Targeting Toll-Like Receptor 2: Polarization of Porcine Macrophages by a Mycoplasma-Derived Pam2cys Lipopeptide
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Generation of Porcine Monocyte-Derived Macrophages and Activation
2.3. Cell Viability
2.4. Flow Cytometry
2.5. Impact of Pam2Cys on moMΦ Morphology
2.6. Phagocytosis Assay
2.7. Cytokine Levels Determination
2.8. Gene Expression
2.9. Statistical Analysis
3. Results
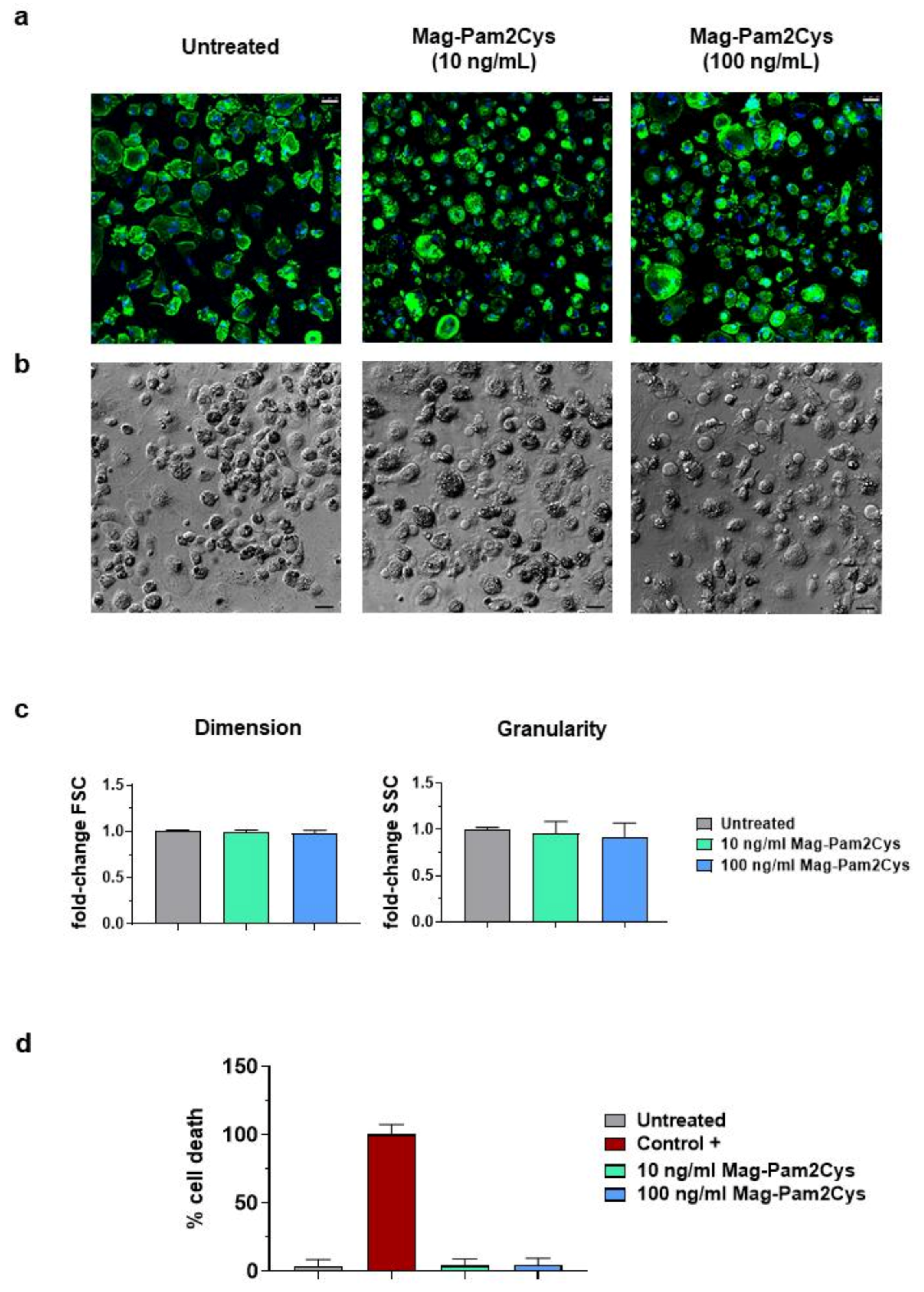
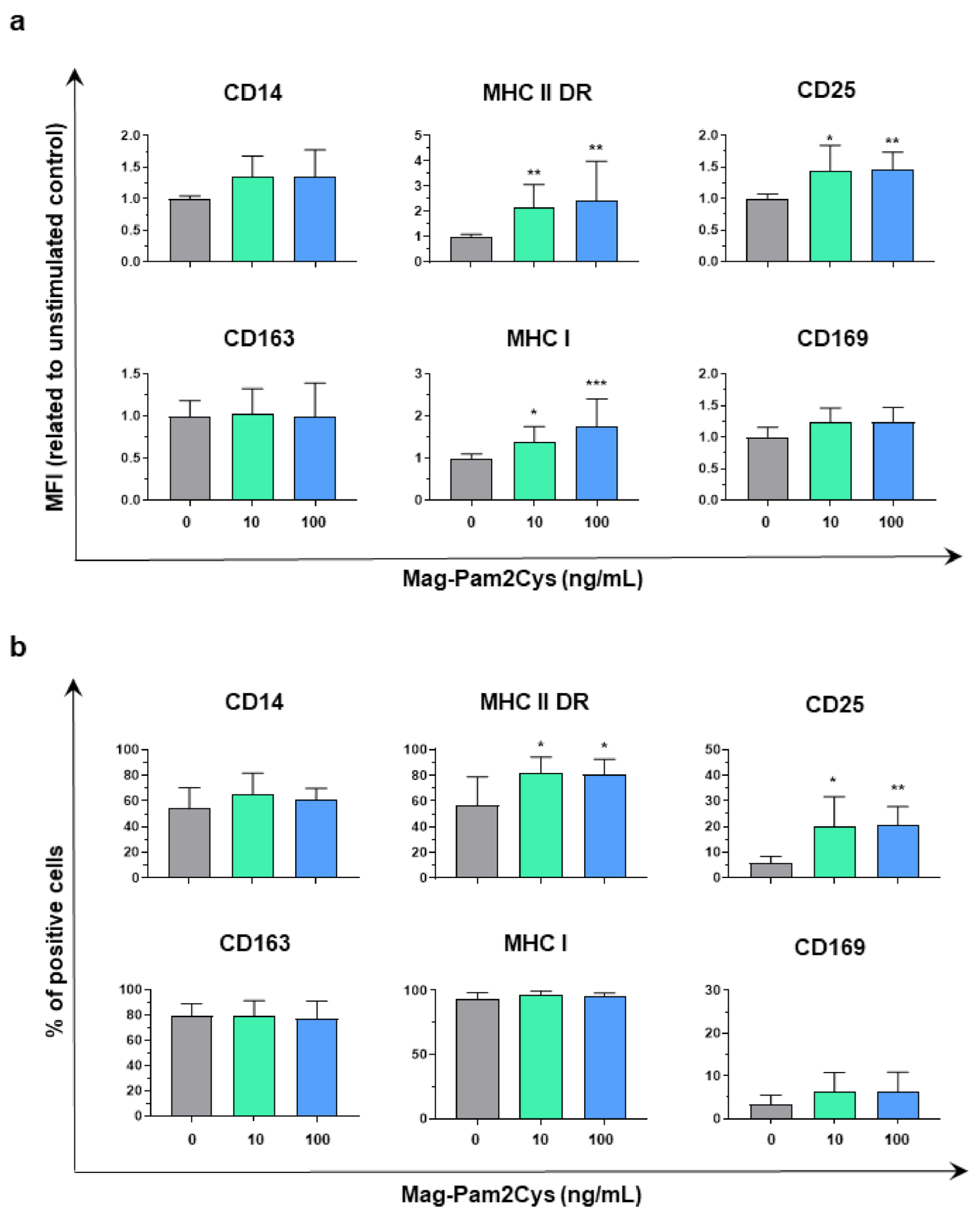
3.1. Impact of Mag-Pam2Cys on moMΦ Phenotype and Viability
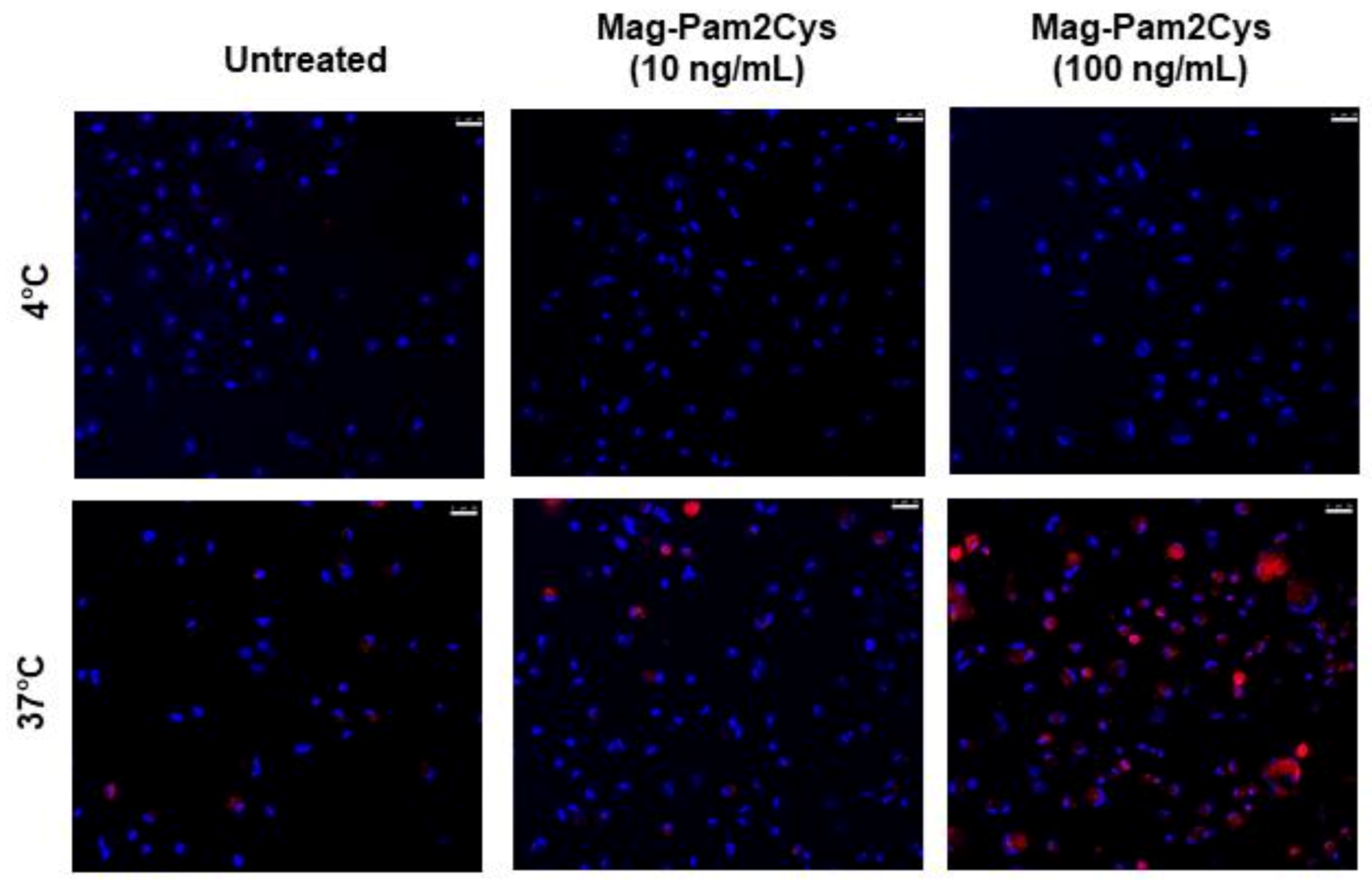
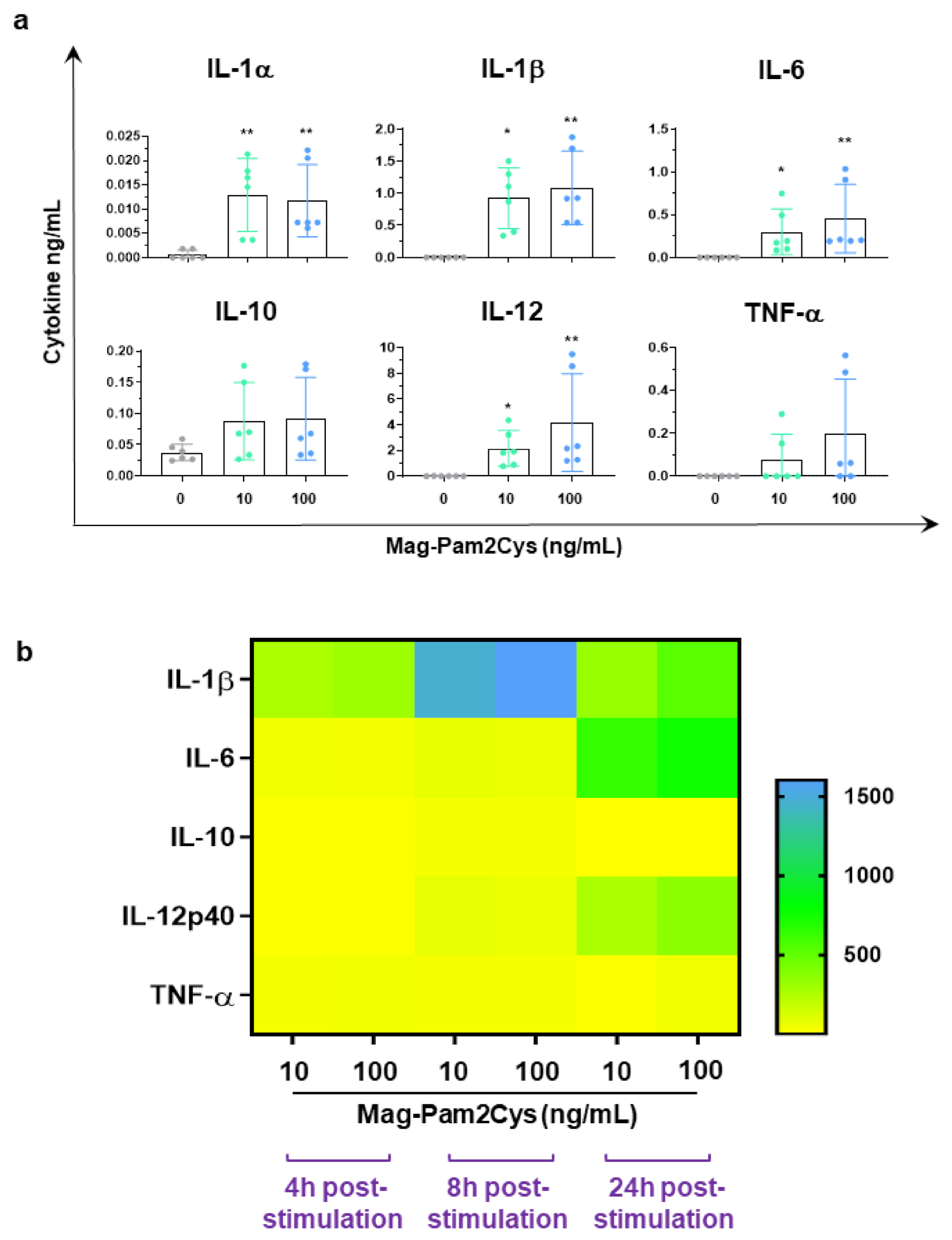
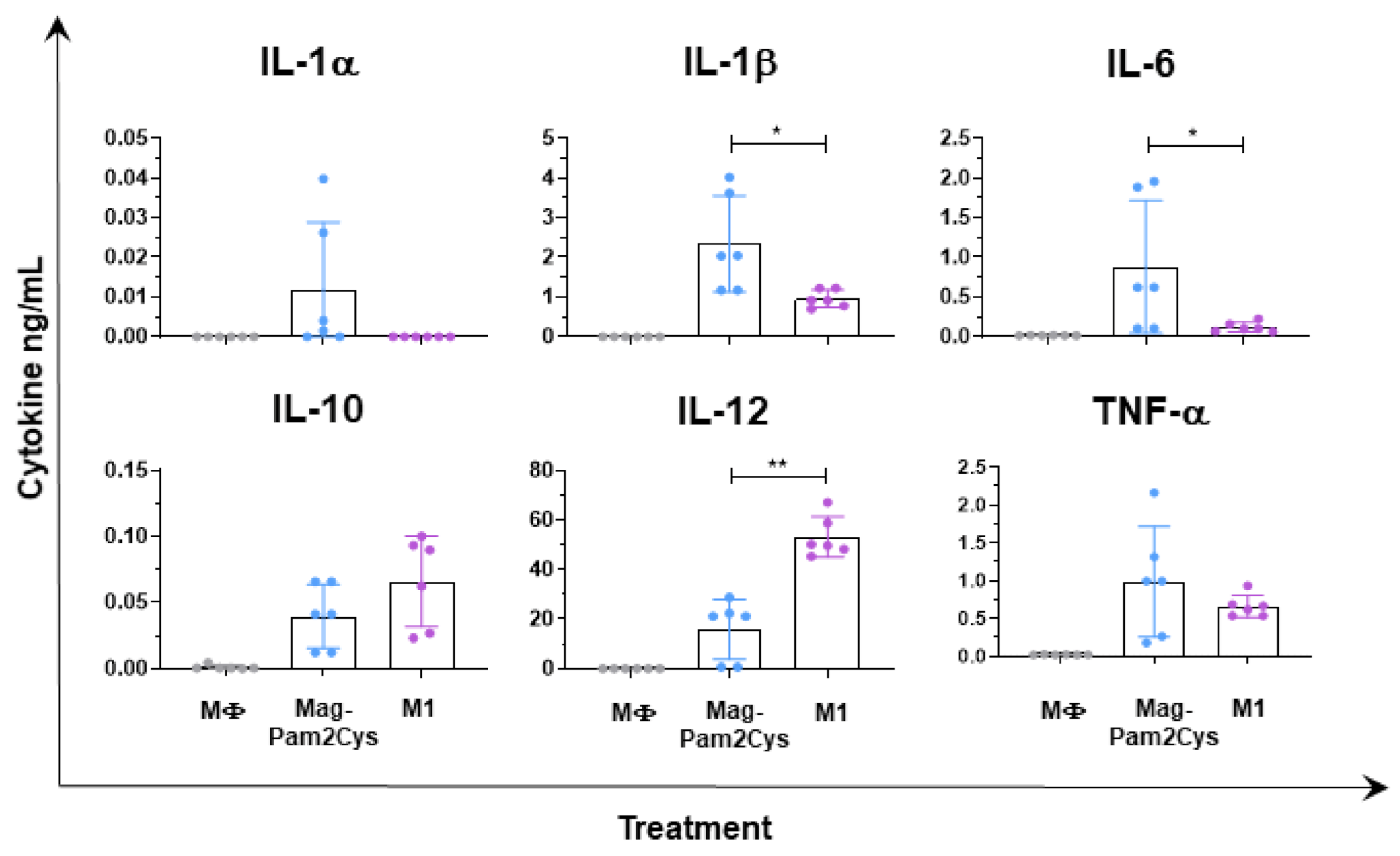
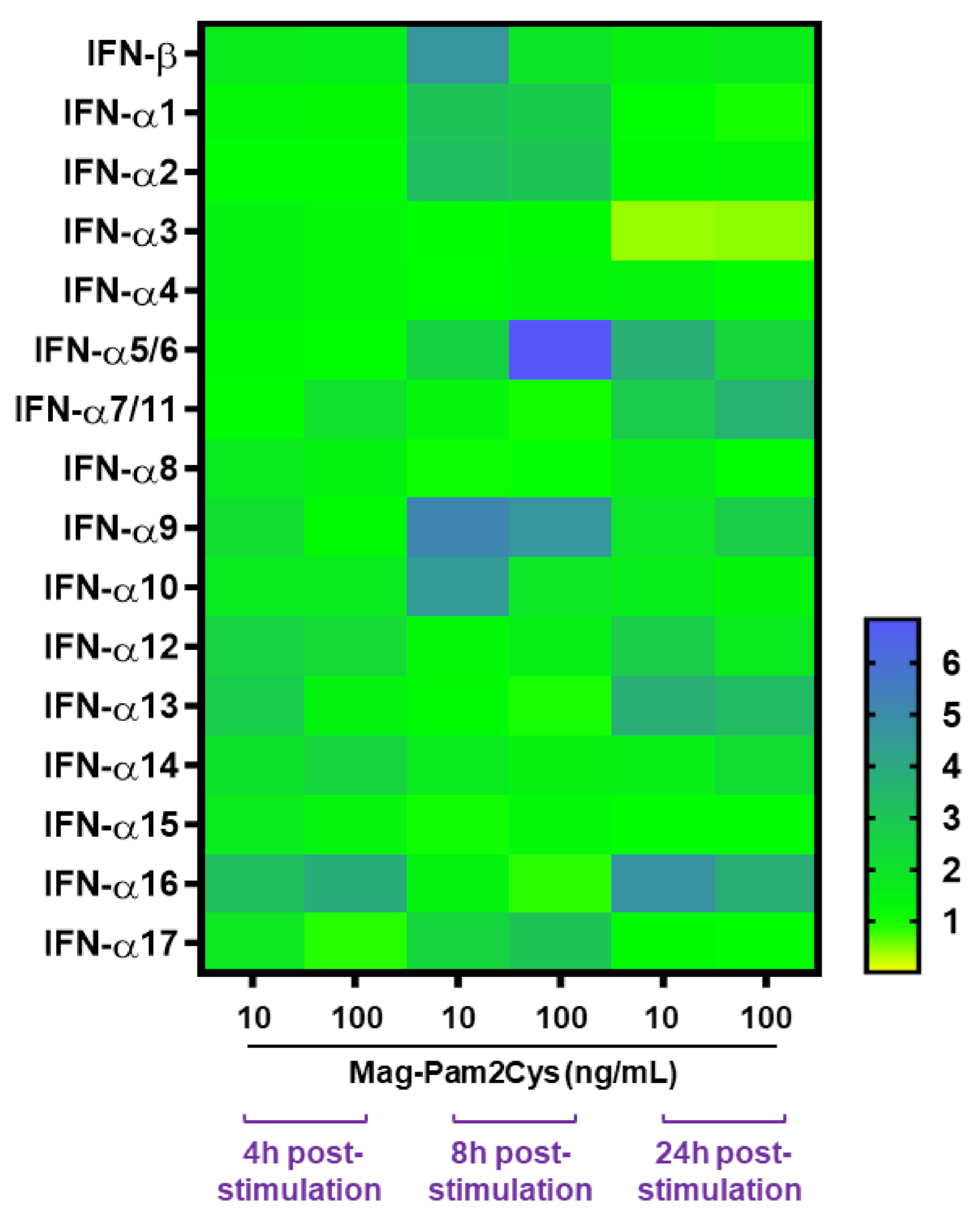
3.2. Evaluation of the Effects of Mag-Pam2Cys on moMΦ, Phagocytic Capacity, and Cytokine Responses
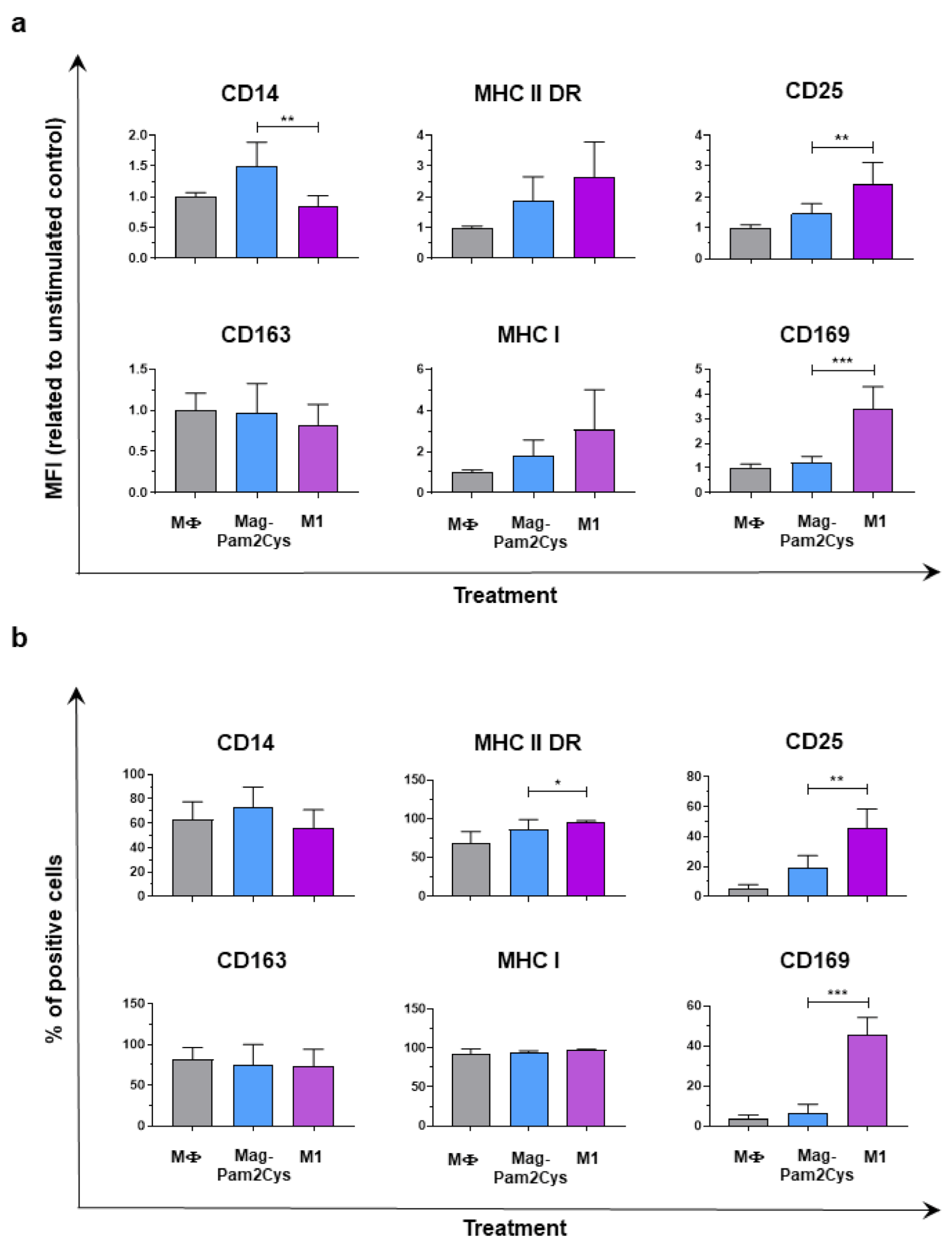
3.3. Comparison of Mag-Pam2Cys and M1-Polarization
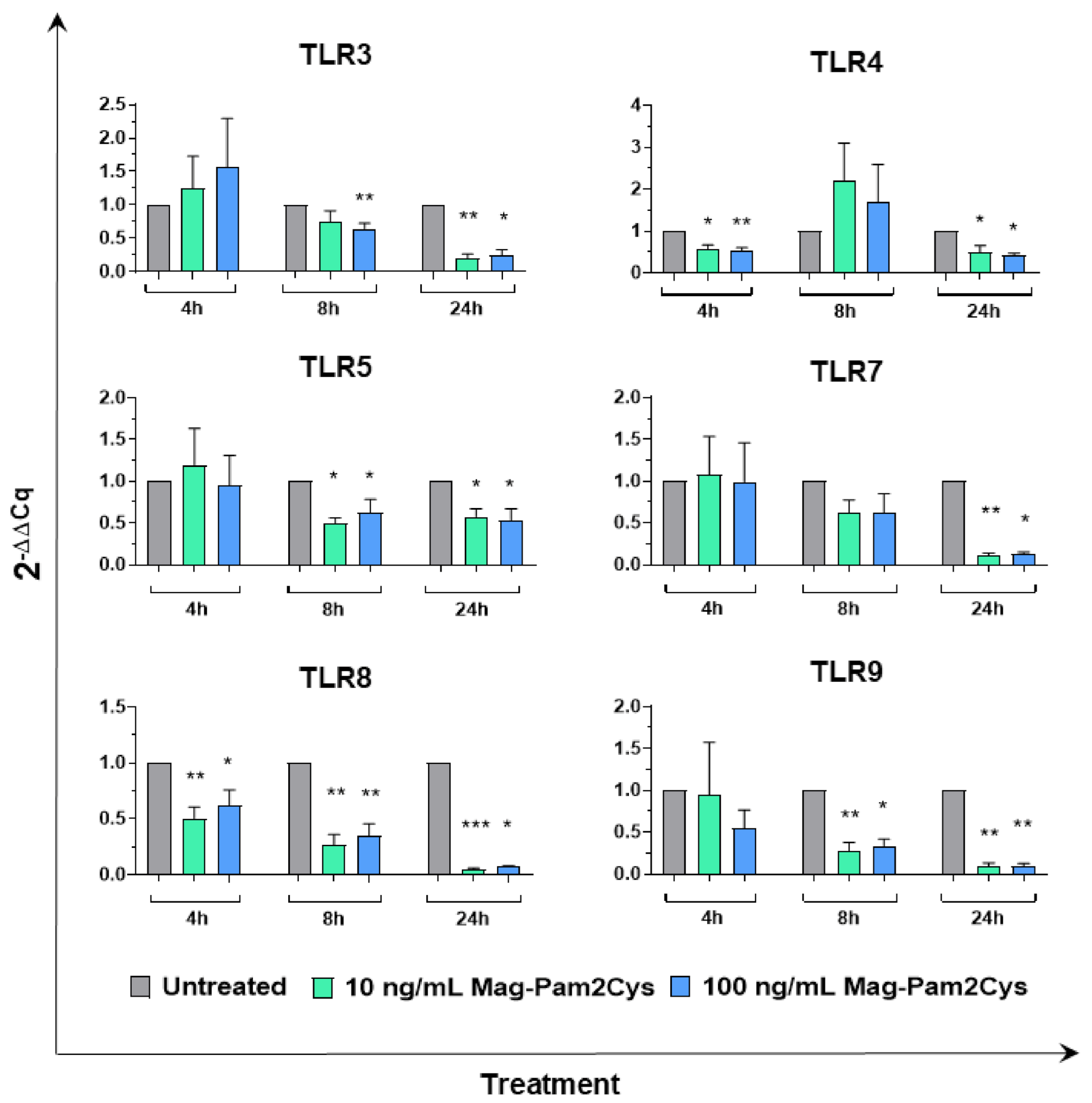
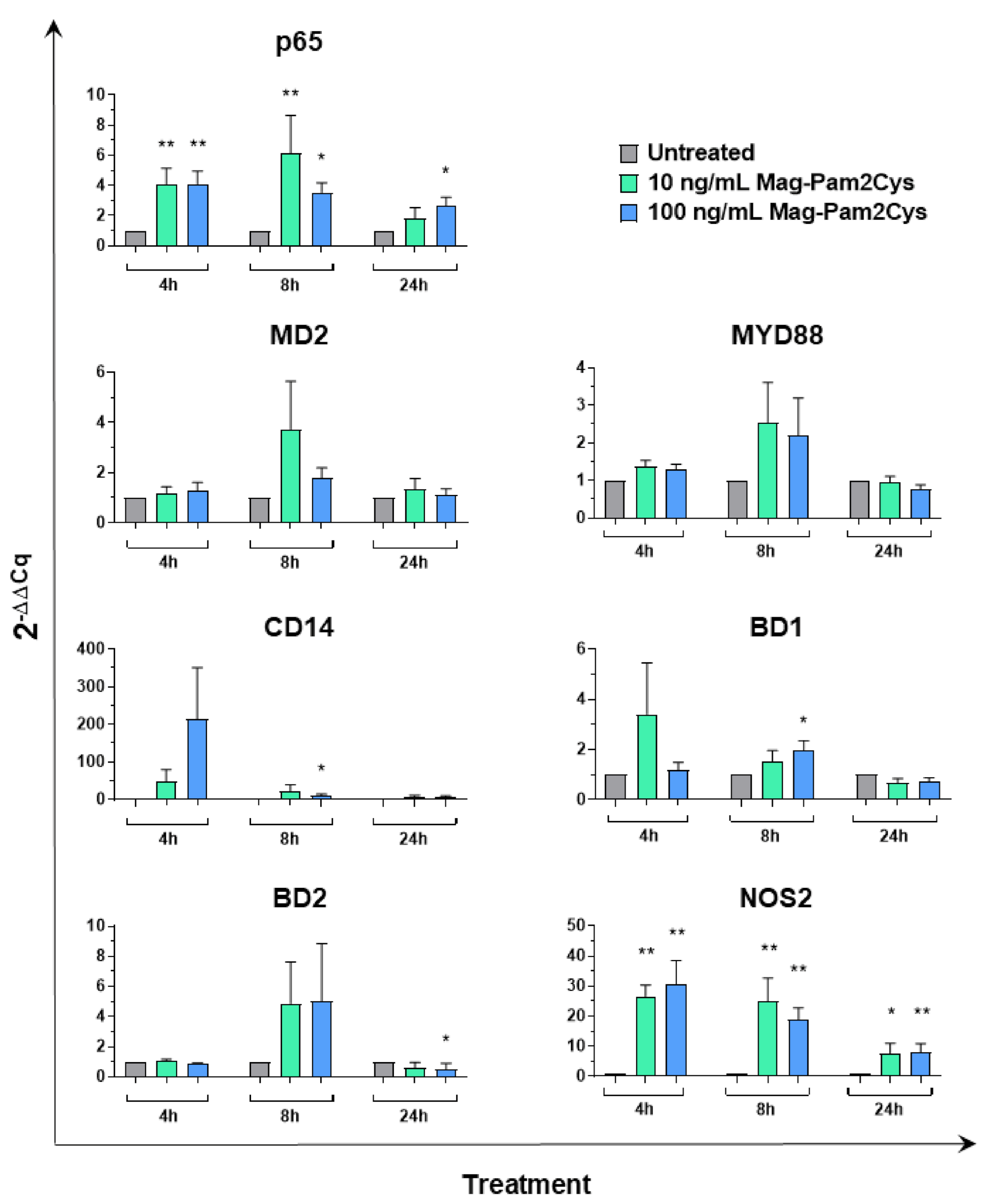
3.4. Effect of Mag-Pam2Cys on Key Macrophage Regulatory Gene Expression
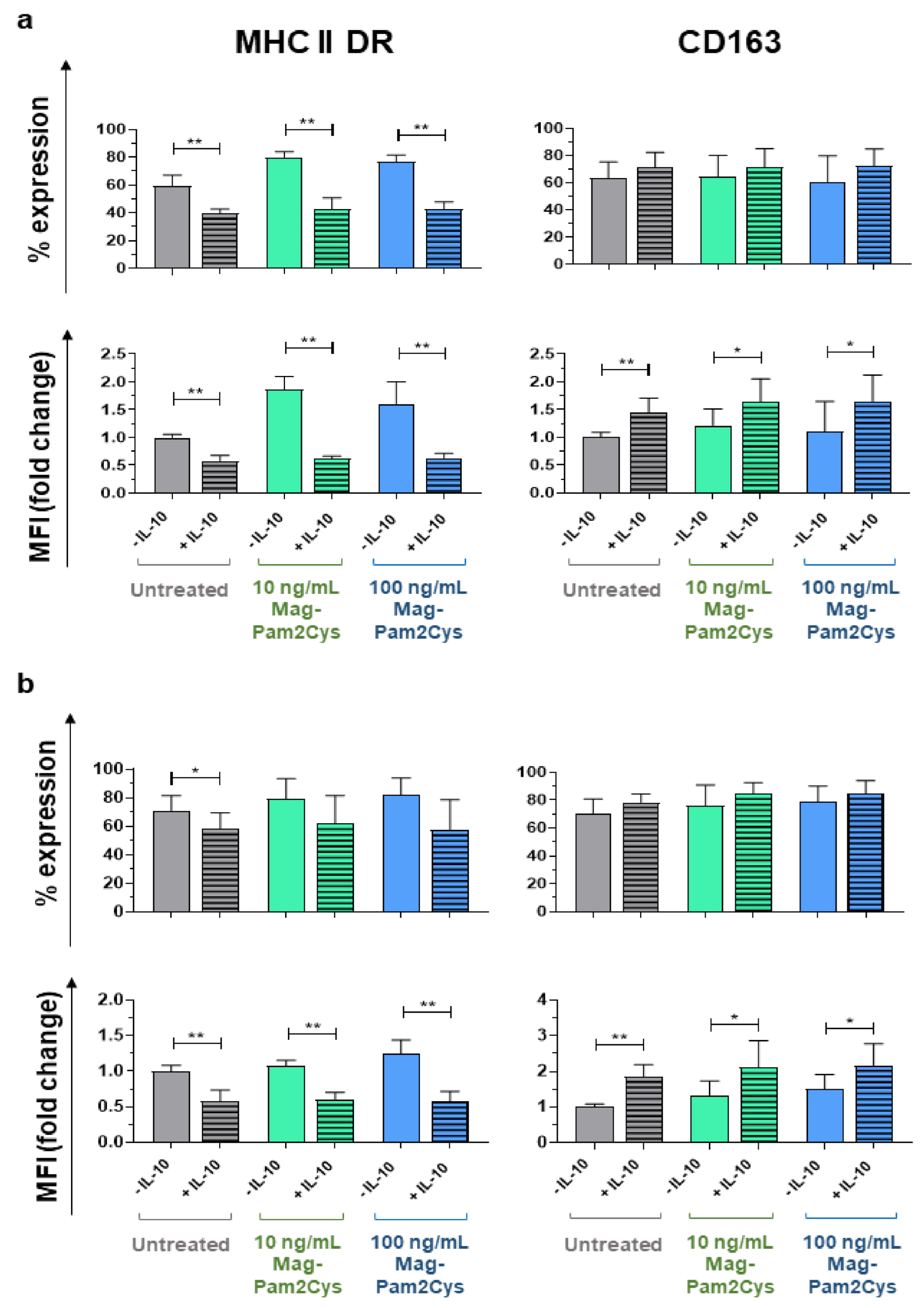
3.5. Mag-Pam2Cys Ability to Affect moMΦ Response to Recombinant Porcine IL-10
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyake, K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin. Immunol. 2007, 19, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 22, 142. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, E.; Tan, A.C.L.; Jackson, D.C. TLR agonists as modulators of the innate immune response and their potential as agents against infectious disease. Front. Immunol. 2014, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Urban-Vojciuk, Z.; Khan, M.M.; Oyler, B.L.; Fåhraeus, R.; Marek-Trzonkowska, N.; Nita-Lazar, A.; Hupp, T.R.; Goodlett, D.R. The Role of TLRs in Anti-cancer Immunity and Tumor Rejection. Front. Immunol. 2019, 10, 2388. