Long-Term Follow-Up of Mesothelioma Patients Treated with Dendritic Cell Therapy in Three Phase I/II Trials
Abstract
1. Introduction
2. Material and Methods
Statistical Analysis
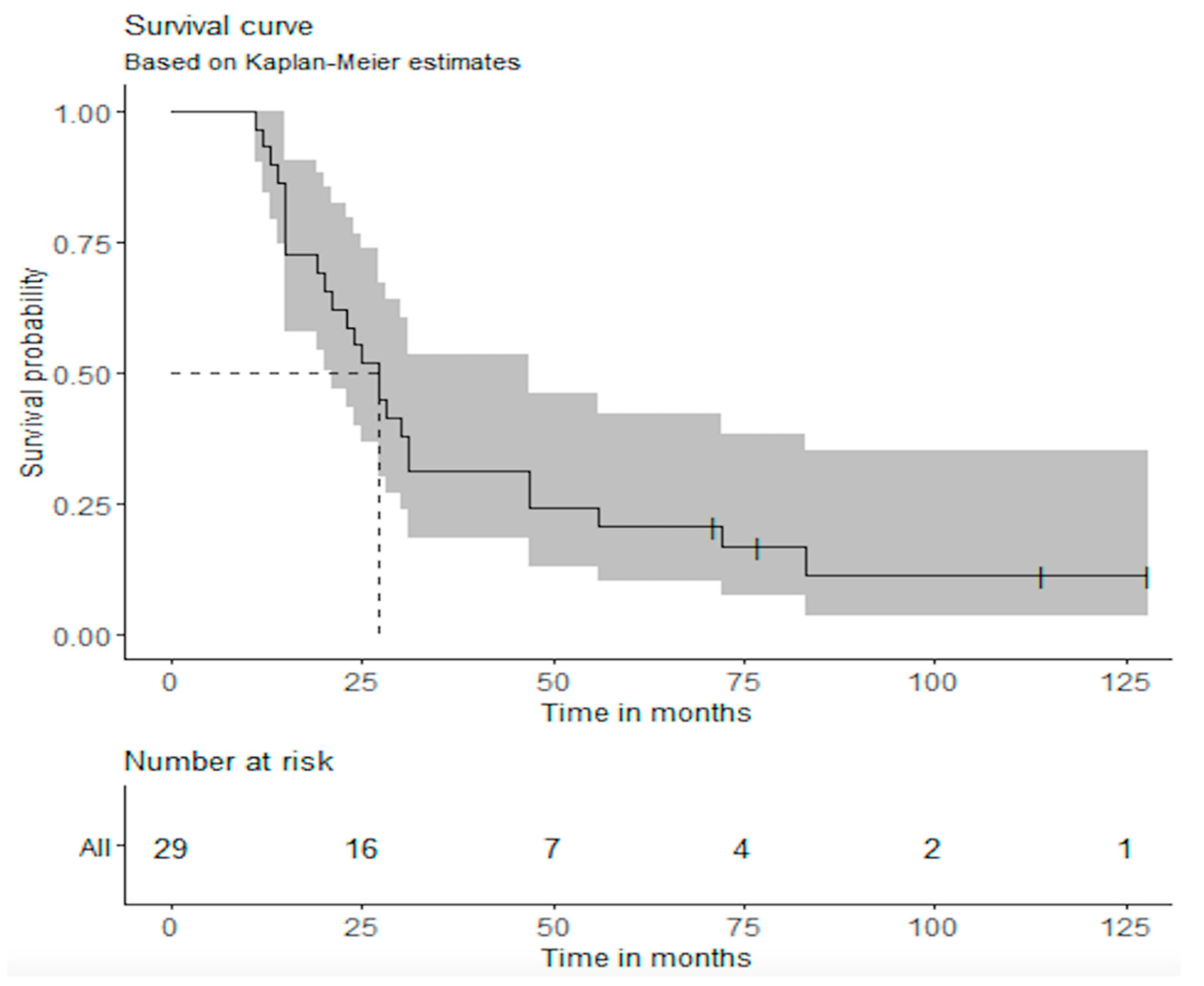
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III Study of Pemetrexed in Combination with Cisplatin versus Cisplatin Alone in Patients with Malignant Pleural Mesothelioma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef]
- van Meerbeeck, J.P.; Gaafar, R.; Manegold, C.; Van Klaveren, R.J.; Van Marck, E.A.; Vincent, M.; Legrand, C.; Bottomley, A.; Debruyne, C.; Giaccone, G.; et al. Randomized Phase III Study of Cisplatin with or without Raltitrexed in Patients with Malignant Pleural Mesothelioma: An Intergroup Study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 6881–6889. [Google Scholar] [CrossRef]
- Popat, S.; Curioni-Fontecedro, A.; Dafni, U.; Shah, R.; O’Brien, M.; Pope, A.; Fisher, P.; Spicer, J.; Roy, A.; Gilligan, D.; et al. A Multicentre Randomised Phase III Trial Comparing Pembrolizumab versus Single-Agent Chemotherapy for Advanced Pre-Treated Malignant Pleural Mesothelioma: The European Thoracic Oncology Platform (ETOP 9-15) PROMISE-Meso Trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1734–1745. [Google Scholar] [CrossRef] [PubMed]
- Approves Nivolumab and Ipilimumab for Unresectable Malignant Pleural Mesothelioma. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-nivolumab-and-ipilimumab-unresectable-malignant-pleural-mesothelioma (accessed on 25 February 2021).
