Poly (I:C)-Potentiated Vaccination Enhances T Cell Response in Olive Flounder (Paralichthys olivaceus) Providing Protection against Viral Hemorrhagic Septicemia Virus (VHSV)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Viruses
2.2. VHSV Challenge Test
2.3. RNA Extraction and RT-PCR for VHSV
2.4. Preparation for Vaccination
2.5. Vaccination
2.6. Preparation of Olive Flounder Leukocytes
2.7. Flow Cytometry
2.8. Measuring Population of CD3+, CD4-1+, and CD4-2+ T Cells in Olive Flounder after VHSV Infection and Vaccination
2.9. Stastical Analysis
3. Results
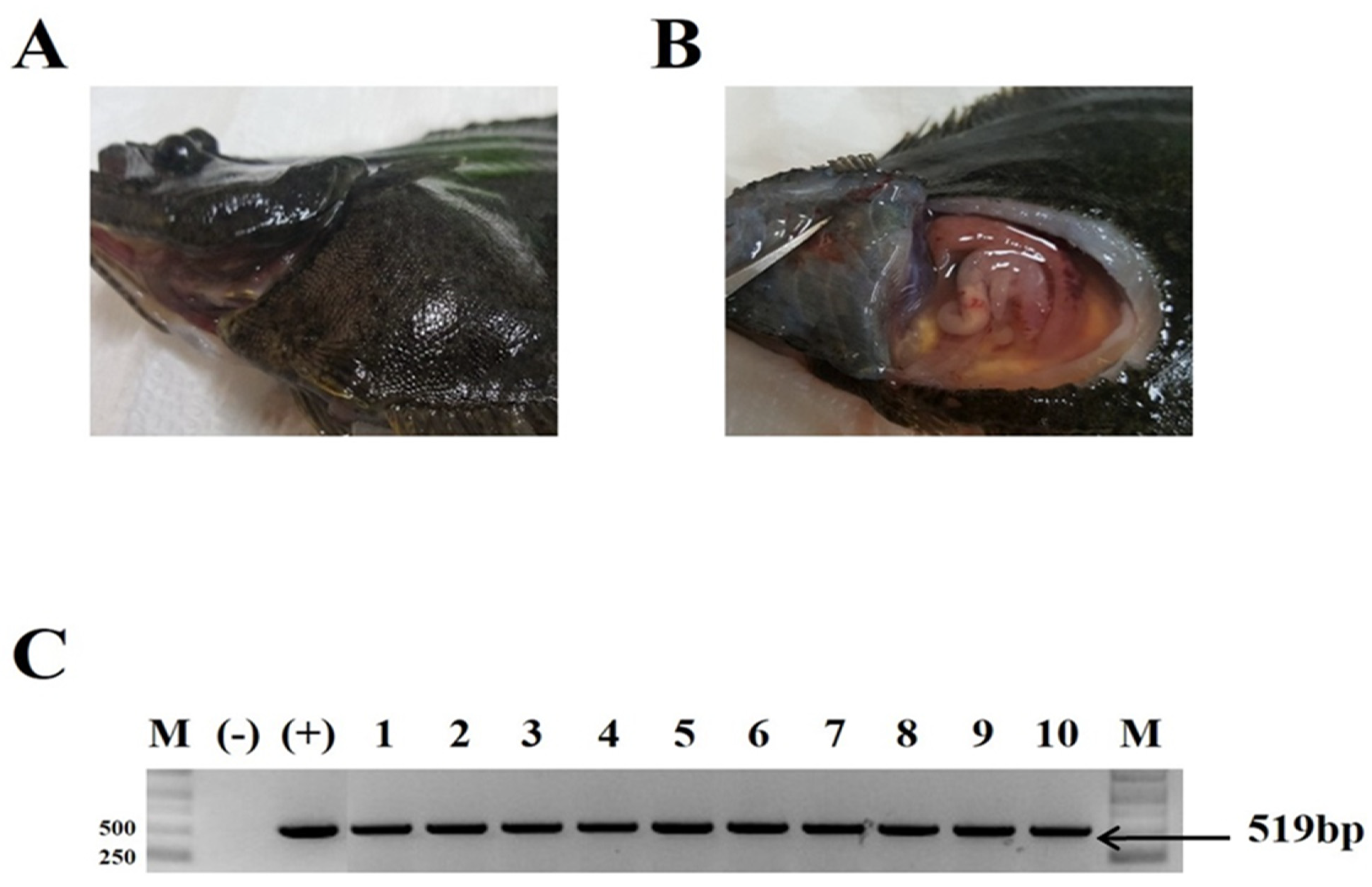
3.1. VHSV Challenge Test
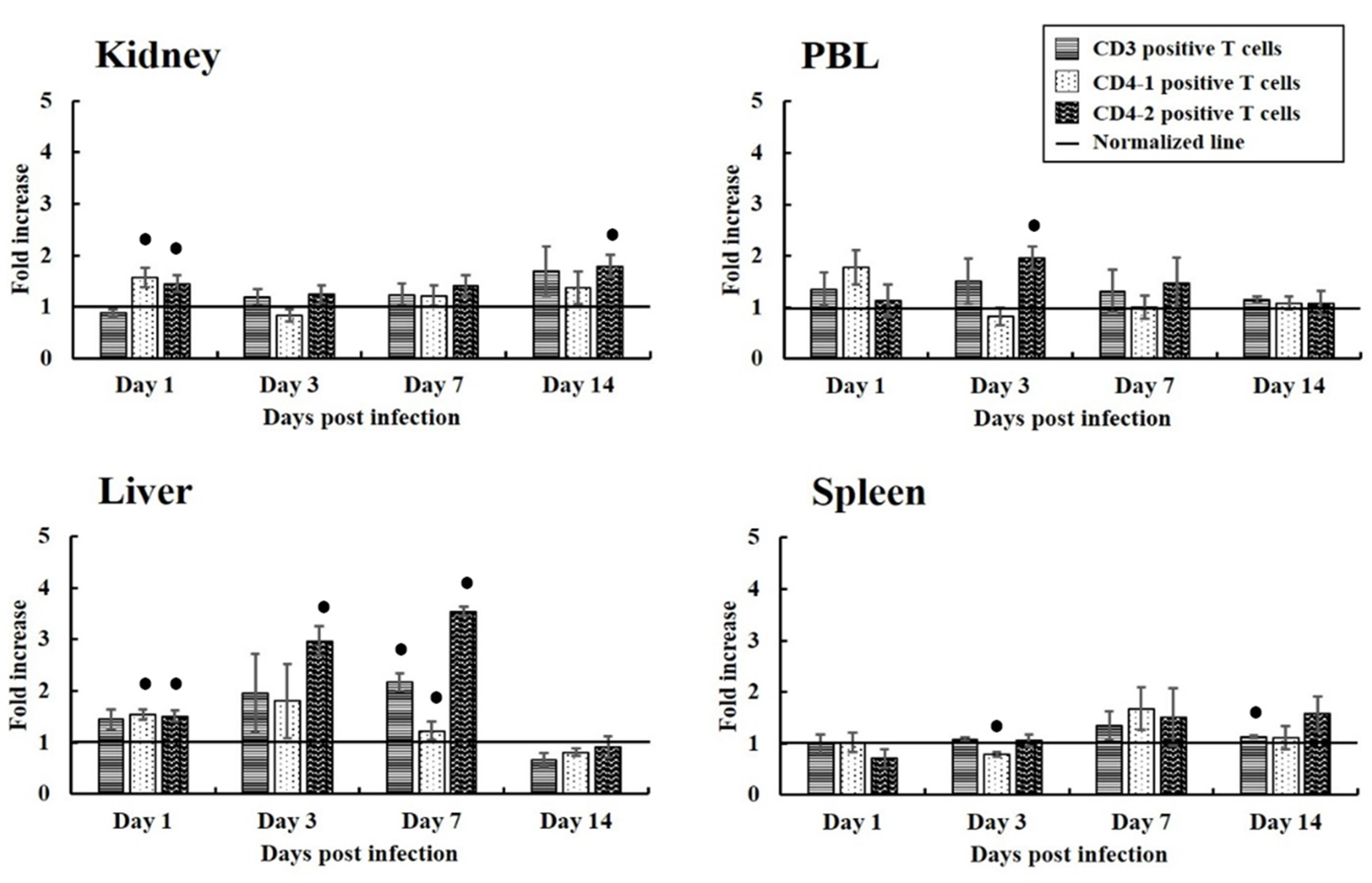
3.2. Proliferation of T Cells Expressing CD3, CD4-1, and CD4-2 in Fish from the Experimental Infection
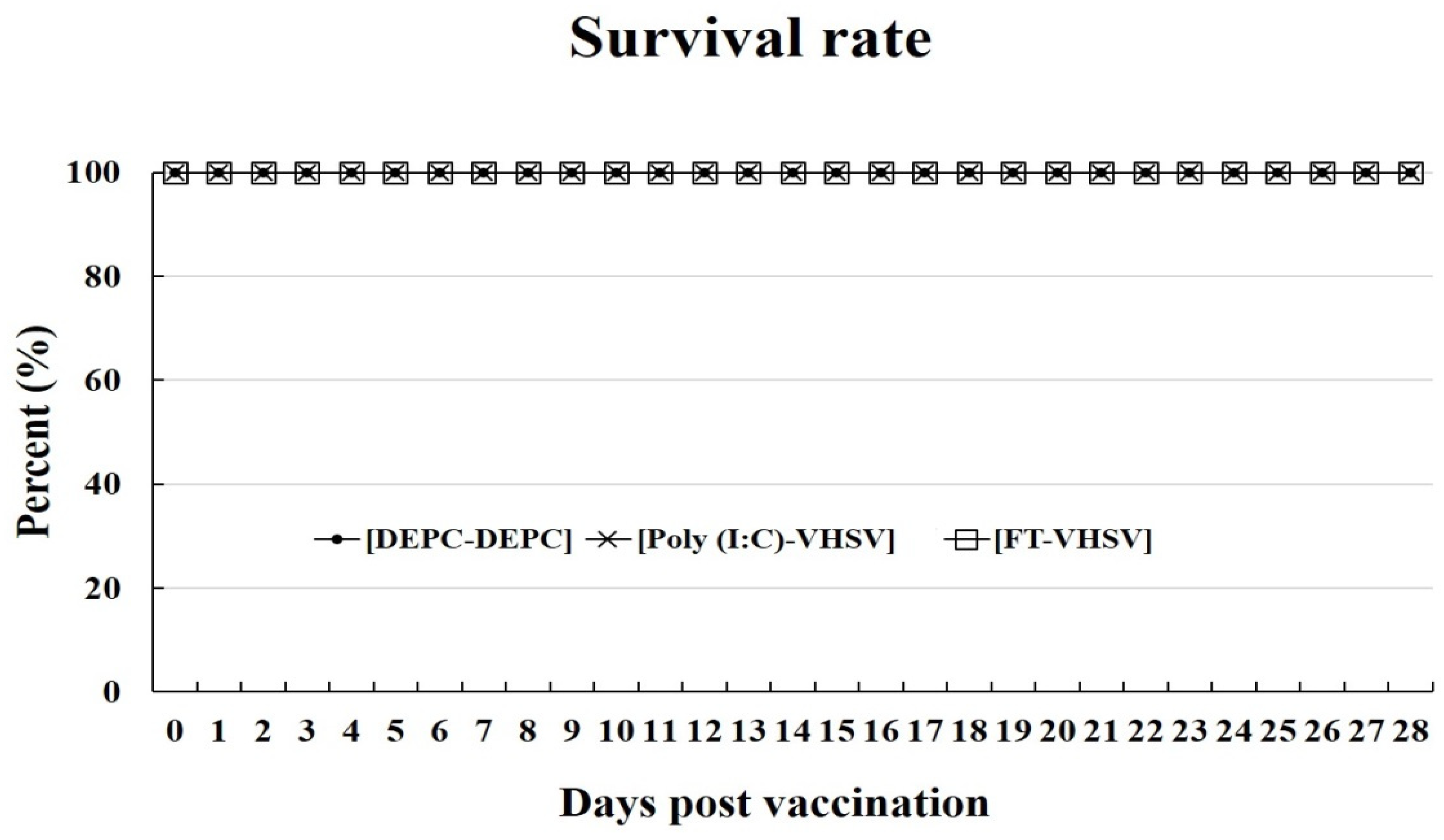
3.3. Changes in Viral Titer due to Formalin Treatment and Survival Rate of Fish after Vaccination
3.4. Proliferation of T Cells Expressing CD3, CD4-1, and CD4-2 In Vivo after Vaccination
3.4.1. CD3+ T Cells
3.4.2. CD4-1+ T Cells
3.4.3. CD4-2+ T Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Tordo, N.; Benmansour, A.; Calisher, C.; Dietzgen, R.G.; Fang, R.X.; Jackson, A.O.; Kurath, G.; Nadin-Davis, S.A.; Tesh, R.B.; Walker, P.J. Family Rhabdoviridae. In Eighth Report of the International Committee on the Taxonomy of Viruses; Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Rasmussen, C.J. A biological study of the Egtved disease (INUL). Ann. N. Y. Acad. Sci. 1965, 126, 427–460. [Google Scholar] [CrossRef]
- Jørgensen, P.V. Egtved virus: The susceptibility of brown trout and rainbow trout to eight virus isolates and the significance of the findings for the VHS control. In Fish Diseases; Springer: Berlin/Heidelberg, Germany, 1980; pp. 3–7. [Google Scholar]
- Smail, D.A. Viral haemorrhagic septicaemia. Fish. Dis Disord. 1999, 3, 123–147. [Google Scholar]
- Kim, S.M.; Lee, J.I.; Hong, M.J.; Park, H.S.; Park, S.I. Genetic relationship of the VHSV (viral hemorrhagic septicaemia virus) isolated from cultured olive flounder, Paralichthysolivaceus in Korea. J. Fish. Pathol. 2003, 16, 1–12. [Google Scholar]