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; Fusco, R.; Ginestra, G.; D’amico, R.; Bisignano, C.; Mandalari, G.; Cuzzocrea, S.; Di Paola, R. Involvement of TLR4 and PPAR-α Receptors in Host Response and NLRP3 Inflammasome Activation, Against Pulmonary Infection with Pseudomonas Aeruginosa. Shock 2019, 51, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Munoz-Planillo, R.; Nunez, G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 2012, 13, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Mu, R.; Wang, Z.; Xing, P.; Zhang, J.; Dong, L.; Wang, C. A toll-like receptor agonist mimicking microbial signal to generate tumor-suppressive macrophages. Nat. Commun. 2019, 10, 2272. [Google Scholar] [CrossRef] [PubMed]
- Muhlradt, P.F.; Kiess, M.; Meyer, H.; Sussmuth, R.; Jung, G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from mycoplasma fermentans acting at picomolar concentration. J. Exp. Med. 1997, 185, 1951–1958. [Google Scholar] [CrossRef]
- Reppe, K.; Tschernig, T.; Luhrmann, A.; Van Laak, V.; Grote, K.; Zemlin, M.V.; Gutbier, B.; Muller, H.C.; Kursar, M.; Schutte, H.; et al. Immunostimulation with macrophage-activating lipopeptide-2 increased survival in murine pneumonia. Am. J. Respir. Cell Mol. Biol. 2009, 40, 474–481. [Google Scholar] [CrossRef]
- Zeng, W.; Ghosh, S.; Lau, Y.F.; Brown, L.E.; Jackson, D.C. Highly immunogenic and totally synthetic lipopeptides as self-adjuvanting immunocontraceptive vaccines. J. Immunol. 2002, 169, 4905–4912. [Google Scholar] [CrossRef]
- Jackson, D.C.; Lau, Y.F.; Le, T.; Suhrbier, A.; Deliyannis, G.; Cheers, C.; Smith, C.; Zeng, W.; Brown, L.E. A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc. Natl. Acad. Sci. USA 2004, 101, 15440–15445. [Google Scholar] [CrossRef]
- Deliyannis, G.; Kedzierska, K.; Lau, Y.F.; Zeng, W.; Turner, S.J.; Jackson, D.C.; Brown, L.E. Intranasal lipopeptide primes lung-resident memory CD8+ T cells for longterm pulmonary protection against influenza. Eur. J. Immunol. 2006, 36, 770–778. [Google Scholar] [CrossRef]
