Association between Antiviral Prophylaxis and Cytomegalovirus and Epstein–Barr Virus DNAemia in Pediatric Recipients of Allogeneic Hematopoietic Stem Cell Transplant
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Statistical Analysis
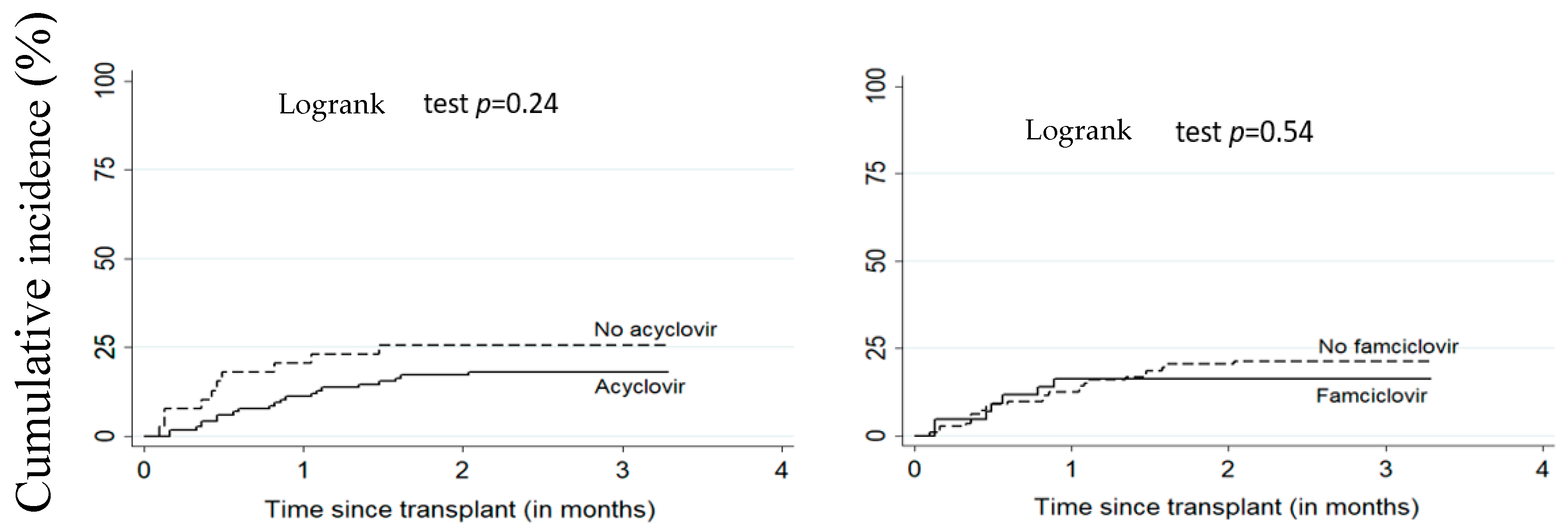
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gratwohl, A.; Brand, R.; Frassoni, F.; Rocha, V.; Niederwieser, D.; Reusser, P.; Einsele, H.; Cordonnier, C. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: An EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant. 2005, 36, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Dominietto, A.; Lamparelli, T.; Raiola, A.M.; Van Lint, M.T.; Gualandi, F.; Berisso, G.; Bregante, S.; Di Grazia, C.; Soracco, M.; Pitto, A.; et al. Transplant-related mortality and long-term graft function are significantly influenced by cell dose in patients undergoing allogeneic marrow transplantation. Blood 2002, 100, 3930–3934. [Google Scholar] [CrossRef] [PubMed]
- Frassoni, F.; Labopin, M.; Gluckman, E.; Prentice, H.G.; Vernant, J.P.; Zwaan, F.; Granena, A.; Gahrton, G.; De Witte, T.; Gratwohl, A.; et al. Results of allogeneic bone marrow transplantation for acute leukemia have improved in Europe with time—A report of the acute leukemia working party of the European group for blood and marrow transplantation (EBMT). Bone Marrow Transplant. 1996, 17, 13–18. [Google Scholar]
- Eapen, M.; Rubinstein, P.; Zhang, M.J.; Camitta, B.M.; Stevens, C.; Cairo, M.S.; Davies, S.M.; Doyle, J.J.; Kurtzberg, J.; Pulsipher, M.A.; et al. Comparable long-term survival after unrelated and HLA-matched sibling donor hematopoietic stem cell transplantations for acute leukemia in children younger than 18 months. J. Clin. Oncol. 2006, 24, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, R.; August, C.; Plotkin, S. Viral-Infections in Pediatric Bone-Marrow Transplant Patients. Pediatr. Infect. Dis. J. 1988, 7, 109–115. [Google Scholar] [CrossRef]
- Olkinuora, H.A.; Taskinen, M.H.; Saarinen-Pihkala, U.M.; Vettenranta, K.K. Multiple viral infections post-hematopoietic stem cell transplantation are linked to the appearance of chronic GVHD among pediatric recipients of allogeneic grafts. Pediatr. Transplant. 2010, 14, 242–248. [Google Scholar] [CrossRef]
- Meyers, J. Infection in Bone-Marrow Transplant Recipients. Am. J. Med. 1986, 81, 27–38. [Google Scholar] [CrossRef]
- Miller, W.; Flynn, P.; Mccullough, J.; Balfour, H.; Goldman, A.; Haake, R.; McGlave, P.; Ramsay, N.; Kersey, J. Cytomegalovirus-Infection After Bone-Marrow Transplantation—An Association with Acute Graft-V-Host Disease. Blood 1986, 67, 1162–1167. [Google Scholar] [CrossRef]
- Ochs, L.; Shu, X.; Miller, J.; Enright, H.; Wagner, J.; Filipovich, A.; Miller, W.; Weisdorf, D. Late Infections After Allogeneic Bone-Marrow Transplantation—Comparison of Incidence in Related and Unrelated Donor Transplant Recipients. Blood 1995, 86, 3979–3986. [Google Scholar] [CrossRef]
