J. Biotechnol. 2004, 3, 7. [Google Scholar]
- Mohammadzadeh, S.; Roohvand, F.; Memarnejadian, A.; Jafari, A.; Ajdary, S.; Salmanian, A.-H.; Ehsani, P. Co-expression of hepatitis C virus polytope–HBsAg and p19-silencing suppressor protein in tobacco leaves. Pharm. Biol. 2016, 54, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Natilla, A.; Piazzolla, G.; Nuzzaci, M.; Saldarelli, P.; Tortorella, C.; Antonaci, S.; Piazzolla, P. Cucumber mosaic virus as carrier of a hepatitis C virus-derived epitope. Arch. Virol. 2003, 149, 137–154. [Google Scholar] [CrossRef]
- Piazzolla, G.; Nuzzaci, M.; Tortorella, C.; Panella, E.; Natilla, A.; Boscia, D.; De Stradis, A.; Piazzolla, P.; Antonaci, S. Immunogenic Properties of a Chimeric Plant Virus Expressing a Hepatitis C Virus (HCV)-Derived Epitope: New Prospects for an HCV Vaccine. J. Clin. Immunol. 2005, 25, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Nuzzaci, M.; Piazzolla, G.; Vitti, A.; Lapelosa, M.; Tortorella, C.; Stella, I.; Natilla, A.; Antonaci, S.; Piazzolla, P. Cucumber mosaic virus as a presentation system for a double hepatitis C virus-derived epitope. Arch. Virol. 2007, 152, 915. [Google Scholar] [CrossRef]
- Nuzzaci, M.; Vitti, A.; Condelli, V.; Lanorte, M.T.; Tortorella, C.; Boscia, D.; Piazzolla, P.; Piazzolla, G. In vitro stability of Cucumber mosaic virus nanoparticles carrying a Hepatitis C virus-derived epitope under simulated gastrointestinal conditions and in vivo efficacy of an edible vaccine. J. Virol. Methods 2010, 165, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.L.; Paruch, L.; Dobrica, M.-O.; Caras, I.; Tucureanu, C.; Onu, A.; Ciulean, S.; Stavaru, C.; Eerde, A.; Wang, Y.; et al. Lettuce-produced hepatitis C virus E1E2 heterodimer triggers immune responses in mice and antibody production after oral vaccination. Plant Biotechnol. J. 2017, 15, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, H.; Zhang, X.; Xiao, H.; Jiang, Y.; Song, Y.; Fang, L.; Xiao, S.; Zhen, Y.; Chen, H. Generation and immunogenicity of Japanese encephalitis virus envelope protein expressed in transgenic rice. Biochem. Biophys. Res. Commun. 2009, 380, 292–297. [Google Scholar] [CrossRef]
- Chen, T.H.; Hu, C.C.; Liao, J.T.; Lee, Y.L.; Huang, Y.W.; Lin, N.S.; Lin, Y.L.; Hsu, Y.H. Production of Japanese Encephalitis Virus Antigens in Plants Using Bamboo Mosaic Virus-Based Vector. Front. Microbiol. 2017, 8, 788. [Google Scholar] [CrossRef]
- Chen, Q. Plant-made vaccines against West Nile virus are potent, safe, and economically feasible. Biotechnol. J. 2015, 10, 671–680. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Peng, L.; Lai, H.; Hurtado, J.; Stahnke, J.; Chen, Q. A plant-produced antigen elicits potent immune responses against West Nile virus in mice. BioMed Res. Int. 2014, 2014, 952865. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Paul, A.M.; Sun, H.; He, J.; Yang, M.; Bai, F.; Chen, Q. A plant-produced vaccine protects mice against lethal West Nile virus infection without enhancing Zika or dengue virus infectivity. Vaccine 2018, 36, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lai, H.; Esqueda, A.; Chen, Q. Plant-Produced Antigen Displaying Virus-Like Particles Evokes Potent Antibody Responses against West Nile Virus in Mice. Vaccines 2021, 9, 60. [Google Scholar] [CrossRef]
- Yang, M.; Lai, H.; Sun, H.; Chen, Q. Virus-like particles that display Zika virus envelope protein domain III induce potent neutralizing immune responses in mice. Sci. Rep. 2017, 7, 7679. [Google Scholar] [CrossRef]
