Neutralizing Antibodies Titers and Side Effects in Response to BNT162b2 Vaccine in Healthcare Workers with and without Prior SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Description of Study Groups
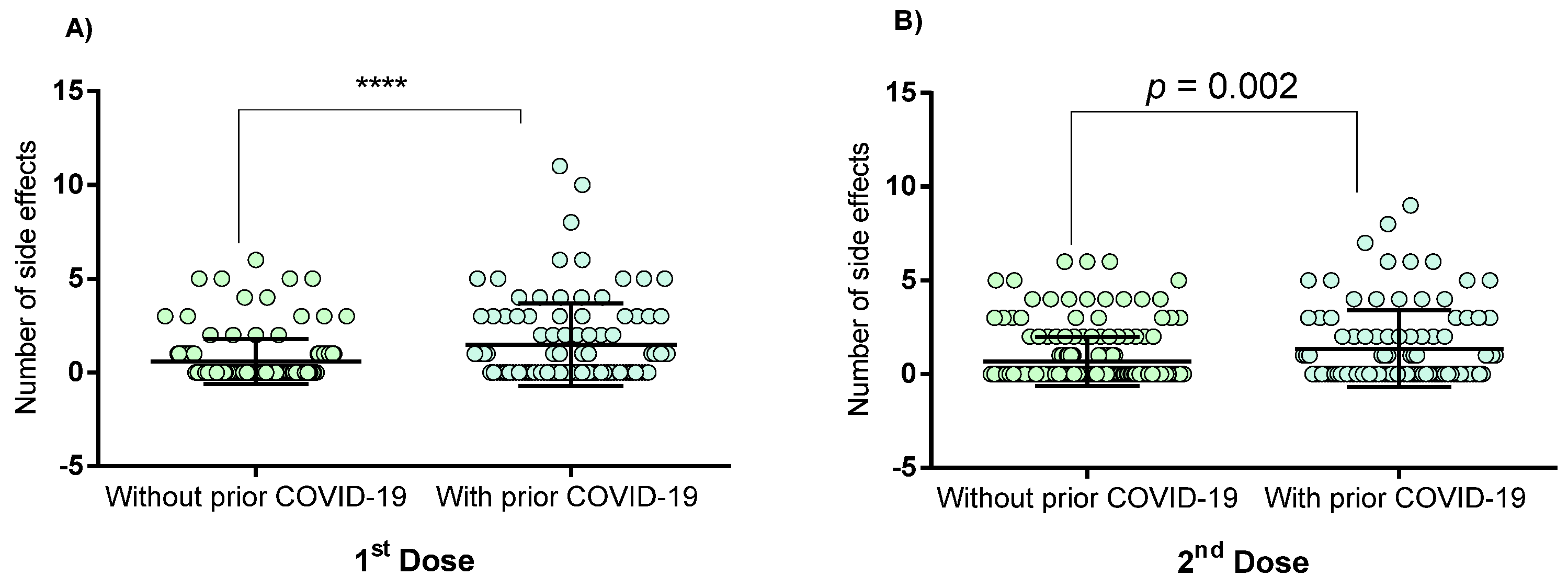
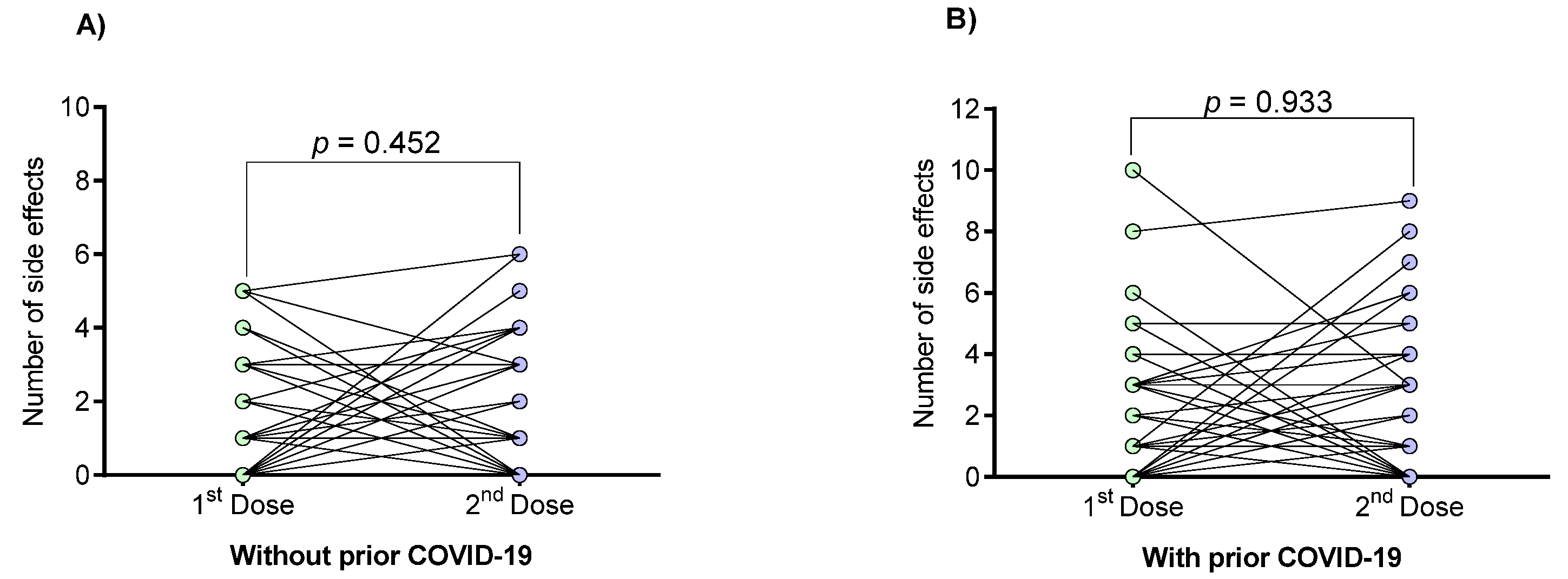
3.2. Vaccine-Associated Side Effects
3.3. Generation of Neutralizing Antibodies in Response to the BNT162b2 Vaccine
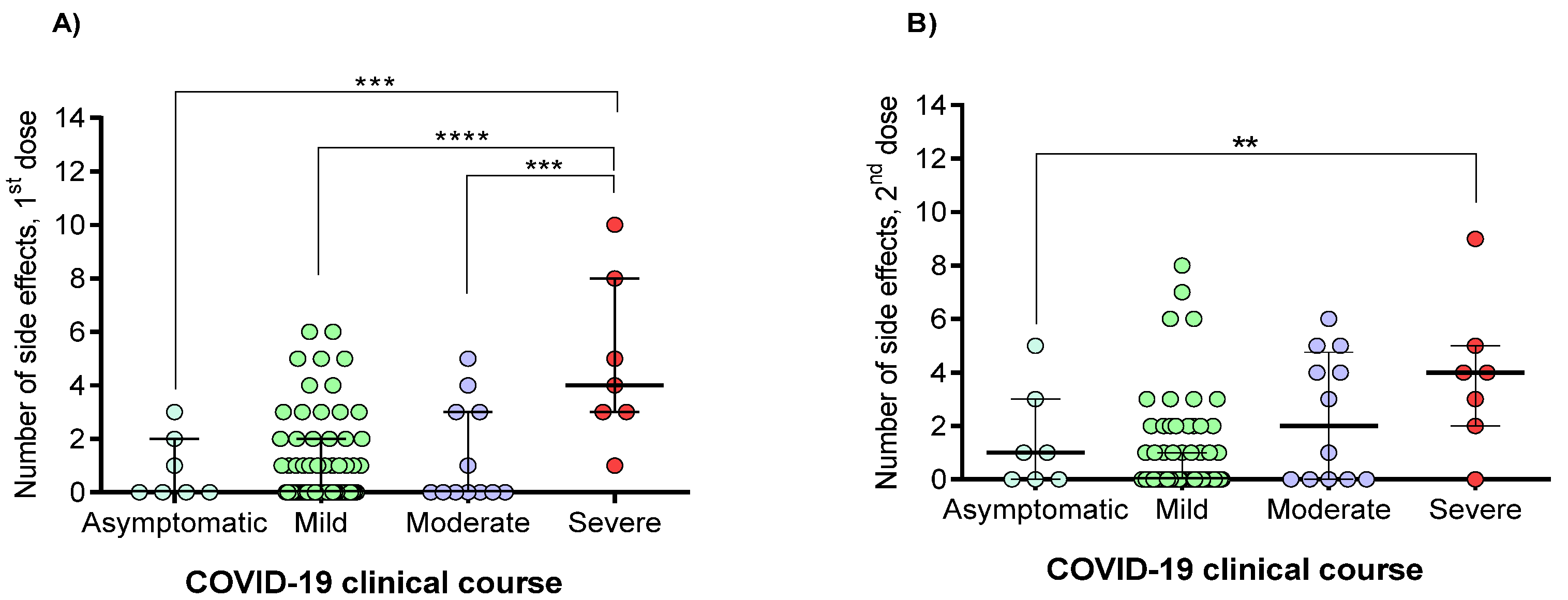
3.4. Correlation between the Percentage of Neutralization with the Clinical and Demographic Variables
3.5. Concordance of the SARS-CoV-2 IgM/IgG Seropositivity with Neutralizing Antibodies Presence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Map—Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 6 September 2020).
- Hodgson, S.H.; Mansatta, K.; Mallett, G.; Harris, V.; Emary, K.R.W.; Pollard, A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect. Dis. 2021, 21, e26–e35. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration, Center for Biologics Evaluation and Research. Development and Licensure of Vaccines to Prevent COVID-19. 2020; p. 24. Available online: https://www.fda.gov/media/139638/download (accessed on 17 June 2021).
- Wibawa, T. COVID-19 vaccine research and development: Ethical issues. Trop. Med. Int. Health 2020. [Google Scholar] [CrossRef]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Nourbakhsh, S.; Sah, P.; Fitzpatrick, M.C.; Galvani, A.P. Evaluation of COVID-19 vaccination strategies with a delayed second dose. medRxiv 2021. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, A.; Meurant, R.; Ardakani, A. COVID-19 Serological Tests: How Well Do They Actually Perform? Diagnostics 2020, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Riggioni, C.; Comberiati, P.; Giovannini, M.; Agache, I.; Akdis, M.; Alves-Correia, M.; Antó, J.M.; Arcolaci, A.; Azkur, A.K.; Azkur, D.; et al. A compendium answering 150 questions on COVID-19 and SARS-CoV-2. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C.; Liu, G.; Luo, W.; Xia, N. COVID-19: Progress in diagnostics, therapy and vaccination. Theranostics 2020, 10, 7821–7835. [Google Scholar] [CrossRef]