- Kim, J.W.; Lee, H.N.; Jee, B.Y.; Woo, S.H.; Kim, Y.J.; Lee, M.K. Monitoring of the mortalities in the aquaculture farms of South Korea. J. Fish. Pathol. 2012, 25, 271–277. [Google Scholar] [CrossRef]
- Kim, W.S.; Kim, S.R.; Kim, D.; Kim, J.O.; Park, M.A.; Kitamura, S.I.; Kim, H.Y.; Kim, D.H.; Han, H.J.; Jung, S.J.; et al. An outbreak of VHSV (viral hemorrhagic septicaemia virus) infection in farmed olive flounder Paralichthysolivaceus in Korea. Aquaculture 2009, 296, 165–168. [Google Scholar] [CrossRef]
- Kim, W.S.; Jung, S.J.; Kim, J.O.; Kim, D.W.; Kim, J.H.; Oh, M.J. Genetic positioning of Korean viral hemorrhagic septicaemia virus (VHSV) from cultured and wild marine fishes. J. Fish. Pathol. 2011, 24, 1–9. [Google Scholar] [CrossRef]
- Lorenzen, N.; Olesen, N.J. Immunization with viral antigens: Viral haemorrhagic septicaemia. Dev. Biol. Stand. 1997, 90, 201–209. [Google Scholar] [PubMed]
- Winton, J.R. Immunization with viral antigens: Infectious haematopoietic necrosis. Dev. Biol. Stand. 1997, 90, 211–220. [Google Scholar]
- Lorenzen, N.; LaPatra, S.E. DNA vaccines for aquacultured fish. Rev. Sci. Tech. Off. Int. Des. Epizoot. 2005, 24, 201. [Google Scholar] [CrossRef]
- Sommerset, I.; Krossøy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, J.S.; Choi, M.C.; Kwon, S.R. Comparison of the efficacy of Poly (I: C) immunization with live vaccine and formalin-killed vaccine against viral hemorrhagic septicaemia virus (VHSV) in olive flounder (Paralichthysolivaceus). Fish. Shellfish Immunol. 2016, 48, 206–211. [Google Scholar] [CrossRef]
- Takami, I.; Kwon, S.R.; Nishizawa, T.; Yoshimizu, M. Protection of Japanese flounder Paralichthysolivaceus from viral hemorrhagic septicaemia (VHS) by Poly (I: C) immunization. Dis. Aquat. Organ. 2010, 89, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Takami, I.; Yang, M.; Oh, M.J. Live vaccine of viral hemorrhagic septicaemia virus (VHSV) for Japanese flounder at fish rearing temperature of 21 °C instead of Poly (I: C) administration. Vaccine 2011, 29, 8397–8404. [Google Scholar] [CrossRef]