- Diamos, A.G.; Pardhe, M.D.; Sun, H.; Hunter, J.G.L.; Mor, T.; Meador, L.; Kilbourne, J.; Chen, Q.; Mason, H.S. Codelivery of improved immune complex and virus-like particle vaccines containing Zika virus envelope domain III synergistically enhances immunogenicity. Vaccine 2020, 38, 3455–3463. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sun, H.; Lai, H.; Hurtado, J.; Chen, Q. Plant-produced Zika virus envelope protein elicits neutralizing immune responses that correlate with protective immunity against Zika virus in mice. Plant Biotechnol. J. 2018, 16, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Reljic, R.; Kilbourne, J.; Ceballos-Olvera, I.; Yang, M.S.; Reyes-del Valle, J.; Mason, H.S. Novel vaccination approach for dengue infection based on recombinant immune complex universal platform. Vaccine 2015, 33, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Saejung, W.; Fujiyama, K.; Takasaki, T.; Ito, M.; Hori, K.; Malasit, P.; Watanabe, Y.; Kurane, I.; Seki, T. Production of dengue 2 envelope domain III in plant using TMV-based vector system. Vaccine 2007, 25, 6646–6654. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Yang, M.-S.; Kim, T.-G. Expression of dengue virus E glycoprotein domain III in non-nicotine transgenic tobacco plants. Biotechnol. Bioprocess Eng. 2009, 14, 725–730. [Google Scholar] [CrossRef]
- Martínez, C.A.; Topal, E.; Giulietti, A.M.; Talou, J.R.; Mason, H. Exploring different strategies to express Dengue virus envelope protein in a plant system. Biotechnol. Lett. 2010, 32, 867–875. [Google Scholar] [CrossRef]
- Gottschamel, J.; Lössl, A.; Ruf, S.; Wang, Y.; Skaugen, M.; Bock, R.; Clarke, J.L. Production of dengue virus envelope protein domain III-based antigens in tobacco chloroplasts using inducible and constitutive expression systems. Plant Mol. Biol. 2016, 91, 497–512. [Google Scholar] [CrossRef] [PubMed]
- van Eerde, A.; Gottschamel, J.; Bock, R.; Hansen, K.E.A.; Munang’andu, H.M.; Daniell, H.; Liu Clarke, J. Production of tetravalent dengue virus envelope protein domain III based antigens in lettuce chloroplasts and immunologic analysis for future oral vaccine development. Plant Biotechnol. J. 2019, 17, 1408–1417. [Google Scholar] [CrossRef]
- Kanagaraj, A.P.; Verma, D.; Daniell, H. Expression of dengue-3 premembrane and envelope polyprotein in lettuce chloroplasts. Plant Mol. Biol. 2011, 76, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Ponndorf, D.; Meshcheriakova, Y.; Thuenemann, E.C.; Dobon Alonso, A.; Overman, R.; Holton, N.; Dowall, S.; Kennedy, E.; Stocks, M.; Lomonossoff, G.P.; et al. Plant-made dengue virus-like particles produced by co-expression of structural and non-structural proteins induce a humoral immune response in mice. Plant Biotechnol. J. 2020. [Google Scholar] [CrossRef]
- Cardona-Ospina, J.A.; Sepúlveda-Arias, J.C.; Mancilla, L.; Gutierrez-López, L.G. Plant expression systems, a budding way to confront chikungunya and Zika in developing countries? F1000Research 2016, 5, 2121. [Google Scholar] [CrossRef]
- Akahata, W.; Yang, Z.Y.; Andersen, H.; Sun, S.; Holdaway, H.A.; Kong, W.P.; Lewis, M.G.; Higgs, S.; Rossmann, M.G.; Rao, S.; et al. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat. Med. 2010, 16, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Salazar-González, J.A.; Angulo, C.; Rosales-Mendoza, S. Chikungunya virus vaccines: Current strategies and prospects for developing plant-made vaccines. Vaccine 2015, 33, 3650–3658. [Google Scholar] [CrossRef] [PubMed]
- Margolin, E.; Chapman, R.; Williamson, A.L.; Rybicki, E.P.; Meyers, A.E. Production of complex viral glycoproteins in plants as vaccine immunogens. Plant Biotechnol. J. 2018, 16, 1531–1545. [Google Scholar] [CrossRef]