- Chua, B.Y.; Zeng, W.; Jackson, D.C. Synthesis of toll-like receptor-2 targeting lipopeptides as self-adjuvanting vaccines. Methods Mol. Biol. 2008, 494, 247–261. [Google Scholar]
- Tan, A.C.L.; Mifsud, E.J.; Zeng, W.; Edenborough, K.; Mcvernon, J.; Brown, L.E.; Jackson, D.C. Intranasal administration of the TLR2 agonist Pam2Cys provides rapid protection against influenza in mice. Mol. Pharm. 2012, 9, 2710–2718. [Google Scholar] [CrossRef]
- Tan, A.C.L.; Eriksson, E.M.Y.; Kedzierska, K.; Deliyannis, G.; Valkenburg, S.A.; Zeng, W.; Jackson, D.C. Polyfunctional CD8+ T cells are associated with the vaccination-induced control of a novel recombinant influenza virus expressing an HCV epitope. Antiviral Res. 2012, 94, 168–178. [Google Scholar] [CrossRef]
- Proud, P.C.; Tsitoura, D.; Watson, R.J.; Chua, B.Y.; Aram, M.J.; Bewley, K.R.; Cavell, B.E.; Cobb, R.; Dowall, S.; Fotheringham, S.A.; et al. Prophylactic intranasal administration of a TLR2/6 agonist reduces upper respiratory tract viral shedding in a SARS-CoV-2 challenge ferret model. EBioMedicine 2021, 63, 103153. [Google Scholar] [CrossRef]
- Cacciotto, C.; Cubeddu, T.; Addis, M.F.; Anfossi, A.G.; Tedde, V.; Tore, G.; Carta, T.; Rocca, S.; Chessa, B.; Pittau, B.; et al. Mycoplasma lipoproteins are major determinants of neutrophil extracellular trap formation. Cell. Microbiol. 2016, 18, 1751–1762. [Google Scholar] [CrossRef]
- Anfossi, A.G.; Coraduzza, E.; Bonelli, P.; Piras, I.; Cacciotto, C.; Puggioni, G.; Masia, M.; Alberti, A.; Pittau, M.; Chessa, B. Sintesi e valutazione delle proprietà immunomodulatorie di un lipopeptide diacilato derivato da Mycoplasma agalactiae. In Proceedings of the Simposio di Immunologia Veterinaria, Florence, Italy, 28 May 2014. [Google Scholar]
- Carta, T.; Razzuoli, E.; Fruscione, F.; Zinellu, S.; Meloni, D.; Anfossi, A.; Chessa, B.; Dei Giudici, S.; Graham, S.P.; Oggiano, A.; et al. Comparative Phenotypic and Functional Analyses of the Effects of IL-10 or TGF-β on Porcine Macrophages. Animals 2021, 11, 1098. [Google Scholar] [CrossRef]