- Nishizawa, T.; Takami, I.; Kokawa, Y.; Yoshimizu, M. Fish immunization using a synthetic double-stranded RNA Poly (I: C), an interferon inducer, offers protection against RGNNV, a fish nodavirus. Dis. Aquat. Organ. 2009, 83, 115–122. [Google Scholar] [CrossRef]
- Nishizawa, T.; Takami, I.; Yoshimizu, M.; Oh, M.J. Required dose of fish nervous necrosis virus (NNV) for Poly (I: C) immunization of sevenband grouper Epinephelus septemfasciatus. Aquaculture 2011, 311, 100–104. [Google Scholar] [CrossRef]
- Kim, H.J.; Oseko, N.; Nishizawa, T.; Yoshimizu, M. Protection of rainbow trout from infectious hematopoietic necrosis (IHN) by injection of infectious pancreatic necrosis virus (IPNV) or Poly (I:C). Dis. Aquat. Organ. 2009, 83, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.J.; Takami, I.; Nishizawa, T.; Kim, W.S.; Kim, C.S.; Kim, S.R.; Park, M.A. Field tests of Poly (I:C) immunization with nervous necrosis virus (NNV) in sevenband grouper, Epinephelusseptemfasciatus (Thunberg). J. Fish. Dis. 2012, 35, 187–191. [Google Scholar] [CrossRef]
- Oh, M.J.; Kim, W.S.; Seo, H.G.; Gye, H.J.; Nishizawa, T. Change in infectivity titre of nervous necrosis virus (NNV) in brain tissue of sevenband grouper, Epinephalus fasciatus Thunberg, with Poly (I:C) administration. J. Fish. Dis. 2013, 36, 159–162. [Google Scholar] [CrossRef]
- Lorenzen, N.; Lapatra, S.E. Immunity to rhabdoviruses in rainbow trout: The antibody response. Fish. Shellfish Immunol. 1999, 9, 345–360. [Google Scholar] [CrossRef]
- Avtalion, R.R.; Clem, L.W. Environmental control of the immune response in fish. Crit. Rev. Environ. Sci. Technol. 1981, 11, 163–188. [Google Scholar] [CrossRef]
- Midtlyng, P.J. Methods for measuring efficacy, safety and potency of fish vaccines. In Fish Vaccines; Springer: Basel, Switzerland, 2016; pp. 119–141. [Google Scholar]
- Bolton, D.L.; Roederer, M. Flow cytometry and the future of vaccine development. Expert Rev. Vaccines 2009, 8, 779–789. [Google Scholar] [CrossRef]
