Type II NKT Cell Agonist, Sulfatide, Is an Effective Adjuvant for Oral Heat-Killed Cholera Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Immunisation of Mice
2.3. Isolation of Splenocytes and Mesenteric Lymph Node Cells
2.4. Cytokine Quantification by Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Collection of Faecal and Serum Samples
2.6. Preparation of Intestinal Tissues
2.7. Measurement of Whole Bacteria-Specific Antibodies by ELISA
2.8. Measurement of CTB-Specific Antibodies by ELISA
2.9. Statistical Analysis
3. Results
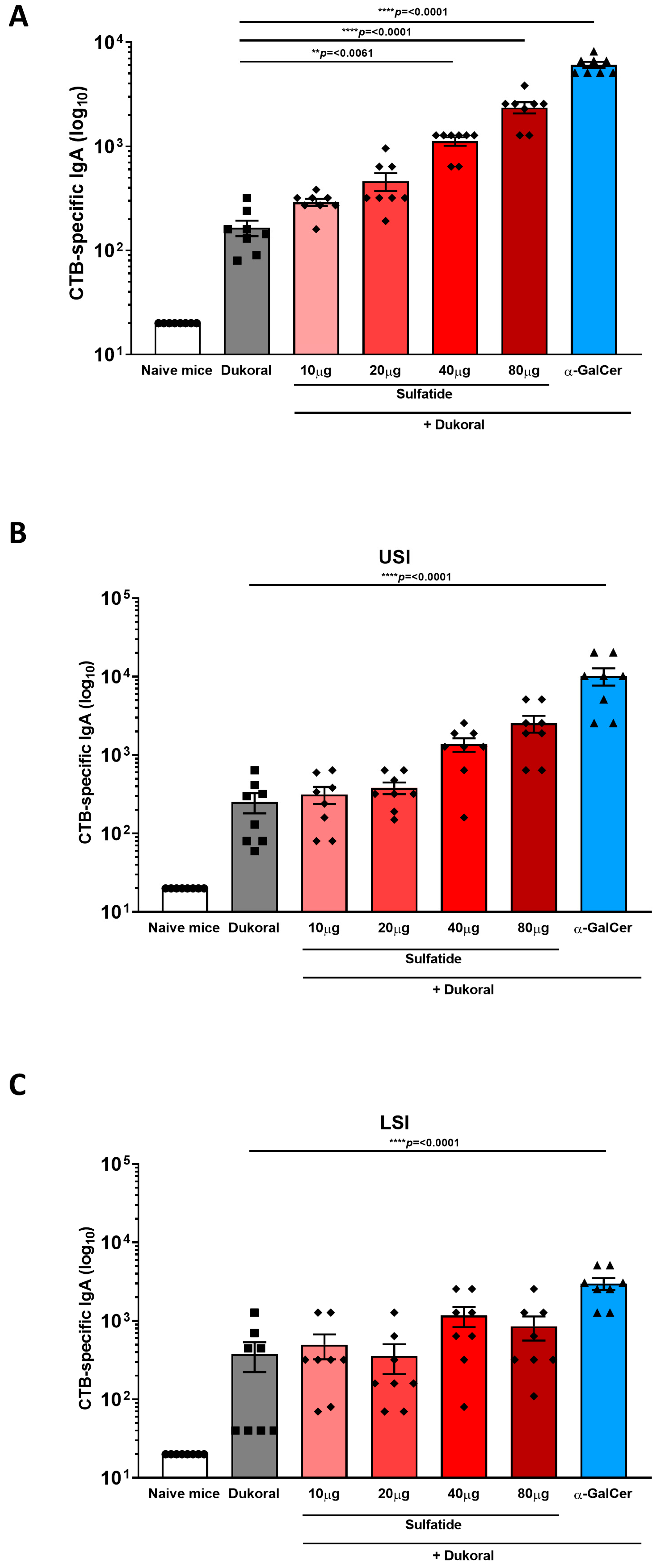
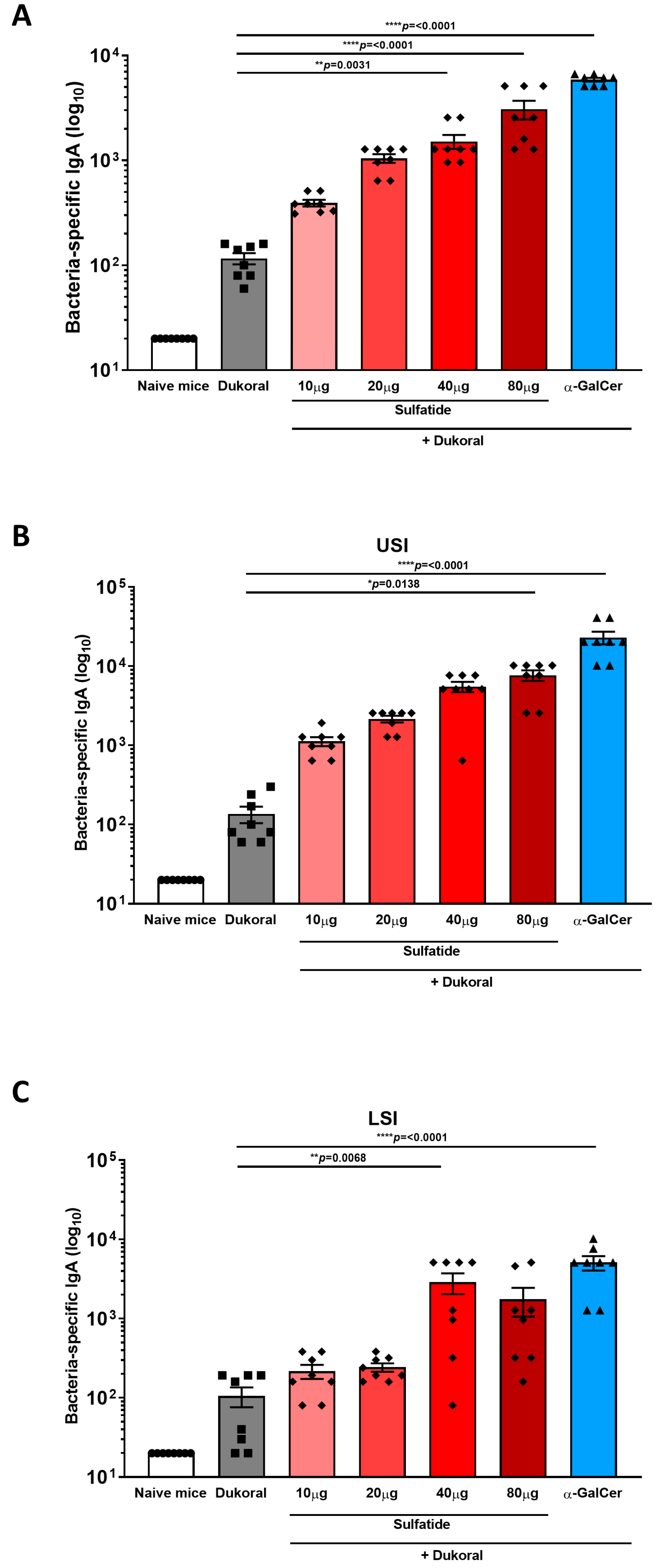
3.1. Oral Co-Administration of Sulfatide with Dukoral® Enhanced CTB- and Bacterium-Specific Intestinal IgA Responses Compared to Vaccination with Dukoral® Alone
3.2. Oral Co-Administration of Sulfatide with Dukoral® Enhanced Mucosal IFNγ and IL-17A Responses, as Well as Systemic IgG Responses
3.3. The Enhancement of Antigen-Specific Intestinal IgA Response by Sulfatide Was Reduced in CD1d−/− Mice but Not in γδ T Cell-Deficient Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Lemoine, C.; Thakur, A.; Krajisnik, D.; Guyon, R.; Longet, S.; Razim, A.; Gorska, S.; Pantelic, I.; Ilic, T.; Nikolic, I.; et al. Technological Approaches for Improving Vaccination Compliance and Coverage. Vaccines 2020, 8, 304. [Google Scholar] [CrossRef]
- Abautret-Daly, A.E.; Davitt, C.J.; Lavelle, E.C. Harnessing the antibacterial and immunological properties of mucosal-associated invariant T cells in the development of novel oral vaccines against enteric infections. Biochem. Pharmacol. 2014, 92, 173–183. [Google Scholar] [CrossRef]
- Davitt, C.J.; McNeela, E.A.; Longet, S.; Tobias, J.; Aversa, V.; McEntee, C.P.; Rosa, M.; Coulter, I.S.; Holmgren, J.; Lavelle, E.C. A novel adjuvanted capsule based strategy for oral vaccination against infectious diarrhoeal pathogens. J. Control. Release 2016, 233, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Longet, S.; Abautret-Daly, A.; Davitt, C.J.H.; McEntee, C.P.; Aversa, V.; Rosa, M.; Coulter, I.S.; Holmgren, J.; Raghavan, S.; Lavelle, E.C. An oral alpha-galactosylceramide adjuvanted Helicobacter pylori vaccine induces protective IL-1R- and IL-17R-dependent Th1 responses. NPJ Vaccines 2019, 4, 45. [Google Scholar] [CrossRef]
- Davitt, C.J.H.; Longet, S.; Albutti, A.; Aversa, V.; Nordqvist, S.; Hackett, B.; McEntee, C.P.; Rosa, M.; Coulter, I.S.; Lebens, M.; et al. Alpha-galactosylceramide enhances mucosal immunity to oral whole-cell cholera vaccines. Mucosal Immunol. 2019, 12, 1055–1064. [Google Scholar] [CrossRef]