- Franzoni, G.; Razzuoli, E.; Dei Giudici, S.; Carta, T.; Galleri, G.; Zinellu, S.; Ledda, M.; Angioi, P.; Modesto, P.; Graham, S.P.; et al. Comparison of macrophage responses to African swine fever viruses reveals that the NH/P68 strain is associated with enhanced sensitivity to type I IFN and cytokine responses from classically associated macrophages. Pathogens 2020, 9, 209. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Franzoni, G.; Dei Giudici, S.; Oggiano, A. Infection, modulation and responses of antigen-presenting cells to African swine fever viruses. Virus Res. 2018, 258, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Amadori, M.; Razzuoli, E. Immune control of PRRS: Lessons to be learned and possible ways forward. Front. Vet. Sci. 2014, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Allan, G.M.; Ellis, J.A. Porcine circoviruses: A review. J. Vet. Diagn. Investig. 2000, 12, 3–14. [Google Scholar] [CrossRef] [PubMed]
- VanderWaal, K.; Deen, J. Global trends in infectious diseases of swine. Proc. Natl. Acad. Sci. USA 2018, 115, 11495–11500. [Google Scholar] [CrossRef]
- Kapentanovic, R.; Fairbairn, L.; Beraldi, D.; Sester, D.P.; Archibald, A.L.; Tuggle, C.K.; Hume, D.A. Pig bone marrow-derived macrophages resemble human macrophages in their response to bacterial lipopolysaccharide. J. Immunol. 2012, 18, 3382–3394. [Google Scholar] [CrossRef]
- Kapentanovic, R.; Fairbairn, L.; Downing, A.; Beraldi, D.; Sester, D.P.; Freeman, T.C.; Tuggle, C.K.; Archibald, A.L.; Hume, D.A. The impact of breed and tissue compartment on the response of pig macrophages to lipopolysaccharide. BMC Genom. 2013, 14, 581. [Google Scholar]
- Fairbairn, L.; Kapetanovic, R.; Sester, D.P.; Hume, D.A. The mononuclear phagocyte system of the pig as a model for understanding human innate immunity and disease. J. Leukoc. Biol. 2011, 8, 855–871. [Google Scholar] [CrossRef]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The immunology of the porcine skin and its value as a model for human skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef]