- Tutschka, P. Infections and Immunodeficiency in Bone-Marrow Transplantation. Pediatr. Infect. Dis. J. 1988, 7, S22–S29. [Google Scholar] [CrossRef] [PubMed]
- Shiley, K.; Blumberg, E. Herpes viruses in transplant recipients: HSV, VZV, human herpes viruses, and EBV. Infect. Dis Clin. N. Am. 2010, 24, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Rieger, C.T.; Rieger, H.; Kolb, H.J.; Peterson, L.; Huppmann, S.; Fiegl, M.; Ostermann, H. Infectious complications after allogeneic stem cell transplantation: Incidence in matched-related and matched-unrelated transplant settings. Transpl. Infect. Dis. 2009, 11, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Björklund, A.; Aschan, J.; Labopin, M.; Remberger, M.; Ringdén, O.; Winiarski, J.; Ljungman, P. Risk factors for fatal infectious complications developing late after allogeneic stem cell transplantation. Bone Marrow Transplant. 2007, 40, 1055–1062. [Google Scholar] [CrossRef]
- Welniak, L.A.; Blazar, B.R.; Murphy, W.J. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu. Rev. Immunol. 2007, 25, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Rosenzwajg, M.; Fery, N.; Bons, V.; Damaj, G.; Gluckman, E.; Gluckman, J. Human herpes virus 8 (HHV8) serology in allogeneic bone marrow transplant recipients. Bone Marrow Transplant. 1999, 24, 351–354. [Google Scholar] [CrossRef]
- Sala, I.; Faraci, M.; Magnano, G.M.; Sementa, A.; di Marco, E.; Garaventa, A.; Micalizzi, C.; Lanino, E.; Morreale, G.; Moroni, C.; et al. HHV-8-related visceral Kaposi’s sarcoma following allogeneic HSCT: Report of a pediatric case and literature review. Pediatr. Transplant. 2011, 15, E8–E11. [Google Scholar] [CrossRef] [PubMed]
- Verdeguer, A.; de Heredia, C.D.; González, M.; Martínez, A.M.; Fernández-Navarro, J.M.; Pérez-Hurtado, J.M.; Badell, I.; Gómez, P.; González, M.E.; Muñoz, A.; et al. Observational prospective study of viral infections in children undergoing allogeneic hematopoietic cell transplantation: A 3-year GETMON experience. Bone Marrow Transplant. 2011, 46, 119–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Styczynski, J.; Reusser, P.; Einsele, H.; de la Camara, R.; Cordonnier, C.; Ward, K.N.; Ljungman, P.; Engelhard, D. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: Guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant. 2009, 43, 757–770. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; Infectious Disease Society of America; American Society of Blood and Marrow Transplantation. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm. Rep. 2000, 49, 1–125. [Google Scholar]
- Lewalle, P.; Pochon, C.; Michallet, M.; Turlure, P.; Brissot, E.; Paillard, C.; Puyade, M.; Roth-Guepin, G.; Yakoub-Agha, I.; Chantepie, S. Prophylaxis of infections post-allogeneic transplantation: Guidelines from the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC). Bull. Cancer 2019, 106 (Suppl. 1), S23–S34. [Google Scholar] [CrossRef]