- Exley, M.A.; Tahir, S.M.; Cheng, O.; Shaulov, A.; Joyce, R.; Avigan, D.; Sackstein, R.; Balk, S.P. A major fraction of human bone marrow lymphocytes are Th2-like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J. Immunol. 2001, 167, 5531–5534. [Google Scholar] [CrossRef]
- Singh, A.K.; Tripathi, P.; Cardell, S.L. Type II NKT Cells: An Elusive Population With Immunoregulatory Properties. Front. Immunol. 2018, 9, 1969. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.V.; Kumar, V. Type II NKT Cells and Their Emerging Role in Health and Disease. J. Immunol. 2017, 198, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Honke, K. Biosynthesis and biological function of sulfoglycolipids. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013, 89, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Jahng, A.; Maricic, I.; Aguilera, C.; Cardell, S.; Halder, R.C.; Kumar, V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 2004, 199, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Fuss, I.J.; Joshi, B.; Yang, Z.; Degheidy, H.; Fichtner-Feigl, S.; de Souza, H.; Rieder, F.; Scaldaferri, F.; Schirbel, A.; Scarpa, M.; et al. IL-13Ralpha2-bearing, type II NKT cells reactive to sulfatide self-antigen populate the mucosa of ulcerative colitis. Gut 2014, 63, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Arrenberg, P.; Maricic, I.; Kumar, V. Sulfatide-mediated activation of type II natural killer T cells prevents hepatic ischemic reperfusion injury in mice. Gastroenterology 2011, 140, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Halder, R.C.; Aguilera, C.; Maricic, I.; Kumar, V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J. Clin. Investig. 2007, 117, 2302–2312. [Google Scholar] [CrossRef]
- Ambrosino, E.; Terabe, M.; Halder, R.C.; Peng, J.; Takaku, S.; Miyake, S.; Yamamura, T.; Kumar, V.; Berzofsky, J.A. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: A new immunoregulatory axis. J. Immunol. 2007, 179, 5126–5136. [Google Scholar] [CrossRef]
- Sundell, I.B.; Halder, R.; Zhang, M.; Maricic, I.; Koka, P.S.; Kumar, V. Sulfatide administration leads to inhibition of HIV-1 replication and enhanced hematopoeisis. J. Stem Cells 2010, 5, 33–42. [Google Scholar] [CrossRef]
- Kwiecinski, J.; Rhost, S.; Lofbom, L.; Blomqvist, M.; Mansson, J.E.; Cardell, S.L.; Jin, T. Sulfatide attenuates experimental Staphylococcus aureus sepsis through a CD1d-dependent pathway. Infect. Immun. 2013, 81, 1114–1120. [Google Scholar] [CrossRef]