- Tremouillaux-Guiller, J.; Moustafa, K.; Hefferon, K.; Gaobotse, G.; Makhzoum, A. Plant-made HIV vaccines and potential candidates. Curr. Opin. Biotechnol. 2020, 61, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Spall, V.E.; Loveland, J.; Johnson, J.E.; Barker, P.J.; Lomonossoff, G.P. Development of cowpea mosaic virus as a high-yielding system for the presentation of foreign peptides. Virology 1994, 202, 949–955. [Google Scholar] [CrossRef]
- McLain, L.; Porta, C.; Lomonossoff, G.P.; Durrani, Z.; Dimmock, N.J. Human immunodeficiency virus type 1-neutralizing antibodies raised to a glycoprotein 41 peptide expressed on the surface of a plant virus. AIDS Res. Hum. Retrovir. 1995, 11, 327–334. [Google Scholar] [CrossRef]
- McLain, L.; Durrani, Z.; Wisniewski, L.A.; Porta, C.; Lomonossoff, G.P.; Dimmock, N.J. Stimulation of neutralizing antibodies to human immunodeficiency virus type 1 in three strains of mice immunized with a 22 amino acid peptide of gp41 expressed on the surface of a plant virus. Vaccine 1996, 14, 799–810. [Google Scholar] [CrossRef]
- Buratti, E.; McLain, L.; Tisminetzky, S.; Cleveland, S.M.; Dimmock, N.J.; Baralle, F.E. The neutralizing antibody response against a conserved region of human immunodeficiency virus type 1 gp41 (amino acid residues 731–752) is uniquely directed against a conformational epitope. J. Gen. Virol. 1998, 79 Pt 11, 2709–2716. [Google Scholar] [CrossRef]
- Zhang, G.G.; Rodrigues, L.; Rovinski, B.; White, K.A. Production of HIV-1 p24 protein in transgenic tobacco plants. Mol. Biotechnol. 2002, 20, 131–136. [Google Scholar] [CrossRef]
- Karasev, A.V.; Foulke, S.; Wellens, C.; Rich, A.; Shon, K.J.; Zwierzynski, I.; Hone, D.; Koprowski, H.; Reitz, M. Plant based HIV-1 vaccine candidate: Tat protein produced in spinach. Vaccine 2005, 23, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Salyaev, R.K.; Pozdnyakov, S.G.; Rekoslavskaya, N.I.; Nesterov, A.E.; Ryzhova, T.S.; Sumtsova, V.M.; Pakova, N.V.; Mishutina, U.O.; Kopytina, T.V.; et al. Immunogenicity of a novel, bivalent, plant-based oral vaccine against hepatitis B and human immunodeficiency viruses. Biotechnol. Lett. 2006, 28, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Michel, M.; Guetard, D.; Cervantes-Gonzalez, M.; Pelucchi, N.; Wain-Hobson, S.; Sala, F.; Sala, M. Production of recombinant HIV-1/HBV virus-like particles in Nicotiana tabacum and Arabidopsis thaliana plants for a bivalent plant-based vaccine. Vaccine 2007, 25, 8228–8240. [Google Scholar] [CrossRef]
- Guetard, D.; Greco, R.; Cervantes Gonzalez, M.; Celli, S.; Kostrzak, A.; Langlade-Demoyen, P.; Sala, F.; Wain-Hobson, S.; Sala, M. Immunogenicity and tolerance following HIV-1/HBV plant-based oral vaccine administration. Vaccine 2008, 26, 4477–4485. [Google Scholar] [CrossRef]
- Meyers, A.; Chakauya, E.; Shephard, E.; Tanzer, F.L.; Maclean, J.; Lynch, A.; Williamson, A.-L.; Rybicki, E.P. Expression of HIV-1 antigens in plants as potential subunit vaccines. BMC Biotechnol. 2008, 8, 53. [Google Scholar] [CrossRef]
- Scotti, N.; Alagna, F.; Ferraiolo, E.; Formisano, G.; Sannino, L.; Buonaguro, L.; De Stradis, A.; Vitale, A.; Monti, L.; Grillo, S.; et al. High-level expression of the HIV-1 Pr55gag polyprotein in transgenic tobacco chloroplasts. Planta 2009, 229, 1109–1122. [Google Scholar] [CrossRef]
- Kessans, S.A.; Linhart, M.D.; Matoba, N.; Mor, T. Biological and biochemical characterization of HIV-1 Gag/dgp41 virus-like particles expressed in Nicotiana benthamiana. Plant Biotechnol. J. 2013, 11, 681–690. [Google Scholar] [CrossRef]