- Kawamura, K.; Wada, H.; Yamasaki, R.; Ishihara, Y.; Sakamoto, K.; Ashizawa, M.; Sato, M.; Machishima, T.; Terasako, K.; Kimura, S.I.; et al. Low-dose acyclovir prophylaxis for the prevention of herpes simplex virus disease after allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2013, 15, 457–465. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Dykewicz, C.A.; Longworth, D.; Boeckh, M.; Baden, L.R.; Rubin, R.H.; Sepkowitz, K.A. Preventing Opportunistic Infections After Hematopoietic Stem Cell Transplantation: The Centers for Disease Control and Prevention, Infectious Diseases Society of America, and American Society for Blood and Marrow Transplantation Practice Guidelines and Beyond. Hematol. Am. Soc. Hematol. Educ. Program. 2001, 2001, 392–421. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Schmidt-Hieber, M.; Bertz, H.; Heinz, W.J.; Kiehl, M.; Krüger, W.; Mousset, S.; Neuburger, S.; Neumann, S.; Penack, O.; et al. Infectious diseases in allogeneic haematopoietic stem cell transplantation: Prevention and prophylaxis strategy guidelines 2016. Ann. Hematol. 2016, 95, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Enok Bonong, P.R.; Buteau, C.; Delage, G.; Tanner, J.E.; Lacroix, J.; Duval, M.; Laporte, L.; Tucci, M.; Robitaille, N.; Spinella, P.C.; et al. Transfusion-related Epstein-Barr virus (EBV) infection: A multicenter prospective cohort study among pediatric recipients of hematopoietic stem cell transplants (TREASuRE study). Transfusion 2021, 61, 144–158. [Google Scholar] [CrossRef]
- Kanda, Y.; Mineishi, S.; Saito, T.; Saito, A.; Yamada, S.; Ohnishi, M.; Chizuka, A.; Niiya, H.; Suenaga, K.; Nakai, K.; et al. Long-term low-dose acyclovir against varicella-zoster virus reactivation after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001, 28, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Boeckh, M.; Kim, H.W.; Flowers, M.E.D.; Meyers, J.D.; Bowden, R.A. Long-term acyclovir for prevention of varicella zoster virus disease after allogeneic hematopoietic cell transplantation—a randomized double-blind placebo-controlled study. Blood 2006, 107, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Steer, C.B.; Szer, J.; Sasadeusz, J.; Matthews, J.P.; Beresford, J.A.; Grigg, A. Varicella-zoster infection after allogeneic bone marrow transplantation: Incidence, risk factors and prevention with low-dose aciclovir and ganciclovir. Bone Marrow Transplant. 2000, 25, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Erard, V.; Wald, A.; Corey, L.; Leisenring, W.M.; Boeckh, M. Use of long-term suppressive acyclovir after hematopoietic stem-cell transplantation: Impact on herpes simplex virus (HSV) disease and drug-resistant HSV disease. J. Infect. Dis. 2007, 196, 266–270. [Google Scholar] [CrossRef]