- Jansson, L.; Tobias, J.; Jarefjall, C.; Lebens, M.; Svennerholm, A.M.; Teneberg, S. Sulfatide recognition by colonization factor antigen CS6 from enterotoxigenic Escherichia coli. PLoS ONE 2009, 4, e4487. [Google Scholar] [CrossRef]
- Choi, B.K.; Schifferli, D.M. Lysine residue 117 of the FasG adhesin of enterotoxigenic Escherichia coli is essential for binding of 987P fimbriae to sulfatide. Infect. Immun. 1999, 67, 5755–5761. [Google Scholar] [CrossRef]
- Kamisago, S.; Iwamori, M.; Tai, T.; Mitamura, K.; Yazaki, Y.; Sugano, K. Role of sulfatides in adhesion of Helicobacter pylori to gastric cancer cells. Infect. Immun. 1996, 64, 624–628. [Google Scholar] [CrossRef]
- Villavedra, M.; Carol, H.; Hjulstrom, M.; Holmgren, J.; Czerkinsky, C. “PERFEXT”: A direct method for quantitative assessment of cytokine production in vivo at the local level. Res. Immunol. 1997, 148, 257–266. [Google Scholar] [CrossRef]
- Frey, A.; Di Canzio, J.; Zurakowski, D. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 1998, 221, 35–41. [Google Scholar] [CrossRef]
- Harris, J.B.; LaRocque, R.C.; Chowdhury, F.; Khan, A.I.; Logvinenko, T.; Faruque, A.S.; Ryan, E.T.; Qadri, F.; Calderwood, S.B. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl. Trop. Dis. 2008, 2, e221. [Google Scholar] [CrossRef]
- Harris, A.M.; Bhuiyan, M.S.; Chowdhury, F.; Khan, A.I.; Hossain, A.; Kendall, E.A.; Rahman, A.; LaRocque, R.C.; Wrammert, J.; Ryan, E.T.; et al. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect. Immun. 2009, 77, 3850–3856. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, T.R.; Lundin, S.B.; Khan, A.I.; Lundgren, A.; Harris, J.B.; Calderwood, S.B.; Qadri, F. Cholera caused by Vibrio cholerae O1 induces T-cell responses in the circulation. Infect. Immun. 2009, 77, 1888–1893. [Google Scholar] [CrossRef]
- Weil, A.A.; Arifuzzaman, M.; Bhuiyan, T.R.; LaRocque, R.C.; Harris, A.M.; Kendall, E.A.; Hossain, A.; Tarique, A.A.; Sheikh, A.; Chowdhury, F.; et al. Memory T-cell responses to Vibrio cholerae O1 infection. Infect. Immun. 2009, 77, 5090–5096. [Google Scholar] [CrossRef][Green Version]
- Hornquist, C.E.; Ekman, L.; Grdic, K.D.; Schon, K.; Lycke, N.Y. Paradoxical IgA immunity in CD4-deficient mice. Lack of cholera toxin-specific protective immunity despite normal gut mucosal IgA differentiation. J. Immunol. 1995, 155, 2877–2887. [Google Scholar] [PubMed]
- Kjerrulf, M.; Grdic, D.; Ekman, L.; Schon, K.; Vajdy, M.; Lycke, N.Y. Interferon-gamma receptor-deficient mice exhibit impaired gut mucosal immune responses but intact oral tolerance. Immunology 1997, 92, 60–68. [Google Scholar] [CrossRef]