- Sapkal, G.N.; Deshpande, G.R.; Tilekar, B.N.; Yadav, P.D.; Gurav, Y.; Gaikwad, S.; Kaushal, H.; Deshpande, K.S.; Kaduskar, O.; Sarkale, P.; et al. Neutralizing antibody responses to SARS-CoV-2 in COVID-19 patients. Indian J. Med Res. 2020, 152, 82–87. [Google Scholar] [CrossRef]
- Meyer, B.; Reimerink, J.; Torriani, G.; Brouwer, F.; Godeke, G.-J.; Yerly, S.; Hoogerwerf, M.; Vuilleumier, N.; Kaiser, L.; Eckerle, I.; et al. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT). Emerg. Microbes Infect. 2020, 9, 2394–2403. [Google Scholar] [CrossRef]
- Tan, C.W.; Ni Chia, W.; Qin, X.; Liu, P.; Chen, M.I.-C.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Hurst, B.; Charlton, C.L.; Bailey, A.; Kanji, J.N.; McCarthy, M.K.; Morrison, T.E.; Huey, L.; Annen, K.; DomBourian, M.G.; et al. A New SARS-CoV-2 Dual-Purpose Serology Test: Highly Accurate Infection Tracing and Neutralizing Antibody Response Detection. J. Clin. Microbiol. 2021, 59. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals after mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef]
- Ni Chia, W.; Zhu, F.; Ong, S.W.X.; Young, B.E.; Fong, S.-W.; Le Bert, N.; Tan, C.W.; Tiu, C.; Zhang, J.; Tan, S.Y.; et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: A longitudinal study. Lancet Microbe 2021, 2, e240–e249. [Google Scholar] [CrossRef]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Heal. 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P.; Hosein, Z.; Padda, I.; Mangat, J.; Altaf, M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Patruno, C.; Nisticò, S.P.; Fabbrocini, G.; Napolitano, M. COVID-19, quarantine, and atopic dermatitis. Med. Hypotheses 2020, 143, 109852. [Google Scholar] [CrossRef]