- Asano-Mori, Y.; Kanda, Y.; Oshima, K.; Kako, S.; Shinohara, A.; Nakasone, H.; Sato, H.; Watanabe, T.; Hosoya, N.; Izutsu, K.; et al. Long-term ultra-low-dose acyclovir against varicella-zoster virus reactivation after allogeneic hematopoietic stem cell transplantation. Am. J. Hematol. 2008, 83, 472–476. [Google Scholar] [CrossRef]
- Klein, A.; Miller, K.B.; Sprague, K.; DesJardin, J.A.; Snydman, D.R. A randomized, double-blind, placebo-controlled trial of valacyclovir prophylaxis to prevent zoster recurrence from months 4 to 24 after BMT. Bone Marrow Transplant. 2011, 46, 294–299. [Google Scholar] [CrossRef][Green Version]
- Pai, V.B.; Davis, D.I.; Clayton, J.; Pietryga, D.W. Efficacy of Low-Dose Acyclovir Prophylaxis Against Varicella Zoster Virus in Pediatric Hematopoietic Stem Cell Transplantation Patients. Biol. Blood Marrow Transplant. 2013, 19 (Suppl. 2), S378–S380. [Google Scholar] [CrossRef][Green Version]
- Jamani, K.; MacDonald, J.; Lavoie, M.; Williamson, T.S.; Brown, C.B.; Chaudhry, A.; Jimenez-Zepeda, V.H.; Duggan, P.; Tay, J.; Stewart, D.; et al. Zoster prophylaxis after allogeneic hematopoietic cell transplantation using acyclovir/valacyclovir followed by vaccination. Blood Adv. 2016, 1, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kumar, D.; Messner, H.A.; Minden, M.; Gupta, V.; Kuruvilla, J.; Chae, Y.S.; Sohn, S.K.; Lipton, J.H. Clinical efficacy of prophylactic strategy of long-term low-dose acyclovir for Varicella-Zoster virus infection after allogeneic peripheral blood stem cell transplantation. Clin. Transplant. 2008, 22, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Bettinger, J.; McConnell, A.; Scheifele, D.; Halperin, S.; Vaudry, W.; Law, B. The Effect of Funded Varicella Immunization Programs on Varicella-related Hospitalizations in IMPACT Centers, Canada, 2000–2008. Pediatr. Infect. Dis. J. 2012, 31, 956–963. [Google Scholar] [CrossRef]
- Haastrup, E.; Müller, K.; Baekgaard, H.; Heilmann, C. Cytomegalovirus infection after allogeneic stem cell transplant in children. Pediatr. Transplant. 2005, 9, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Lee, J.H.; Choi, E.S.; Seo, J.J.; Moon, H.N.; Kim, M.-N.; Im, H.J. Cytomegalovirus infection in children who underwent hematopoietic stem cell transplantation at a single center: A retrospective study of the risk factors. Pediatr. Transplant. 2009, 13, 898–905. [Google Scholar] [CrossRef]
- Selby, P.J.; Powles, R.L.; Easton, D.; Perren, T.J.; Stolle, K.; Jameson, B.; Fiddian, A.P.; Tryhorn, Y.; Stern, H. The prophylactic role of intravenous and long-term oral acyclovir after allogeneic bone marrow transplantation. Br. J. Cancer 1989, 59, 434–438. [Google Scholar] [CrossRef]