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-Line Nivolumab plus Ipilimumab in Unresectable Malignant Pleural Mesothelioma (CheckMate 743): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef]
- Gettinger, S.; Borghaei, H.; Brahmer, J.; Chow, L.; Burgio, M.; Carpeno, J.D.C.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. OA14.04 Five-Year Outcomes From the Randomized, Phase 3 Trials CheckMate 017/057: Nivolumab vs Docetaxel in Previously Treated NSCLC. J. Thorac. Oncol. 2019, 14, S244–S245. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Galluzzi, L.; Chan, T.A.; Kroemer, G.; Wolchok, J.D.; López-Soto, A. The Hallmarks of Successful Anticancer Immunotherapy. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Dammeijer, F.; van Gulijk, M.; Mulder, E.E.; Lukkes, M.; Klaase, L.; van den Bosch, T.; van Nimwegen, M.; Lau, S.P.; Latupeirissa, K.; Schetters, S.; et al. The PD-1/PD-L1-Checkpoint Restrains T Cell Immunity in Tumor-Draining Lymph Nodes. Cancer Cell 2020, 38, 685–700.e8. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, D.W.; Aerts, J.G.; Cornelissen, R. Is Immunotherapy a Viable Option in Treating Mesothelioma? Future Oncol. 2017, 13, 1747–1750. [Google Scholar] [CrossRef]
- Belderbos, R.A.; Cornelissen, R.; Aerts, J.G.J.V. Immunotherapy of Mesothelioma: Vaccines and Cell Therapy. In Mesothelioma: From Research to Clinical Practice; Ceresoli, G.L., Bombardieri, E., D’Incalci, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 271–280. ISBN 978-3-030-16884-1. [Google Scholar]
- Aerts, J.G.J.V.; de Goeje, P.L.; Cornelissen, R.; Kaijen-Lambers, M.E.H.; Bezemer, K.; van der Leest, C.H.; Mahaweni, N.M.; Kunert, A.; Eskens, F.A.L.M.; Waasdorp, C.; et al. Autologous Dendritic Cells Pulsed with Allogeneic Tumor Cell Lysate in Mesothelioma: From Mouse to Human. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, R.; Hegmans, J.P.J.J.; Maat, A.P.W.M.; Kaijen-Lambers, M.E.H.; Bezemer, K.; Hendriks, R.W.; Hoogsteden, H.C.; Aerts, J.G.J.V. Extended Tumor Control after Dendritic Cell Vaccination with Low-Dose Cyclophosphamide as Adjuvant Treatment in Patients with Malignant Pleural Mesothelioma. Am. J. Respir. Crit. Care Med. 2016, 193, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Hegmans, J.P.; Veltman, J.D.; Lambers, M.E.; de Vries, I.J.M.; Figdor, C.G.; Hendriks, R.W.; Hoogsteden, H.C.; Lambrecht, B.N.; Aerts, J.G. Consolidative Dendritic Cell-Based Immunotherapy Elicits Cytotoxicity against Malignant Mesothelioma. Am. J. Respir. Crit. Care Med. 2010, 181, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Aerts, J.; Cornelissen, R.; Leest, C.V.D.; Hegmans, J.; Bezemer, K.; Kaijen-Lambers, M.; Eskens, F.; Braakman, E.; Holt, B.V.D.; Hendriks, R.; et al. OA13.06 Autologous Dendritic Cells Loaded with Allogeneic Tumor Cell Lysate (Pheralys®) in Patients with Mesothelioma: Final Results of a Phase I Study. J. Thorac. Oncol. 2017, 12, S295. [Google Scholar] [CrossRef][Green Version]
- Aerts, J.G.; Hegmans, J.P. Tumor-Specific Cytotoxic T Cells Are Crucial for Efficacy of Immunomodulatory Antibodies in Patients with Lung Cancer. Cancer Res. 2013. [Google Scholar] [CrossRef]