- Redondo-Sendino, Á.; Sánchez, I.C.G.; de Victoria Fernández, B. Skin manifestations associated with the new coronavirus SARS-CoV-2 disease. Med. Clin. 2020, 155, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Srivastava, K.; Simon, V. Robust spike antibody responses and increased reactogenicity in seropositive individuals after a single dose of SARS-CoV-2 mRNA vaccine. medRxiv 2021. [Google Scholar] [CrossRef]
- Wang, Z.; Schmidt, F.; Weisblum, Y.; Muecksch, F.; Barnes, C.O.; Finkin, S.; Schaefer-Babajew, D.; Cipolla, M.; Gaebler, C.; Lieberman, J.A.; et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nat. Cell Biol. 2021, 592, 616–622. [Google Scholar] [CrossRef]
- Pieper, K.; Grimbacher, B.; Eibel, H. B-cell biology and development. J. Allergy Clin. Immunol. 2013, 131, 959–971. [Google Scholar] [CrossRef]
- Martinez-Liu, C.; Martínez-Acuña, N.; Arellanos-Soto, D.; Galan-Huerta, K.; Lozano-Sepulveda, S.; Martínez-Guzmán, M.; Rivas-Estilla, A. SARS-CoV-2 in Mexico: Beyond Detection Methods, Scope and Limitations. Diagnostics 2021, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.G.; Zhang, Z.; Gao, Q.; Pan, M.; Rowan, E.G.; Zhang, J. Recent advances in therapeutic applications of neutralizing antibodies for virus infections: An overview. Immunol. Res. 2020, 68, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Mohsen, M.O.; Zha, L.; Vogel, M.; Speiser, D.E. SARS-CoV-2 structural features may explain limited neutralizing-antibody responses. Npj Vaccines 2021, 6, 2. [Google Scholar] [CrossRef]
- Manian, D.V.; Jensen, C.; Theel, E.S.; Mills, J.R.; Joshi, A. Non-neutralizing antibodies and limitations of serologic testing for severe acute respiratory syndrome coronavirus 2 in patients receiving immunoglobulin replacement products. Ann. Allergy Asthma Immunol. 2020, 126, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv 2020. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Xia, H.; Zhang, X.; Fontes-Garfias, C.R.; Swanson, K.A.; Cai, H.; Sarkar, R.; Chen, W.; Cutler, M.; et al. Neutralizing Activity of BNT162b2-Elicited Serum. N. Engl. J. Med. 2021, 384, 1466–1468. [Google Scholar] [CrossRef]