- Kaser, T. Swine as biomedical animal model for T-cell research—Success and potential for transmittable and non-transmittable human diseases. Mol. Immunol. 2021, 135, 95–115. [Google Scholar] [CrossRef]
- Pabst, R. The pig as a model for immunology research. Cell Tissue Res. 2020, 380, 287–304. [Google Scholar] [CrossRef]
- Franzoni, G.; Dei Giudici, S.; Loi, F.; Sanna, D.; Floris, M.; Fiori, M.; Sanna, M.L.; Madrau, P.; Scarpa, F.; Zinellu, S.; et al. African Swine Fever Circulation among Free-Ranging Pigs in Sardinia: Data from the Eradication Program. Vaccines 2020, 8, 549. [Google Scholar] [CrossRef]
- Franzoni, G.; Bonelli, P.; Graham, S.P.; Anfossi, A.G.; Dei Giudici, S.; Pilo, G.; Pittau, M.; Nicolussi, P.; Oggiano, A. Comparative phenotypic and functional analyses of the effects of autologous plasma and recombinant human macrophage-colony stimulating factor (M-CSF) on porcine monocyte to macrophage differentiation. Vet. Immunol. Immunopathol. 2017, 187, 80–88. [Google Scholar] [CrossRef]
- Razzuoli, E.; Mignone, G.; Lazzara, F.; Vencia, W.; Ferraris, M.; Masiello, L.; Vivaldi, B.; Ferrari, A.; Bozzetta, E.; Amadori, M. Impact of cadmium exposure on swine enterocytes. Toxicol. Lett. 2018, 287, 92–99. [Google Scholar] [CrossRef]
- Mecocci, S.; Porcellato, I.; Armando, F.; Mechelli, L.; Brachelente, C.; Pepe, M.; Gialletti, R.; Passeri, B.; Modesto, P.; Ghelardi, A.; et al. Equine Genital Squamous Cell Carcinoma Associated with EcPV2 Infection: RANKL Pathway Correlated to Inflammation and Wnt Signaling Activation. Biology 2021, 10, 244. [Google Scholar] [CrossRef]
- Razzuoli, E.; Amadori, M.; Lazzara, F.; Bilato, D.; Ferraris, M.; Vito, G.; Ferrari, A. Salmonella serovar-specific interaction with jejunal epithelial cells. Vet. Microbiol. 2017, 207, 219–225. [Google Scholar] [CrossRef]