- Kessans, S.A.; Linhart, M.D.; Meador, L.R.; Kilbourne, J.; Hogue, B.G.; Fromme, P.; Matoba, N.; Mor, T.S. Immunological Characterization of Plant-Based HIV-1 Gag/Dgp41 Virus-Like Particles. PLoS ONE 2016, 11, e0151842. [Google Scholar] [CrossRef]
- Rosenberg, Y.; Sack, M.; Montefiori, D.; Forthal, D.; Mao, L.; Abanto, S.H.; Urban, L.; Landucci, G.; Fischer, R.; Jiang, X. Rapid High-Level Production of Functional HIV Broadly Neutralizing Monoclonal Antibodies in Transient Plant Expression Systems. PLoS ONE 2013, 8, e58724. [Google Scholar] [CrossRef]
- Margolin, E.; Chapman, R.; Meyers, A.E.; van Diepen, M.T.; Ximba, P.; Hermanus, T.; Crowther, C.; Weber, B.; Morris, L.; Williamson, A.-L.; et al. Production and Immunogenicity of Soluble Plant-Produced HIV-1 Subtype C Envelope gp140 Immunogens. Front. Plant Sci. 2019, 10, 1378. [Google Scholar] [CrossRef]
- Brown, V.R.; Bevins, S.N. A review of virulent Newcastle disease viruses in the United States and the role of wild birds in viral persistence and spread. Vet. Res. 2017, 48, 68. [Google Scholar] [CrossRef] [PubMed]
- Pantua, H.D.; McGinnes, L.W.; Peeples, M.E.; Morrison, T.G. Requirements for the assembly and release of Newcastle disease virus-like particles. J. Virol. 2006, 80, 11062–11073. [Google Scholar] [CrossRef]
- Cox, R.M.; Plemper, R.K. Structure and organization of paramyxovirus particles. Curr. Opin. Virol. 2017, 24, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Parks, G.D.; Lamb, R.A. Folding and oligomerization properties of a soluble and secreted form of the paramyxovirus hemagglutinin-neuraminidase glycoprotein. Virology 1990, 178, 498–508. [Google Scholar] [CrossRef]
- Yuan, P.; Swanson, K.A.; Leser, G.P.; Paterson, R.G.; Lamb, R.A.; Jardetzky, T.S. Structure of the Newcastle disease virus hemagglutinin-neuraminidase (HN) ectodomain reveals a four-helix bundle stalk. Proc. Natl. Acad. Sci. USA 2011, 108, 14920–14925. [Google Scholar] [CrossRef]
- Natilla, A.; Hammond, R.W.; Nemchinov, L.G. Epitope presentation system based on cucumber mosaic virus coat protein expressed from a potato virus X-based vector. Arch. Virol. 2006, 151, 1373–1386. [Google Scholar] [CrossRef]
- Natilla, A.; Nemchinov, L.G. Improvement of PVX/CMV CP expression tool for display of short foreign antigens. Protein Expr. Purif. 2008, 59, 117–121. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Hammond, R.W. Development of a candidate vaccine for Newcastle disease virus by epitope display in the Cucumber mosaic virus capsid protein. Biotechnol. Lett. 2005, 27, 375–382. [Google Scholar] [CrossRef]
- Berinstein, A.; Vazquez-Rovere, C.; Asurmendi, S.; Gómez, E.; Zanetti, F.; Zabal, O.; Tozzini, A.; Conte Grand, D.; Taboga, O.; Calamante, G.; et al. Mucosal and systemic immunization elicited by Newcastle disease virus (NDV) transgenic plants as antigens. Vaccine 2005, 23, 5583–5589. [Google Scholar] [CrossRef]
- Yang, Z.-Q.; Liu, Q.-Q.; Pan, Z.-M.; Yu, H.-X.; Jiao, X.-A. Expression of the fusion glycoprotein of newcasstle disease virus in transgenic rice and its immunogenicity in mice. Vaccine 2007, 25, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Andrade, O.; Loza-Rubio, E.; Olivera-Flores, T.; Fehérvári-Bone, T.; Gómez-Lim, M.A. Expression of the Newcastle disease virus fusion protein in transgenic maize and immunological studies. Transgenic Res. 2006, 15, 455–463. [Google Scholar] [CrossRef]