- Nakanishi, T.; Shibasaki, Y.; Matsuura, Y. T cells in fish. Biology 2015, 4, 640–663. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Lee, J.S.; Kim, Y.R.; Im, S.P.; Kim, S.W.; Lazarte, J.M.S.; Kim, J.; Thompson, K.D.; Jung, T.S. Development of a monoclonal antibody against the CD3ε of olive flounder (Paralichthysolivaceus) and its application in evaluating immune response related to CD3ε. Fish. Shellfish Immunol. 2017, 65, 179–185. [Google Scholar] [CrossRef]
- Jung, J.W.; Lee, J.S.; Kim, J.; Im, S.P.; Kim, S.W.; Lazarte, J.M.S.; Kim, Y.R.; Chun, J.H.; Ha, M.W.; Kim, N.N.; et al. Involvement of CD4-1 T cells in the cellular immune response of olive flounder (Paralichthysolivaceus) against viral hemorrhagic septicaemia virus (VHSV) and nervous necrosis virus (NNV) infection. Dev. Comp. Immunol. 2020, 103, 103518. [Google Scholar] [CrossRef]
- Jung, J.W.; Chun, J.H.; Lee, J.S.; Kim, S.W.; Lee, A.R.; Kim, J.; Lazarte, J.M.S.; Kim, Y.R.; Kim, H.J.; Thompson, K.D.; et al. Characterization of CD4 positive lymphocytes in the antiviral response of olive flounder (Paralichthysoliveceus) to nervous necrosis virus. Int. J. Mol. Sci. 2020, 21, 4180. [Google Scholar] [CrossRef]
- Xing, J.; Wang, L.; Zhen, M.; Tang, X.; Zhan, W. Variations of T and B lymphocytes of flounder (Paralichthysolivaceus) after Hiramenovirhabdovirus infection and immunization. Mol. Immunol. 2018, 96, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Luo, K.; Tang, X.; Zhan, W. Influence of CD4-1+, CD4-2+ and CD8+ T lymphocytes subpopulations on the immune response of B lymphocytes in flounder (Paralichthysolivaceus) immunized with thymus-dependent or thymus-independent antigen. Fish. Shellfish Immunol. 2019, 84, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Tubo, N.J.; Jenkins, M.K. TCR signal quantity and quality in CD4+ T cell differentiation. Trends Immunol. 2014, 35, 591–596. [Google Scholar] [CrossRef]