- Kuchta, A.; Rahman, T.; Sennott, E.L.; Bhuyian, T.R.; Uddin, T.; Rashu, R.; Chowdhury, F.; Kahn, A.I.; Arifuzzaman, M.; Weil, A.A.; et al. Vibrio cholerae O1 infection induces proinflammatory CD4+ T-cell responses in blood and intestinal mucosa of infected humans. Clin. Vaccine Immunol. 2011, 18, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, Y.; Masuda, S.; Tomaru, U.; Ishizu, A. CD1d-Restricted Type II NKT Cells Reactive With Endogenous Hydrophobic Peptides. Front. Immunol. 2018, 9, 548. [Google Scholar] [CrossRef]
- Bai, L.; Picard, D.; Anderson, B.; Chaudhary, V.; Luoma, A.; Jabri, B.; Adams, E.J.; Savage, P.B.; Bendelac, A. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vdelta1 TCR. Eur. J. Immunol. 2012, 42, 2505–2510. [Google Scholar] [CrossRef] [PubMed]
- Luoma, A.M.; Castro, C.D.; Mayassi, T.; Bembinster, L.A.; Bai, L.; Picard, D.; Anderson, B.; Scharf, L.; Kung, J.E.; Sibener, L.V.; et al. Crystal structure of Vdelta1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human gammadelta T cells. Immunity 2013, 39, 1032–1042. [Google Scholar] [CrossRef]
- Dieude, M.; Striegl, H.; Tyznik, A.J.; Wang, J.; Behar, S.M.; Piccirillo, C.A.; Levine, J.S.; Zajonc, D.M.; Rauch, J. Cardiolipin binds to CD1d and stimulates CD1d-restricted gammadelta T cells in the normal murine repertoire. J. Immunol. 2011, 186, 4771–4781. [Google Scholar] [CrossRef]
- Shah, H.B.; Devera, T.S.; Rampuria, P.; Lang, G.A.; Lang, M.L. Type II NKT cells facilitate Alum-sensing and humoral immunity. J. Leukoc. Biol. 2012, 92, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Turner, J.E.; Villa, M.; Duarte, J.H.; Demengeot, J.; Steinmetz, O.M.; Stockinger, B. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 2013, 14, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.T.; Yao, S.; Gong, B.; Elson, C.O.; Cong, Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J. Immunol. 2012, 189, 4666–4673. [Google Scholar] [CrossRef]
- Datta, S.K.; Sabet, M.; Nguyen, K.P.; Valdez, P.A.; Gonzalez-Navajas, J.M.; Islam, S.; Mihajlov, I.; Fierer, J.; Insel, P.A.; Webster, N.J.; et al. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc. Natl. Acad. Sci. USA 2010, 107, 10638–10643. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, E.E.; Matsumoto, T.; Lindenbergh, D.; Willemsen, R.; Kaser, A.; Simons-Oosterhuis, Y.; Brugman, S.; Yamaguchi, K.; Ishikawa, H.; Aiba, Y.; et al. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J. Clin. Investig. 2009, 119, 1241–1250. [Google Scholar] [CrossRef]