- Manisty, C.; Otter, A.D.; A Treibel, T.; McKnight, Á.; Altmann, D.M.; Brooks, T.; Noursadeghi, M.; Boyton, R.J.; Semper, A.; Moon, J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 2021, 397, 1057–1058. [Google Scholar] [CrossRef]
- Gobbi, F.; Buonfrate, D.; Moro, L.; Rodari, P.; Piubelli, C.; Caldrer, S.; Riccetti, S.; Sinigaglia, A.; Barzon, L. Antibody Response to the BNT162b2 mRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Viruses 2021, 13, 422. [Google Scholar] [CrossRef]
- Sprent, J.; King, C. COVID-19 vaccine side effects: The positives about feeling bad. Sci. Immunol. 2021, 6. [Google Scholar] [CrossRef]
- Bereshchenko, O.; Bruscoli, S.; Riccardi, C. Glucocorticoids, Sex Hormones, and Immunity. Front. Immunol. 2018, 9, 1332. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Bowdish, D.M.E. Immunosenescence and Novel Vaccination Strategies for the Elderly. Front. Immunol. 2013, 4, 171. [Google Scholar] [CrossRef] [Green Version]
- Grubeck-Loebenstein, B.; Della Bella, S.; Iorio, A.M.; Michel, J.-P.; Pawelec, G.; Solana, R. Immunosenescence and vaccine failure in the elderly. Aging Clin. Exp. Res. 2009, 21, 201–209. [Google Scholar] [CrossRef]
- Galipeau, Y.; Greig, M.; Liu, G.; Driedger, M.; Langlois, M.-A. Humoral Responses and Serological Assays in SARS-CoV-2 Infections. Front. Immunol. 2020, 11, 610688. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M. What level of neutralizing antibody protects from COVID-19? medRxiv 2021. [Google Scholar] [CrossRef]



| Immunized with BNT162b2 Vaccine | p-Value | |||
|---|---|---|---|---|
| Yes | No | |||
| without Prior COVID-19 n = 143 | with Prior COVID-19 n = 100 | with Prior COVID-19 n = 60 | ||
| Age (years), mean ± SD | 45 ± 12 | 41 ± 12 | 42 ± 11 | 0.06 |
| Sex, n (%) | ||||