- Lundgren, G.; Wilczek, H.; Lönnqvist, B.; Lindholm, A.; Wahren, B.; Ringdén, O. Acyclovir prophylaxis in bone marrow transplant recipients. Scand. J. Infect. Dis. Suppl. 1985, 47, 137–144. [Google Scholar]
- Ljungman, P.; Wilczek, H.; Gahrton, G.; Gustavsson, A.; Lundgren, G.; Lonnqvist, B.; Ringdén, O.; Wahren, B. Long-term acyclovir prophylaxis in bone marrow transplant recipients and lymphocyte proliferation responses to herpes virus antigens in vitro. Bone Marrow Transplant. 1986, 1, 185–192. [Google Scholar]
- Prentice, H.G. Use of acyclovir for prophylaxis of herpes infections in severely immunocompromised patients. J. Antimicrob. Chemother. 1983, 12 (Suppl. B), 153–159. [Google Scholar] [CrossRef]
- Prentice, H.G.; Gluckman, E.; Powles, R.; Ljungman, P.; Milpied, N.J.; Fernandez-Rañada, J.M.; Mandelli, F.; Kho, P. Impact of long-term acyclovir on cytomegalovirus infection and survival after allogeneic bone marrow transplantation. Lancet 1994, 343, 749–753. [Google Scholar] [CrossRef]
- Meyers, J.D.; Reed, E.C.; Shepp, D.H.; Thornquist, M.; Dandliker, P.S.; Vicary, C.A.; Flournoy, N.; Kirk, L.E.; Kersey, J.H.; Thomas, E.D.; et al. Acyclovir for Prevention of Cytomegalovirus Infection and Disease after Allogeneic Marrow Transplantation. N. Engl. J. Med. 1988, 318, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, E.; Lotsberg, J.; Devergie, A.; Zhao, X.M.; Melo, R.; Gomez-Morales, M.; Nebout, T.; Mazeron, M.C.; Perol, Y. Prophylaxis of herpes infections after bone-marrow transplantation by oral acyclovir. Lancet 1983, 2, 706–708. [Google Scholar] [CrossRef]
- Graef, T.; Bobrikova, T.; Adams, O.; Fenk, R.; Ruf, L.; Zohren, F.; Gattermann, N.; Haas, R.; Kobbe, G. Prognostic Factors for CMV Reactivation/Infection after Stem Cell Transplantation and Value of Oral Valganciclovir for Preemptive Therapy. Blood 2005, 106, 5369. [Google Scholar] [CrossRef]
- Bordon, V.; Padalko, E.; Benoit, Y.; Dhooge, C.; Laureys, G. Incidence, kinetics, and risk factors of Epstein–Barr virus viremia in pediatric patients after allogeneic stem cell transplantation. Pediatr. Transplant. 2012, 16, 144–150. [Google Scholar] [CrossRef]
- Düver, F.; Weißbrich, B.; Eyrich, M.; Wölfl, M.; Schlegel, P.G.; Wiegering, V. Viral reactivations following hematopoietic stem cell transplantation in pediatric patients—A single center 11-year analysis. PLoS ONE 2020, 15, e0228451. [Google Scholar] [CrossRef]
- Tsoumakas, K.; Giamaiou, K.; Goussetis, E.; Graphakos, S.; Kossyvakis, A.; Horefti, E.; Mentis, A.; Elefsiniotis, I.; Pavlopoulou, I.D. Epidemiology of viral infections among children undergoing hematopoietic stem cell transplant: A prospective single-center study. Transpl. Infect. Dis. 2019, 21, e13095. [Google Scholar] [CrossRef] [PubMed]
- Hann, I.M.; Prentice, H.G.; Blacklock, H.A.; Ross, M.G.; Brigden, D.; Rosling, A.E.; Burke, C.; Crawford, D.H.; Brumfitt, W.; Hoffbrand, A.V. Acyclovir prophylaxis against herpes virus infections in severely immunocompromised patients: Randomised double blind trial. Br. Med. J. Clin. Res. Ed. 1983, 287, 384–388. [Google Scholar] [CrossRef]