- Razzuoli, E.; Villa, R.; Amadori, M. IPEC-J2 cells as reporter system of the anti-inflammatory control actions of interferon-alpha. J. Interferon Cytokine Res. 2013, 33, 597–605. [Google Scholar] [CrossRef]
- Razzuoli, E.; Villa, R.; Sossi, E.; Amadori, M. Reverse Transcription Real-Time PCR for Detection of Porcine Interferon α and β Genes. Scand. J. Immunol. 2011, 74, 412–418. [Google Scholar] [CrossRef]
- Cheng, G.; Chen, W.; Li, Z.; Yan, W.; Zhao, X.; Xie, J.; Liu, M.; Zhang, H.; Zhong, Y.; Zheng, Z. Characterization of the porcine alpha interferon multigene family. Gene 2006, 382, 28–38. [Google Scholar] [CrossRef]
- Yoo, I.; Han, J.; Lee, S.; Jung, W.; Kim, J.H.; Kim, Y.W.; Kim, H.J.; Hong, M.; Ka, H. Analysis of stage-specific expression of the toll-like receptor family in the porcine endometrium throughout the estrous cycle and pregnancy. Theriogenology 2019, 125, 173–183. [Google Scholar] [CrossRef]
- Sautter, C.A.; Auray, G.; Python, S.; Liniger, M.; Summerfield, A. Phenotypic and functional modulations of porcine macrophages by interferons and interleukin-4. Dev. Comp. Immunol. 2018, 84, 181–192. [Google Scholar] [CrossRef]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef]
- Petersen, C.B.; Nygård, A.; Fredholm, M.; Aasted, B.; Salomonsen, J. Cloning, characterization and mapping of porcine CD14 reveals a high conservation of mammalian CD14 structure, expression and locus organization. Dev. Comp. Immunol. 2006, 31, 729–737. [Google Scholar] [CrossRef]
- Elahi, S.; Buchanan, R.M.; Attah-Poku, S.; Townsend, H.G.; Babiuk, L.A.; Gerdts, V. The host defense peptide beta-defensin 1 confers protection against Bordetella pertussis in newborn piglets. Infect. Immun. 2006, 74, 2338–2352. [Google Scholar] [CrossRef]
- Li, C.L.; Xu, T.T.; Chen, R.B.; Huang, X.X.; Zhao, Y.C.; Bao, Y.Y.; Zhao, W.; Zheng, Z. Cloning, expression and characterization of antimicrobial porcine beta defensin 1 in Escherichia coli. Protein Expr. Purif. 2013, 88, 47–53. [Google Scholar] [CrossRef]