- Shahid, N.; Samiullah, T.R.; Shakoor, S.; Latif, A.; Yasmeen, A.; Azam, S.; Shahid, A.A.; Husnain, T.; Rao, A.Q. Early Stage Development of a Newcastle Disease Vaccine Candidate in Corn. Front. Vet. Sci. 2020, 7, 499. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, M.J.; Ebrahimi, M.M.; Shahsavandi, S.; Amani, J.; Kazemi, R.; Jafari, M.; Salmanian, A.H. The Immunogenicity of a Novel Chimeric Hemagglutinin-Neuraminidase-Fusion Antigen from Newcastle Disease Virus by Oral Delivery of Transgenic Canola Seeds to Chickens. Mol. Biotechnol. 2020, 62, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Gómez, E.; Zoth, S.C.; Asurmendi, S.; Vázquez Rovere, C.; Berinstein, A. Expression of Hemagglutinin-Neuraminidase glycoprotein of Newcastle Disease Virus in agroinfiltrated Nicotiana benthamiana plants. J. Biotechnol. 2009, 144, 337–340. [Google Scholar] [CrossRef]
- Hahn, B.-S.; Jeon, I.-S.; Jung, Y.-J.; Kim, J.-B.; Park, J.-S.; Ha, S.-H.; Kim, K.-H.; Kim, H.-M.; Yang, J.-S.; Kim, Y.-H. Expression of hemagglutinin-neuraminidase protein of Newcastle disease virus in transgenic tobacco. Plant Biotechnol. Rep. 2007, 1, 85–92. [Google Scholar] [CrossRef]
- Lai, K.S.; Yusoff, K.; Mahmood, M. Functional ectodomain of the hemagglutinin-neuraminidase protein is expressed in transgenic tobacco cells as a candidate vaccine against Newcastle disease virus. Plant Cell Tissue Organ Cult. 2013, 112, 117–121. [Google Scholar] [CrossRef]
- News in Brief. USDA approves the first plant-based vaccine. Nat. Biotechnol. 2006, 24, 233–234. [Google Scholar] [CrossRef]
- Thomas, D.R.; Walmsley, A.M. Plant-made veterinary vaccines for newcastle disease virus. In Prospects of Plant-Based Vaccines in Veterinary Medicine; MacDonald, J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 149–167. [Google Scholar] [CrossRef]
- Nieto-Gómez, R.; Angulo, C.; Monreal-Escalante, E.; Govea-Alonso, D.O.; De Groot, A.S.; Rosales-Mendoza, S. Design of a multiepitopic Zaire ebolavirus protein and its expression in plant cells. J. Biotechnol. 2019, 295, 41–48. [Google Scholar] [CrossRef]
- Phoolcharoen, W.; Bhoo, S.H.; Lai, H.; Ma, J.; Arntzen, C.J.; Chen, Q.; Mason, H.S. Expression of an immunogenic Ebola immune complex in Nicotiana benthamiana. Plant Biotechnol. J. 2011, 9, 807–816. [Google Scholar] [CrossRef]
- Phoolcharoen, W.; Dye, J.M.; Kilbourne, J.; Piensook, K.; Pratt, W.D.; Arntzen, C.J.; Chen, Q.; Mason, H.S.; Herbst-Kralovetz, M.M. A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge. Proc. Natl. Acad. Sci. USA 2011, 108, 20695–20700. [Google Scholar] [CrossRef]
- Belanger, H.; Fleysh, N.; Cox, S.; Bartman, G.; Deka, D.; Trudel, M.; Koprowski, H.; Yusibov, V. Human respiratory syncytial virus vaccine antigen produced in plants. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2000, 14, 2323–2328. [Google Scholar] [CrossRef] [PubMed]
- Yusibov, V.; Mett, V.; Mett, V.; Davidson, C.; Musiychuk, K.; Gilliam, S.; Farese, A.; Macvittie, T.; Mann, D. Peptide-based candidate vaccine against respiratory syncytial virus. Vaccine 2005, 23, 2261–2265. [Google Scholar] [CrossRef] [PubMed]
- Bouche, F.B.; Marquet-Blouin, E.; Yanagi, Y.; Steinmetz, A.; Muller, C.P. Neutralising immunogenicity of a polyepitope antigen expressed in a transgenic food plant: A novel antigen to protect against measles. Vaccine 2003, 21, 2065–2072. [Google Scholar] [CrossRef]
- Huang, Z.; Dry, I.; Webster, D.; Strugnell, R.; Wesselingh, S. Plant-derived measles virus hemagglutinin protein induces neutralizing antibodies in mice. Vaccine 2001, 19, 2163–2171. [Google Scholar] [CrossRef]