- Catalán, P.; Alba, A. Prophylaxis against Epstein Barr disease in pediatric and adult patients undergoing solid organ and hematopoietic stem cells transplantation. Rev. Chil. Infectol. 2012, 29 (Suppl. 1), S29–S31. [Google Scholar] [CrossRef]
- Czyzewski, K.; Dziedzic, M.; Salamonowicz, M.; Fraczkiewicz, J.; Zajac-Spychala, O.; Zaucha-Prazmo, A.; Gozdzik, J.; Galazka, P.; Bartoszewicz, N.; Demidowicz, E.; et al. Epidemiology, Outcome and Risk Factors Analysis of Viral Infections in Children and Adolescents Undergoing Hematopoietic Cell Transplantation: Antiviral Drugs Do Not Prevent Epstein–Barr Virus Reactivation. Infect. Drug Resist. 2019, 12, 3893–3902. [Google Scholar] [CrossRef]
- Zutter, M.M.; Martin, P.J.; Sale, G.E.; Shulman, H.M.; Fisher, L.; Thomas, E.D.; Durnam, D.M. Epstein-Barr virus lymphoproliferation after bone marrow transplantation. Blood 1988, 72, 520–529. [Google Scholar] [CrossRef] [PubMed]
- AlDabbagh, M.A.; Gitman, M.R.; Kumar, D.; Humar, A.; Rotstein, C.; Husain, S. The Role of Antiviral Prophylaxis for the Prevention of Epstein–Barr Virus–Associated Posttransplant Lymphoproliferative Disease in Solid Organ Transplant Recipients: A Systematic Review. Am. J. Transplant. 2017, 17, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Paintsil, E.; Cheng, Y.C. Antiviral Agents, Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 176–225. [Google Scholar]
- Pagano, J.S.; Whitehurst, C.B.; Andrei, G. Antiviral Drugs for EBV. Cancers 2018, 10, 197. [Google Scholar] [CrossRef]
- Marty, F.M.; Ljungman, P.; Chemaly, R.F.; Maertens, J.; Dadwal, S.S.; Duarte, R.F.; Haider, S.; Ullmann, A.J.; Katayama, Y.; Brown, J.; et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2017, 377, 2433–2444. [Google Scholar] [CrossRef] [PubMed]

| Variables | Overall | HSV-1+ | VZV+ | EBV+ | CMV+ | HHV-6+ | Total Positive HHV | Total Negative HHV | |
|---|---|---|---|---|---|---|---|---|---|
| Number of Patients (n) | n = 156 | n = 3 | n = 1 | n = 53 | n = 31 | n = 4 | n = 77 | n = 79 | |
| Sex, n (%) | Male | 83 (53.2) | 2(66.7) | 0 | 26 (49.1) | 14 (45.2) | 3 (75.0) | 44 (57.1) | 39 (49.4) |
| Female | 73(46.8) | 1(33.3) | 1 (100) | 27 (50.9) | 17 (54.8) | 1 (25.0) | 33 (42.9) | 40 (50.6) | |
| Recipient age at transplant (years) | Mean (SD) | 7.3 (5.3) | 4.0 (2.5) | 6.8 | 8.0 (5.6) | 6.8 (4.9) | 4.9 (2.8) | 7.3 (5.4) | 7.3 (5.3) |
| Median (IQR) | 6.3 (2.5,10.4) | 5.2 (1.2,5.6) | 6.8 | 7.3 (3.8, 10.6) | 5.8 (2.3, 9.6) | 4.9 (2.7, 7.1) | 6.1 (2.4, 11.2) | 6.5 (2.9, 10.1) | |
| Primary diagnosis, n (%) | Malignant | 69 (44.2) | 1(33.3) | 1 (100) | 21 (39.6) | 12 (38.7) | 2 (50.0) | 35 (45.5) | 34 (43) |
| Non-malignant | 87 (55.8) | 2(66.7) | 0 | 32 (60.4) | 19 (61.3) | 2 (50.0) | 42 (54.5) | 45 (57) | |