- Nutt, S.L.; Hodgkin, P.D.; Tarlinton, D.M.; Corcoran, L.M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 2015, 15, 160–171. [Google Scholar] [CrossRef]
- Ashfaq, H.; Soliman, H.; Saleh, M.; El-Matbouli, M. CD4: A vital player in the teleost fish immune system. Vet. Res. 2019, 50, 1–11. [Google Scholar] [CrossRef]
- Robertsen, B. The interferon system of teleost fish. Fish. Shellfish Immunol. 2006, 20, 172–191. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Koppang, E.O.; Nakanishi, T. Teleost T and NK cell immunity. Fish. Shellfish Immunol. 2013, 35, 197–206. [Google Scholar] [CrossRef]
- Rombout, J.H.; Yang, G.; Kiron, V. Adaptive immune responses at mucosal surfaces of teleost fish. Fish. Shellfish Immunol. 2014, 40, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Roederer, M.; Brenchley, J.M.; Betts, M.R.; De Rosa, S.C. Flow cytometric analysis of vaccine responses: How many colors are enough? Clin. Immunol. 2004, 110, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Lee, C.K.; Julander, J.G.; Brown, A.; Beasley, D.W.; Watts, D.M.; Hayman, E.; Guertin, P.; Makowiecki, J.; Crowell, J.; et al. Inactivated yellow fever 17D vaccine: Development and nonclinical safety, immunogenicity and protective activity. Vaccine 2010, 28, 3827–3840. [Google Scholar] [CrossRef]
- Guidotti, L.G.; Chisari, F.V. Noncytolytic control of viral infections by the innate and adaptive immuneresponse. Ann. Rev. Immunol. 2001, 19, 65–91. [Google Scholar] [CrossRef]
- Philip, A.M.; Jørgensen, E.H.; Maule, A.G.; Vijayan, M.M. Tissue-specific molecular immune response to lipopolysaccharide challenge in emaciated anadromous Arctic charr. Dev. Comp. Immunol. 2014, 45, 133–140. [Google Scholar] [CrossRef]
- Philip, A.M.; Kim, S.D.; Vijayan, M.M. Cortisol modulates the expression of cytokines and suppressors of cytokine signaling (SOCS) in rainbow trout hepatocytes. Dev. Comp. Immunol 2012, 38, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Philip, A.M.; Vijayan, M.M. Stress-immune-growth interactions: Cortisol modulates suppressors of cytokine signaling and JAK/STAT pathway in rainbow trout liver. PLoS ONE 2015, 10, e0129299. [Google Scholar] [CrossRef]