| Male | 58 (41) | 42 (42) | 24 (40) | 0.962 |
| Female | 85 (59) | 58 (58) | 36 (60) | |
| Comorbidity, n (%) | ||||
| At least one comorbidity | 62 (43) | 70 (70) | 31 (52) | 0.0001 |
| Diabetes | 2 (1.4) | 2 (2) | 2 (3.3) | 0.66 |
| SAH | 8 (6) | 9 (9) | 4 (6.6) | 0.581 |
| Allergic diseases | 15 (10) | 20 (20) | 6 (10) | 0.089 |
| Dermatitis | 2 (1.4) | 12 (12) | 2 (3.3) | 0.001 |
| Overweight | 35 (24) | 27 (27) | 17 (28) | 0.793 |
| Positivity to antibodies, n (%) | ||||
| 1st dose | ||||
| IgM | 8 (5.6) | 10 (10) | 0.297 | |
| IgG | 128 (89.5) | 100 (100) | 0.0005 | |
| 2nd dose, n (%) | ||||
| IgM | 0 (0) | 0 (0) | - | - |
| IgG | 100 (100) | 100 (100) | - | 1.00 |
| Vitamin D intake (4000 IU/day), n (%) | 42 (29) | 32 (32) | 20 (33) | 0.812 |
| Side Effects to the Vaccine | Dose | Immunized with BNT162b2 Vaccine | p-Value | |
|---|---|---|---|---|
| Without Prior COVID-19 N = 143 n (%) | With prIor COVID-19 N = 100 n (%) | |||
| None | 1st | 46 (32) | 10 (10) | <0.0001 |
| 2nd | 100 (70) | 50 (50) | 0.002 | |
| At least 1 side effect | 1st | 97 (68) | 90 (90) | <0.0001 |
| 2nd | 43 (30) | 50 (50) | 0.002 | |
| Myalgia | 1st | 16 (11) | 27 (27) | 0.002 |
| 2nd | 15 (10.5) | 28 (28) | 0.0006 | |
| Odynophagia | 1st | 5 (3.5) | 2 (2) | 0.703 |
| 2nd | 3 (2.1) | 4 (4) | 0.450 | |
| Cough | 1st | 4 (2.8) | 4 (4) | 0.720 |
| 2nd | 3 (2.1) | 1 (1) | 0.645 | |
| Shivers | 1st | 8 (5.56) | 14 (14) | 0.038 |
| 2nd | 11 (7.7) | 17 (17) | 0.039 | |
| Rhinorrhea | 1st | 4 (2.8) | 7 (7) | 0.207 |
| 2nd | 6 (4.2) | 7 (7) | 0.391 | |
| Headache | 1st | 34 (24) | 26 (26) | 0.692 |
| 2nd | 22 (15.4) | 24 (24) | 0.091 | |
| Arthralgias | 1st | 4 (2.8) | 20 (20) | <0.0001 |
| 2nd | 9 (6.3) | 14 (14) | 0.048 | |
| Fever | 1st | 7 (4.8) | 13 (13) | 0.031 |
| 2nd | 5 (3.5) | 16 (16) | 0.0009 | |
| Dysgeusia | 1st | 2 (1.4) | 4 (4) | 0.232 |
| 2nd | 2 (1.4) | 1 (1) | 1.00 | |
| Irritability | 1st | 4 (2.8) | 10 (10) | 0.024 |