| Recipient pre-transplant EBV serology, n (%) | Negative | 42 (26.9) | 0 | 0 | 8 (15.1) | 8 (25.8) | 1 (25.0) | 26 (33.8) | 16 (20.3) |
| Positive | 101(64.8) | 3(100) | 1 (100) | 40 (75.5) | 21 (67.7) | 3 (75.0) | 43 (55.8) | 58 (73.4) | |
| Unknown | 13 (8.3) | 0 | 0 | 5 (9.4) | 2 (6.5) | 0 | 8 (10.4) | 5 (6.3) | |
| Graft EBV serostatus, n (%) | Negative | 62 (39.7) | 2 (66.7) | 1 (100) | 8 (15.1) | 12 (38.7) | 3 (75.0) | 39 (50.6) | 23 (29.1) |
| Positive | 63 (40.4) | 1 (33.3) | 0 | 32 (60.4) | 15 (48.4) | 1 (25.0) | 20 (26) | 43 (54.4) | |
| Unknown | 31 (19.9) | 0 | 0 | 13 (24.5) | 4 (12.9) | 0 | 18 (23.4) | 13 (16.5) | |
| Donor match, n (%) | Donor matched | 53 (34.0) | 0 | 1 (100) | 18 (34.0) | 14 (45.2) | 0 | 28 (36.4) | 25 (31.6) |
| Alternative donor | 103 (66.0) | 3 (100) | 0 | 35 (66.0) | 17 (54.8) | 4 (100) | 49 (63.6) | 54 (68.4) | |
| Graft source, n (%) | CB | 39 (25.0) | 2 (66.7) | 0 | 5 (9.4) | 9 (29.0) | 3 (75.0) | 23 (29.9) | 16 (20.3) |
| BM/PBSC | 117 (75.0) | 1 (33.3) | 1 (100) | 48 (90.6) | 22 (71.0) | 1 (25.0) | 54 (70.1) | 63 (79.7) | |
| GvHD, n (%) | No | 97 (62.18) | 1 (33.33) | 1 (100) | 34 (64.15) | 23 (74.19) | 0 | 51 (66.23) | 46 (58.23) |
| Yes | 59 (37.82) | 2 (66.67) | 0 | 19 (35.85) | 8 (25.81) | 4 (100) | 26 (33.77) | 33 (41.77) | |
| Conditioning regimen, n (%) | Other | 98 (62.8) | 2 (66.7) | 0 | 35 (66.0) | 18 (58.1) | 1 (25.0) | 50 (64.9) | 48 (60.8) |
| MAC | 58 (37.2) | 1 (33.3) | 1 (100) | 18 (34.0) | 13 (41.9) | 3 (75.0) | 27 (35.1) | 31 (39.2) | |
| Antithymocyte globulin, n (%) | No | 92 (59.0) | 3 (100) | 1 (100) | 19 (35.8) | 19 (61.3) | 3 (75.0) | 52 (67.5) | 40 (50.6) |
| Yes | 64 (41.0) | 0 | 0 | 34 (64.2) | 12 (38.7) | 1 (25.0) | 25 (32.5) | 39 (49.4) | |
| Alemtuzumab, n (%) | No | 118(75.6) | 1 (33.3) | 1 (100) | 44 (83.0) | 24 (77.4) | 3 (75.0) | 55 (71.4) | 63 (79.7) |
| Yes | 38 (24.4) | 2 (66.7) | 0 | 9 (17.0) | 7 (22.6) | 1 (25.0) | 22 (28.6) | 16 (20.3) | |
| Tacrolimus or CsA, n (%) | No | 15 (9.6) | 0 | 0 | 3 (5.7) | 3 (9.7) | 2 (50.0) | 8 (10.4) | 7 (8.9) |
| Yes | 141(90.4) | 3 (100) | 1 (100) | 50 (94.3) | 28 (90.3) | 2 (50.0) | 69 (89.6) | 72 (91.1) | |
| MTX, n (%) | No | 92 (59.0) | 1 (33.3) | 0 | 23 (43.4) | 18 (58.1) | 3 (75.0) | 52 (67.5) | 40 (50.6) |
| Yes | 64 (41.0) | 2 (66.7) | 1 (100) | 30 (56.6) | 13 (41.9) | 1 (25.0) | 25 (32.5) | 39 (49.4) | |
| MMF, n (%) | No | 100(64.1) | 2 (66.7) | 1 (100) | 46 (86.8) | 21 (67.7) | 2 (50.0) | 39 (50.6) | 61 (77.2) |
| Yes | 56 (35.9) | 1 (33.3) | 0 | 7 (13.2) | 10 (32.3) | 2 (50.0) | 38 (49.4) | 18 (22.7) | |
| Acyclovir, n (%) | No | 39 (25.0) | 0 | 1 (100) | 9 (17.0) | 10 (32.3) | 0 | 21 (27.3) | 18 (22.8) |
| Yes | 117(75.0) | 3 (100) | 0 | 44 (83.0) | 21 (67.7) | 4 (100) | 56 (72.7) | 61 (77.2) | |
| Famciclovir, n (%) | No | 113(72.4) | 2 (66.7) | 1 (100) | 39 (73.6) | 24 (77.4) | 4 (100) | 54 (70.1) | 59 (74.7) |