- Veldhuizen, E.J.; Rijnders, M.; Claassen, E.A.; van Dijk, A.; Haagsman, H.P. Porcine beta-defensin 2 displays broad antimicrobial activity against pathogenic intestinal bacteria. Mol. Immunol. 2008, 45, 386–394. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Schenk, M.; Belisle, J.T.; Modlin, R.L. TLR2 looks at lipoproteins. Immunity 2009, 31, 847–849. [Google Scholar] [CrossRef]
- Kaufmann, A.; Muhlradt, P.F.; Gemsa, D.; Sprenger, H. Induction of Cytokines and Chemokines in Human Monocytes by Mycoplasma fermentans-Derived Lipoprotein MALP-2. Infect. Immun. 1999, 67, 6303–6308. [Google Scholar] [CrossRef]
- Singleton, H.; Graham, S.P.; Bodman-Smith, K.B.; Frossard, J.; Steinbachet, F. Establishing porcine monocyte-derived macrophage and dendritic cell systems for studying the interaction with PRRSV-1. Front. Microbiol. 2016, 7, 832. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, C.; Razzuoli, E.; Crooke, H.; Soule, O.; Pezzoni, G.; Ferraris, M.; Ferrari, A.; Amadori, M. Different biological activities of swine IFN-α subtypes. J. Interferon Cytokine Res. 2015, 35, 990–1002. [Google Scholar] [CrossRef]
- Sang, Y.; Rowland, R.R.R.; Hesse, R.A.; Blecha, F. Differential expression and activity of the porcine type I interferon family. Physiol. Genom. 2010, 42, 248–258. [Google Scholar] [CrossRef]
- Fernandez-Sainz, I.; Ramanathan, P.; O’Donnell, V.; Diaz-San Segundo, F.; Velazquez-Salinas, L.; Sturza, D.F.; Zhu, J.; de Jos Santos, T.; Borca, M.V. Treatment with interferon-alpha delays disease in swine infected with a highly virulent CSFV strain. Virology 2015, 483, 284–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chinsangaram, J.; Moraes, M.P.; Koster, M.; Grubman, M.J. Novel viral disease control strategy: Adenovirus expressing alpha interferon rapidily protects swine from foot-and-mouth disease. J. Virol. 2003, 77, 1621–1625. [Google Scholar] [CrossRef]