| 2nd | 4 (2.8) | 10 (10) | 0.024 | |
| Chest pain | 1st | 3 (2.1) | 4 (4) | 0.450 |
| 2nd | 2 (2.8) | 1 (1) | 1.00 | |
| Diarrhea | 1st | 3 (2.1) | 4 (4) | 0.450 |
| 2nd | 7 (4.8) | 4 (4) | 0.207 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Núñez, J.J.; Muñoz-Valle, J.F.; Meza-López, C.; Wang, L.-F.; Machado Sulbarán, A.C.; Torres-Hernández, P.C.; Bedolla-Barajas, M.; De la O-Gómez, B.; Balcázar-Félix, P.; Hernández-Bello, J. Neutralizing Antibodies Titers and Side Effects in Response to BNT162b2 Vaccine in Healthcare Workers with and without Prior SARS-CoV-2 Infection. Vaccines 2021, 9, 742. https://doi.org/10.3390/vaccines9070742
Morales-Núñez JJ, Muñoz-Valle JF, Meza-López C, Wang L-F, Machado Sulbarán AC, Torres-Hernández PC, Bedolla-Barajas M, De la O-Gómez B, Balcázar-Félix P, Hernández-Bello J. Neutralizing Antibodies Titers and Side Effects in Response to BNT162b2 Vaccine in Healthcare Workers with and without Prior SARS-CoV-2 Infection. Vaccines. 2021; 9(7):742. https://doi.org/10.3390/vaccines9070742
Chicago/Turabian StyleMorales-Núñez, José Javier, José Francisco Muñoz-Valle, Carlos Meza-López, Lin-Fa Wang, Andrea Carolina Machado Sulbarán, Paola Carolina Torres-Hernández, Martín Bedolla-Barajas, Brenda De la O-Gómez, Paulina Balcázar-Félix, and Jorge Hernández-Bello. 2021. "Neutralizing Antibodies Titers and Side Effects in Response to BNT162b2 Vaccine in Healthcare Workers with and without Prior SARS-CoV-2 Infection" Vaccines 9, no. 7: 742. https://doi.org/10.3390/vaccines9070742
APA StyleMorales-Núñez, J. J., Muñoz-Valle, J. F., Meza-López, C., Wang, L.-F., Machado Sulbarán, A. C., Torres-Hernández, P. C., Bedolla-Barajas, M., De la O-Gómez, B., Balcázar-Félix, P., & Hernández-Bello, J. (2021). Neutralizing Antibodies Titers and Side Effects in Response to BNT162b2 Vaccine in Healthcare Workers with and without Prior SARS-CoV-2 Infection. Vaccines, 9(7), 742. https://doi.org/10.3390/vaccines9070742