- Shepherd, B.S.; Spear, A.R.; Philip, A.M.; Leaman, D.W.; Stepien, C.A.; Sepulveda-Villet, O.J.; Palmquist, D.E.; Vijayan, M.M. Effects of cortisol and lipopolysaccharide on expression of select growth-, stress- and immune-related genes in rainbow trout liver. Fish. Shellfish Immunol. 2018, 74, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Abós, B.; Pignatelli, J.; von GersdorffJørgensen, L.; Granja, A.G.; Buchmann, K.; Tafalla, C. Early immune responses in rainbow trout liver upon viral hemorrhagic septicaemia virus (VHSV) infection. PLoS ONE 2014, 9, e111084. [Google Scholar] [CrossRef]
- Kole, S.; Avunje, S.; Jung, S.J. Differential expression profile of innate immune genes in the liver of olive flounder (Paralichthysolivaceus) against viral haemorrhagic septicaemia virus (VHSV) at host susceptible and non-susceptible temperatures. Aquaculture 2019, 503, 51–58. [Google Scholar] [CrossRef]
- Möller, A.M.; Korytář, T.; Köllner, B.; Schmidt-Posthaus, H.; Segner, H. The teleostean liver as an immunological organ: Intrahepatic immune cells (IHICs) in healthy and benzo [a] pyrene challenged rainbow trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 2014, 46, 518–529. [Google Scholar] [CrossRef]
- Longhi, M.P.; Trumpfheller, C.; Idoyaga, J.; Caskey, M.; Matos, I.; Kluger, C.; Salazar, A.M.; Colonna, M.; Steinman, R.M. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 2009, 206, 1589–1602. [Google Scholar] [CrossRef]
- Avunje, S.; Jung, S.J. Poly (I: C) and imiquimod induced immune responses and their effects on the survival of olive flounder (Paralichthysolivaceus) from viral haemorrhagic septicaemia. Fish. Shellfish Immunol. 2017, 71, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Weaver, W.; Pore, A.; Gorgoglione, B.; Wildschutte, J.H.; Xiao, P.; Shepherd, B.S.; Spear, A.; Malathi, K.; Stepien, C.A.; et al. Role of viral hemorrhagicsepticemia virus matrix (M) protein in suppressing host transcription. J. Virol. 2017, 91, e00269-17. [Google Scholar] [CrossRef] [PubMed]