- Sosan, O.; Graham, S.P.; Everett, H.; Crudgington, B.; Bodman-Smith, K.; Crooke, H. Differential antiviral effect of porcine interferon alpha subtypes on classical swine fever virus infection of porcine monocytes. Cytokine 2012, 59, 552. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Rath, M.; Muller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via arginase or nitric oxide synthase: Two competing arginine pathways in macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef]
- Mosser, D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzoni, G.; Anfossi, A.; De Ciucis, C.G.; Mecocci, S.; Carta, T.; Dei Giudici, S.; Fruscione, F.; Zinellu, S.; Vito, G.; Graham, S.P.; et al. Targeting Toll-Like Receptor 2: Polarization of Porcine Macrophages by a Mycoplasma-Derived Pam2cys Lipopeptide. Vaccines 2021, 9, 692. https://doi.org/10.3390/vaccines9070692
Franzoni G, Anfossi A, De Ciucis CG, Mecocci S, Carta T, Dei Giudici S, Fruscione F, Zinellu S, Vito G, Graham SP, et al. Targeting Toll-Like Receptor 2: Polarization of Porcine Macrophages by a Mycoplasma-Derived Pam2cys Lipopeptide. Vaccines. 2021; 9(7):692. https://doi.org/10.3390/vaccines9070692
Chicago/Turabian StyleFranzoni, Giulia, Antonio Anfossi, Chiara Grazia De Ciucis, Samanta Mecocci, Tania Carta, Silvia Dei Giudici, Floriana Fruscione, Susanna Zinellu, Guendalina Vito, Simon Paul Graham, and et al. 2021. "Targeting Toll-Like Receptor 2: Polarization of Porcine Macrophages by a Mycoplasma-Derived Pam2cys Lipopeptide" Vaccines 9, no. 7: 692. https://doi.org/10.3390/vaccines9070692
APA StyleFranzoni, G., Anfossi, A., De Ciucis, C. G., Mecocci, S., Carta, T., Dei Giudici, S., Fruscione, F., Zinellu, S., Vito, G., Graham, S. P., Oggiano, A., Chessa, B., & Razzuoli, E. (2021). Targeting Toll-Like Receptor 2: Polarization of Porcine Macrophages by a Mycoplasma-Derived Pam2cys Lipopeptide. Vaccines, 9(7), 692. https://doi.org/10.3390/vaccines9070692