- Dowds, C.M.; Blumberg, R.S.; Zeissig, S. Control of intestinal homeostasis through crosstalk between natural killer T cells and the intestinal microbiota. Clin. Immunol. 2015, 159, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; He, W.; Shi, X.; He, X.; Dou, L.; Gao, Y. The Role of CD1d and MR1 Restricted T Cells in the Liver. Front. Immunol. 2018, 9, 2424. [Google Scholar] [CrossRef]
- Iinuma, C.; Waki, M.; Kawakami, A.; Yamaguchi, M.; Tomaru, U.; Sasaki, N.; Masuda, S.; Matsui, Y.; Iwasaki, S.; Baba, T.; et al. Establishment of a vascular endothelial cell-reactive type II NKT cell clone from a rat model of autoimmune vasculitis. Int. Immunol. 2015, 27, 105–114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sagami, S.; Ueno, Y.; Tanaka, S.; Fujita, A.; Niitsu, H.; Hayashi, R.; Hyogo, H.; Hinoi, T.; Kitadai, Y.; Chayama, K. Choline Deficiency Causes Colonic Type II Natural Killer T (NKT) Cell Loss and Alleviates Murine Colitis under Type I NKT Cell Deficiency. PLoS ONE 2017, 12, e0169681. [Google Scholar] [CrossRef] [PubMed]
- Shamshiev, A.; Gober, H.J.; Donda, A.; Mazorra, Z.; Mori, L.; De Libero, G. Presentation of the same glycolipid by different CD1 molecules. J. Exp. Med. 2002, 195, 1013–1021. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albutti, A.; Longet, S.; McEntee, C.P.; Quinn, S.; Liddicoat, A.; Rîmniceanu, C.; Lycke, N.; Lynch, L.; Cardell, S.; Lavelle, E.C. Type II NKT Cell Agonist, Sulfatide, Is an Effective Adjuvant for Oral Heat-Killed Cholera Vaccines. Vaccines 2021, 9, 619. https://doi.org/10.3390/vaccines9060619
Albutti A, Longet S, McEntee CP, Quinn S, Liddicoat A, Rîmniceanu C, Lycke N, Lynch L, Cardell S, Lavelle EC. Type II NKT Cell Agonist, Sulfatide, Is an Effective Adjuvant for Oral Heat-Killed Cholera Vaccines. Vaccines. 2021; 9(6):619. https://doi.org/10.3390/vaccines9060619
Chicago/Turabian StyleAlbutti, Aqel, Stephanie Longet, Craig P. McEntee, Shauna Quinn, Alex Liddicoat, Cristiana Rîmniceanu, Nils Lycke, Lydia Lynch, Susanna Cardell, and Ed C. Lavelle. 2021. "Type II NKT Cell Agonist, Sulfatide, Is an Effective Adjuvant for Oral Heat-Killed Cholera Vaccines" Vaccines 9, no. 6: 619. https://doi.org/10.3390/vaccines9060619
APA StyleAlbutti, A., Longet, S., McEntee, C. P., Quinn, S., Liddicoat, A., Rîmniceanu, C., Lycke, N., Lynch, L., Cardell, S., & Lavelle, E. C. (2021). Type II NKT Cell Agonist, Sulfatide, Is an Effective Adjuvant for Oral Heat-Killed Cholera Vaccines. Vaccines, 9(6), 619. https://doi.org/10.3390/vaccines9060619