- Swiecki, M.; McCartney, S.A.; Wang, Y.; Colonna, M. TLR7/9 versus TLR3/MDA5 signaling during virus infections and diabetes. J. Leukoc. Biol. 2011, 90, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Castro, R.; Tafalla, C. Overview of fish immunity. In Mucosal Health in Aquaculture; Academic Press: Cambridge, UK, 2015; pp. 3–54. [Google Scholar]
- Liu, Y.; Zhang, S.; Jiang, G.; Yang, D.; Lian, J.; Yang, Y. The development of the lymphoid organs of flounder, Paralichthysolivaceus, from hatching to 13 months. Fish. Shellfish Immunol. 2004, 16, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, I.; Katakura, F.; Moritomo, T. Isolation and characterization of hematopoietic stem cells in teleost fish. Dev. Comp. Immunol. 2016, 58, 86–94. [Google Scholar] [CrossRef]
- Ganassin, R.C.; Bols, N.C. A stromal cell line from rainbow trout spleen, RTS34st, that supports the growth of rainbow trout macrophages and produces conditioned medium with mitogenic effects of leukocytes. In Vitro Cell Dev. Biol. Anim. 1999, 35, 80–86. [Google Scholar] [CrossRef]
- Williams, M.A.; Bevan, M.J. Effector and memory CTL differentiation. Ann. Rev. Immunol. 2007, 25, 171–192. [Google Scholar] [CrossRef]
- Castro, R.; Bernard, D.; Lefranc, M.P.; Six, A.; Benmansour, A.; Boudinot, P. T cell diversity and TcR repertoires in teleost fish. Fish. Shellfish Immunol. 2011, 31, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Secombes, C.J. Teleost fish interferons and their role in immunity. Dev. Comp. Immunol. 2011, 35, 1376–1387. [Google Scholar] [CrossRef]
- Tang, X.; Guo, M.; Du, Y.; Xing, J.; Sheng, X.; Zhan, W. Interleukin-2 (IL-2) in flounder (Paralichthys olivaceus): Molecular cloning, characterization and bioactivity analysis. Fish. Shellfish Immunol. 2019, 93, 55–65. [Google Scholar] [CrossRef]