- Vroman, H.; Balzaretti, G.; Belderbos, R.A.; Klarenbeek, P.L.; van Nimwegen, M.; Bezemer, K.; Cornelissen, R.; Niewold, I.T.G.; van Schaik, B.D.; van Kampen, A.H.; et al. T Cell Receptor Repertoire Characteristics Both before and Following Immunotherapy Correlate with Clinical Response in Mesothelioma. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Katsurada, M.; Nagano, T.; Tachihara, M.; Kiriu, T.; Furukawa, K.; Koyama, K.; Otoshi, T.; Sekiya, R.; Hazama, D.; Tamura, D.; et al. Baseline Tumor Size as a Predictive and Prognostic Factor of Immune Checkpoint Inhibitor Therapy for Non-Small Cell Lung Cancer. Anticancer Res. 2019, 39, 815–825. [Google Scholar] [CrossRef]
- Chardin, D.; Paquet, M.; Schiappa, R.; Darcourt, J.; Bailleux, C.; Poudenx, M.; Sciazza, A.; Ilie, M.; Benzaquen, J.; Martin, N.; et al. Baseline Metabolic Tumor Volume as a Strong Predictive and Prognostic Biomarker in Patients with Non-Small Cell Lung Cancer Treated with PD1 Inhibitors: A Prospective Study. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.W.; Elassaiss-Schaap, J.; Kefford, R.; Hwu, W.-J.; Wolchok, J.D.; Joshua, A.M.; Ribas, A.; Hodi, F.S.; Hamid, O.; Robert, C.; et al. Baseline Tumor Size Is an Independent Prognostic Factor for Overall Survival in Patients with Melanoma Treated with Pembrolizumab. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 4960–4967. [Google Scholar] [CrossRef]
- Veltman, J.D.; Lambers, M.E.H.; van Nimwegen, M.; de Jong, S.; Hendriks, R.W.; Hoogsteden, H.C.; Aerts, J.G.J.V.; Hegmans, J.P.J.J. Low-Dose Cyclophosphamide Synergizes with Dendritic Cell-Based Immunotherapy in Antitumor Activity. J. Biomed. Biotechnol. 2010, 2010, 798467. [Google Scholar] [CrossRef] [PubMed]
- Noordam, L.; Kaijen, M.E.H.; Bezemer, K.; Cornelissen, R.; Maat, L.A.P.W.M.; Hoogsteden, H.C.; Aerts, J.G.J.V.; Hendriks, R.W.; Hegmans, J.P.J.J.; Vroman, H. Low-Dose Cyclophosphamide Depletes Circulating Naïve and Activated Regulatory T Cells in Malignant Pleural Mesothelioma Patients Synergistically Treated with Dendritic Cell-Based Immunotherapy. Oncoimmunology 2018, 7, e1474318. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of Tumour Mutational Burden with Outcomes in Patients with Advanced Solid Tumours Treated with Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- de Gooijer, C.J.; van der Noort, V.; Stigt, J.A.; Baas, P.; Biesma, B.; Cornelissen, R.; van Walree, N.; van Heemst, R.C.; Soud, M.Y.-E.; Groen, H.J.M.; et al. Switch-Maintenance Gemcitabine after First-Line Chemotherapy in Patients with Malignant Mesothelioma (NVALT19): An Investigator-Initiated, Randomised, Open-Label, Phase 2 Trial. Lancet Respir. Med. 2021. [Google Scholar] [CrossRef]
- Belderbos, R.A.; Baas, P.; Berardi, R.; Cornelissen, R.; Fennell, D.A.; van Meerbeeck, J.P.; Scherpereel, A.; Vroman, H.; Aerts, J.G.J.V. A Multicenter, Randomized, Phase II/III Study of Dendritic Cells Loaded with Allogeneic Tumor Cell Lysate (MesoPher) in Subjects with Mesothelioma as Maintenance Therapy after Chemotherapy: DENdritic Cell Immunotherapy for Mesothelioma (DENIM) Trial. Transl. Lung Cancer Res. 2019, 8, 280–285. [Google Scholar] [CrossRef] [PubMed]
| Study | Gender | Age | Histology | Extended P/D | Chemotherapy | Response on DC | Survival (Months) | Alive |
|---|---|---|---|---|---|---|---|---|
| MM01 (N= 10) | ||||||||
| male | 68 | epithelial | no | yes | PR | 23 | no | |
| male | 63 | epithelial | no | yes | PD | 72 | no | |
| male | 55 | epithelial | no | yes | PR | 19 | no | |