- Pickering, R.J.; Smith, S.D.; Strugnell, R.A.; Wesselingh, S.L.; Webster, D.E. Crude saponins improve the immune response to an oral plant-made measles vaccine. Vaccine 2006, 24, 144–150. [Google Scholar] [CrossRef]
- Marquet-Blouin, E.; Bouche, F.B.; Steinmetz, A.; Muller, C.P. Neutralizing immunogenicity of transgenic carrot (Daucus carota L.)-derived measles virus hemagglutinin. Plant Mol. Biol. 2003, 51, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.E.; Smith, S.D.; Pickering, R.J.; Strugnell, R.A.; Dry, I.B.; Wesselingh, S.L. Measles virus hemagglutinin protein expressed in transgenic lettuce induces neutralising antibodies in mice following mucosal vaccination. Vaccine 2006, 24, 3538–3544. [Google Scholar] [CrossRef]
- Webster, D.E.; Thomas, M.C.; Huang, Z.; Wesselingh, S.L. The development of a plant-based vaccine for measles. Vaccine 2005, 23, 1859–1865. [Google Scholar] [CrossRef]
- Peyret, H.; Lomonossoff, G.P. When plant virology met Agrobacterium: The rise of the deconstructed clones. Plant Biotechnol. J. 2015, 13, 1121–1135. [Google Scholar] [CrossRef]
- Peyret, H.; Brown, J.K.M.; Lomonossoff, G.P. Improving plant transient expression through the rational design of synthetic 5’ and 3’ untranslated regions. Plant Methods 2019, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Margolin, E.; Oh, Y.J.; Verbeek, M.; Naude, J.; Ponndorf, D.; Meshcheriakova, Y.A.; Peyret, H.; van Diepen, M.T.; Chapman, R.; Meyers, A.E.; et al. Co-expression of human calreticulin significantly improves the production of HIV gp140 and other viral glycoproteins in plants. Plant Biotechnol. J. 2020, 18, 2109–2117. [Google Scholar] [CrossRef]
- Margolin, E.A.; Strasser, R.; Chapman, R.; Williamson, A.-L.; Rybicki, E.P.; Meyers, A.E. Engineering the Plant Secretory Pathway for the Production of Next-Generation Pharmaceuticals. Trends Biotechnol. 2020, 38, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Buyel, J.F.; Twyman, R.M.; Fischer, R. Extraction and downstream processing of plant-derived recombinant proteins. Biotechnol. Adv. 2015, 33 Pt 1, 902–913. [Google Scholar] [CrossRef]
- Schillberg, S.; Finnern, R. Plant molecular farming for the production of valuable proteins—Critical evaluation of achievements and future challenges. J. Plant Physiol. 2021, 258–259, 153359. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Buyel, J.F. Molecular farming—The slope of enlightenment. Biotechnol. Adv. 2020, 40, 107519. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J. Prospects of Plant-Based Vaccines in Veterinary Medicine; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peyret, H.; Steele, J.F.C.; Jung, J.-W.; Thuenemann, E.C.; Meshcheriakova, Y.; Lomonossoff, G.P. Producing Vaccines against Enveloped Viruses in Plants: Making the Impossible, Difficult. Vaccines 2021, 9, 780. https://doi.org/10.3390/vaccines9070780
Peyret H, Steele JFC, Jung J-W, Thuenemann EC, Meshcheriakova Y, Lomonossoff GP. Producing Vaccines against Enveloped Viruses in Plants: Making the Impossible, Difficult. Vaccines. 2021; 9(7):780. https://doi.org/10.3390/vaccines9070780
Chicago/Turabian StylePeyret, Hadrien, John F. C. Steele, Jae-Wan Jung, Eva C. Thuenemann, Yulia Meshcheriakova, and George P. Lomonossoff. 2021. "Producing Vaccines against Enveloped Viruses in Plants: Making the Impossible, Difficult" Vaccines 9, no. 7: 780. https://doi.org/10.3390/vaccines9070780
APA StylePeyret, H., Steele, J. F. C., Jung, J.-W., Thuenemann, E. C., Meshcheriakova, Y., & Lomonossoff, G. P. (2021). Producing Vaccines against Enveloped Viruses in Plants: Making the Impossible, Difficult. Vaccines, 9(7), 780. https://doi.org/10.3390/vaccines9070780