| Yes | 43 (27.6) | 1 (33.3) | 0 | 14 (26.4) | 7 (22.6) | 0 | 23 (29.9) | 20 (25.3) | |
| Other antivirals (Ganciclovir) n (%) | No | 152 (97.44) | 2 (66.67) | 1 (100) | 52 (98.11) | 28 (90.32) | 4 (100) | 75 (94.94) | 77 (100) |
| Yes | 4 (2.56) | 1 (33.33) | 0 | 1 (1.89) | 3 (9.68) | 0 | 4 (5.06) | 0 | |
| Variable | Number of Cases | Person-Time (Months) | Incidence Rate (95% CI) | HR Crude (95% CI) | HR Adjusted (95% CI) | |
|---|---|---|---|---|---|---|
| EBV DNAemia | 53 | 406.87 | 0.13 (0.1–0.17) | |||
| Acyclovir (a) | No | 9 | 106.28 | 0.08 (0.04–0.16) | 1 | 1 |
| Yes | 44 | 300.58 | 0.15 (0.11–0.20) | 1.68 (0.82–3.44) | 1.41 (0.63–3.14) | |
| Famciclovir (b) | No | 39 | 287.61 | 0.14 (0.10–0.19) | 1 | 1 |
| Yes | 14 | 119.26 | 0.12 (0.07–0.20) | 0.85 (0.46–1.58) | 0.79 (0.36–1.72) | |
| CMV DNAemia | 31 | 425.95 | 0.07 (0.05–0.10) | |||
| Acyclovir (c) | No | 10 | 100.47 | 0.10 (0.05–0.18) | 1 | 1 |
| Yes | 21 | 325.49 | 0.06 (0.04–0.10) | 0.64 (0.30–1.36) | 0.55 (0.24–1.26) | |
| Famciclovir (d) | No | 24 | 304.23 | 0.08 (0.05–0.12) | 1 | 1 |
| Yes | 7 | 121.72 | 0.06 (0.03–0.12) | 0.77 (0.33–1.78) | 0.82 (0.30–2.29) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diop, N.S.; Enok Bonong, P.R.; Buteau, C.; Duval, M.; Lacroix, J.; Laporte, L.; Tucci, M.; Robitaille, N.; Spinella, P.C.; Cuvelier, G.; et al. Association between Antiviral Prophylaxis and Cytomegalovirus and Epstein–Barr Virus DNAemia in Pediatric Recipients of Allogeneic Hematopoietic Stem Cell Transplant. Vaccines 2021, 9, 610. https://doi.org/10.3390/vaccines9060610
Diop NS, Enok Bonong PR, Buteau C, Duval M, Lacroix J, Laporte L, Tucci M, Robitaille N, Spinella PC, Cuvelier G, et al. Association between Antiviral Prophylaxis and Cytomegalovirus and Epstein–Barr Virus DNAemia in Pediatric Recipients of Allogeneic Hematopoietic Stem Cell Transplant. Vaccines. 2021; 9(6):610. https://doi.org/10.3390/vaccines9060610
Chicago/Turabian StyleDiop, Ndeye Soukeyna, Pascal Roland Enok Bonong, Chantal Buteau, Michel Duval, Jacques Lacroix, Louise Laporte, Marisa Tucci, Nancy Robitaille, Philip C. Spinella, Geoffrey Cuvelier, and et al. 2021. "Association between Antiviral Prophylaxis and Cytomegalovirus and Epstein–Barr Virus DNAemia in Pediatric Recipients of Allogeneic Hematopoietic Stem Cell Transplant" Vaccines 9, no. 6: 610. https://doi.org/10.3390/vaccines9060610
APA StyleDiop, N. S., Enok Bonong, P. R., Buteau, C., Duval, M., Lacroix, J., Laporte, L., Tucci, M., Robitaille, N., Spinella, P. C., Cuvelier, G., Vercauteren, S. M., Lewis, V., Alfieri, C., & Trottier, H. (2021). Association between Antiviral Prophylaxis and Cytomegalovirus and Epstein–Barr Virus DNAemia in Pediatric Recipients of Allogeneic Hematopoietic Stem Cell Transplant. Vaccines, 9(6), 610. https://doi.org/10.3390/vaccines9060610