| male | 66 | epithelial | no | yes | PR | 30 | no | |
| male | 71 | epithelial | no | yes | SD | 15 | no | |
| male | 64 | epithelial | no | yes | PD | 13 | no | |
| male | 75 | epithelial | no | yes | PD | 11 | no | |
| male | 77 | epithelial | no | yes | PD | 15 | no | |
| male | 70 | epithelial | no | yes | PD | 15 | no | |
| male | 58 | epithelial | no | yes | PD | 15 | no | |
| MM02 (N = 10) | ||||||||
| male | 62 | epithelial | no | yes | SD | 24 | no | |
| male | 71 | epithelial | no | yes | SD | 25 | no | |
| male | 78 | epithelial | no | yes | SD | 14 | no | |
| male | 55 | epithelial | no | yes | CR | 114 | yes | |
| male | 75 | epithelial | no | yes | SD | 27 | no | |
| male | 63 | epithelial | yes | yes | SD | 20 | no | |
| male | 58 | biphasic | yes | yes | PD | 12 | no | |
| female | 35 | epithelial | yes | yes | PR | 128 | yes | |
| female | 55 | biphasic | yes | yes | SD | 56 | no | |
| male | 48 | epithelial | yes | yes | SD | 83 | no | |
| MM03 (N = 9) | ||||||||
| male | 79 | epithelial | no | no | SD | 47 | no | |
| male | 69 | epithelial | no | no | SD | 31 | no | |
| male | 44 | epithelial | no | yes | SD | 77 | yes | |
| female | 59 | epithelial | no | yes | PR | 47 | no | |
| male | 73 | epithelial | no | no | PR | 71 | yes | |
| male | 67 | epithelial | no | yes | SD | 28 | no | |
| male | 68 | epithelial | no | no | SD | 21 | no | |
| male | 71 | epithelial | no | yes | SD | 31 | no | |
| male | 60 | epithelial | no | yes | SD | 27 | no |
| Study | Median OS (95% CI) | OS—2 Years (95% CI) | OS—5 Years (95% CI) |
|---|---|---|---|
| Overall | 27 months (21–47) | 55.2% (39.7%–76.6%) | 20.7% (10.1%–42.2%) |
| MM01 | 15 months (15–Inf) | 20.0% (5.8%–69.1%) | 10.0% (1.6%–64.2%) |
| MM02 | 26 months (20–Inf) | 60.0% (36.2–99.5%) | 30.0% (11.6%–77.3%) |
| MM03 | 31 months (28–Inf) | 88.9% (70.6%–100%) | 22.2% (6.6%–75.4%) |
| Toxicity (N = 29) | Any Grade, n (%) | Grade 3–4, n (%) |
|---|---|---|
| Any AE | 29 (100) | 0 |
| Injection site reaction | 29 (100) | 0 |
| Fever | 21 (72) | 0 |
| Dyspnea | 8 (28) | 0 |
| Lab abnormalities | 8 (28) | 0 |
| Gastrointestinal | 8 (28) | 0 |
| Rash | 3 (10) | 0 |
| Lethargia | 3 (10) | 0 |
| Depression | 1 (3) | 0 |
| Cardiomyopathy | 1 (3) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumoulin, D.W.; Cornelissen, R.; Bezemer, K.; Baart, S.J.; Aerts, J.G.J.V. Long-Term Follow-Up of Mesothelioma Patients Treated with Dendritic Cell Therapy in Three Phase I/II Trials. Vaccines 2021, 9, 525. https://doi.org/10.3390/vaccines9050525
Dumoulin DW, Cornelissen R, Bezemer K, Baart SJ, Aerts JGJV. Long-Term Follow-Up of Mesothelioma Patients Treated with Dendritic Cell Therapy in Three Phase I/II Trials. Vaccines. 2021; 9(5):525. https://doi.org/10.3390/vaccines9050525
Chicago/Turabian StyleDumoulin, Daphne W., Robin Cornelissen, Koen Bezemer, Sara J. Baart, and Joachim G. J. V. Aerts. 2021. "Long-Term Follow-Up of Mesothelioma Patients Treated with Dendritic Cell Therapy in Three Phase I/II Trials" Vaccines 9, no. 5: 525. https://doi.org/10.3390/vaccines9050525
APA StyleDumoulin, D. W., Cornelissen, R., Bezemer, K., Baart, S. J., & Aerts, J. G. J. V. (2021). Long-Term Follow-Up of Mesothelioma Patients Treated with Dendritic Cell Therapy in Three Phase I/II Trials. Vaccines, 9(5), 525. https://doi.org/10.3390/vaccines9050525