- Díaz-Rosales, P.; Bird, S.; Wang, T.H.; Fujiki, K.; Davidson, W.S.; Zou, J.; Secombes, C.J. Rainbow trout interleukin-2: Cloning, expression and bioactivity analysis. Fish. Shellfish Immunol. 2009, 27, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Mu, P.; Wang, Y.; Ao, J.; Ai, C.; Chen, X. Molecular cloning and bioactivity of an IL-2 homologue in large yellow croaker (Larimichthyscrocea). Fish. Shellfish Immunol. 2018, 81, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.; Zou, J.; Kono, T.; Sakai, M.; Dijkstra, J.M.; Secombes, C. Characterisation and expression analysis of interleukin 2 (IL-2) and IL-21 homologues in the Japanese pufferfish, Fugu rubripes, following their discovery by synteny. Immunogenetics 2005, 56, 909–923. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, J.H.; Jung, J.W.; Kim, Y.R.; Lazarte, J.M.S.; Kim, S.W.; Kim, J.; Thompson, K.D.; Kim, H.J.; Jung, T.S. Poly (I:C)-Potentiated Vaccination Enhances T Cell Response in Olive Flounder (Paralichthys olivaceus) Providing Protection against Viral Hemorrhagic Septicemia Virus (VHSV). Vaccines 2021, 9, 482. https://doi.org/10.3390/vaccines9050482
Chun JH, Jung JW, Kim YR, Lazarte JMS, Kim SW, Kim J, Thompson KD, Kim HJ, Jung TS. Poly (I:C)-Potentiated Vaccination Enhances T Cell Response in Olive Flounder (Paralichthys olivaceus) Providing Protection against Viral Hemorrhagic Septicemia Virus (VHSV). Vaccines. 2021; 9(5):482. https://doi.org/10.3390/vaccines9050482
Chicago/Turabian StyleChun, Jin Hong, Jae Wook Jung, Young Rim Kim, Jassy Mary S. Lazarte, Si Won Kim, Jaesung Kim, Kim D. Thompson, Hyoung Jun Kim, and Tae Sung Jung. 2021. "Poly (I:C)-Potentiated Vaccination Enhances T Cell Response in Olive Flounder (Paralichthys olivaceus) Providing Protection against Viral Hemorrhagic Septicemia Virus (VHSV)" Vaccines 9, no. 5: 482. https://doi.org/10.3390/vaccines9050482
APA StyleChun, J. H., Jung, J. W., Kim, Y. R., Lazarte, J. M. S., Kim, S. W., Kim, J., Thompson, K. D., Kim, H. J., & Jung, T. S. (2021). Poly (I:C)-Potentiated Vaccination Enhances T Cell Response in Olive Flounder (Paralichthys olivaceus) Providing Protection against Viral Hemorrhagic Septicemia Virus (VHSV). Vaccines, 9(5), 482. https://doi.org/10.3390/vaccines9050482






