Comparative Evaluation of Lumpy Skin Disease Virus-Based Live Attenuated Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Challenge Virus and Cell Line
2.2. Serological Analysis
2.3. Virus Isolation and Titration
2.4. Animal Trial and Sampling Design
2.4.1. Vaccines
2.4.2. Animal Trial Design
2.4.3. Clinical Evaluation and Scoring
2.4.4. Sampling
2.5. Real-Time PCR (RT-PCR)
2.5.1. Pan Capripox RT-PCR
2.5.2. DIVA Real-Time PCR (DIVA RT-PCR)
2.6. IFNg Release Assay
2.7. Statistical Analysis
3. Results
3.1. Unvaccinated Infected Control Group: Clinical Observations and Scoring
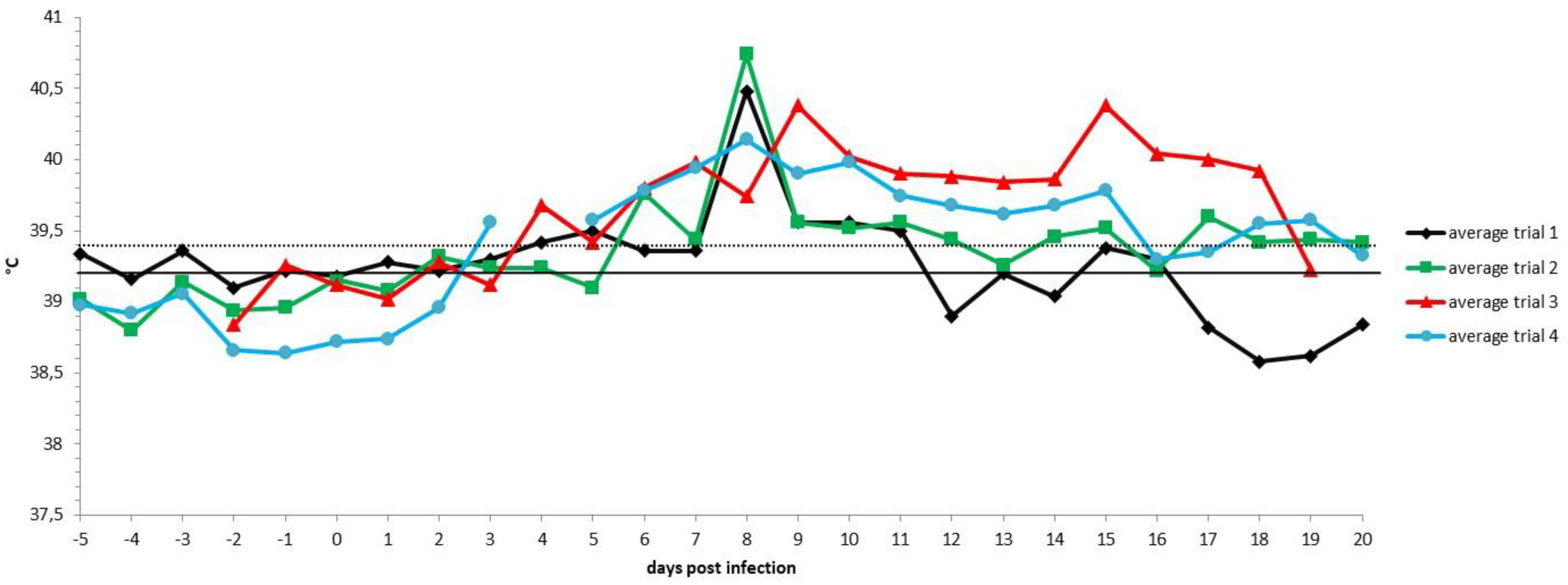
3.2. Unvaccinated Infected Control Group: Virology and Serology
3.3. Vaccinated Groups: Clinical Observations and Scoring
3.3.1. Post-Vaccination
3.3.2. Post-Challenge with a Virulent Field LSDV Strain
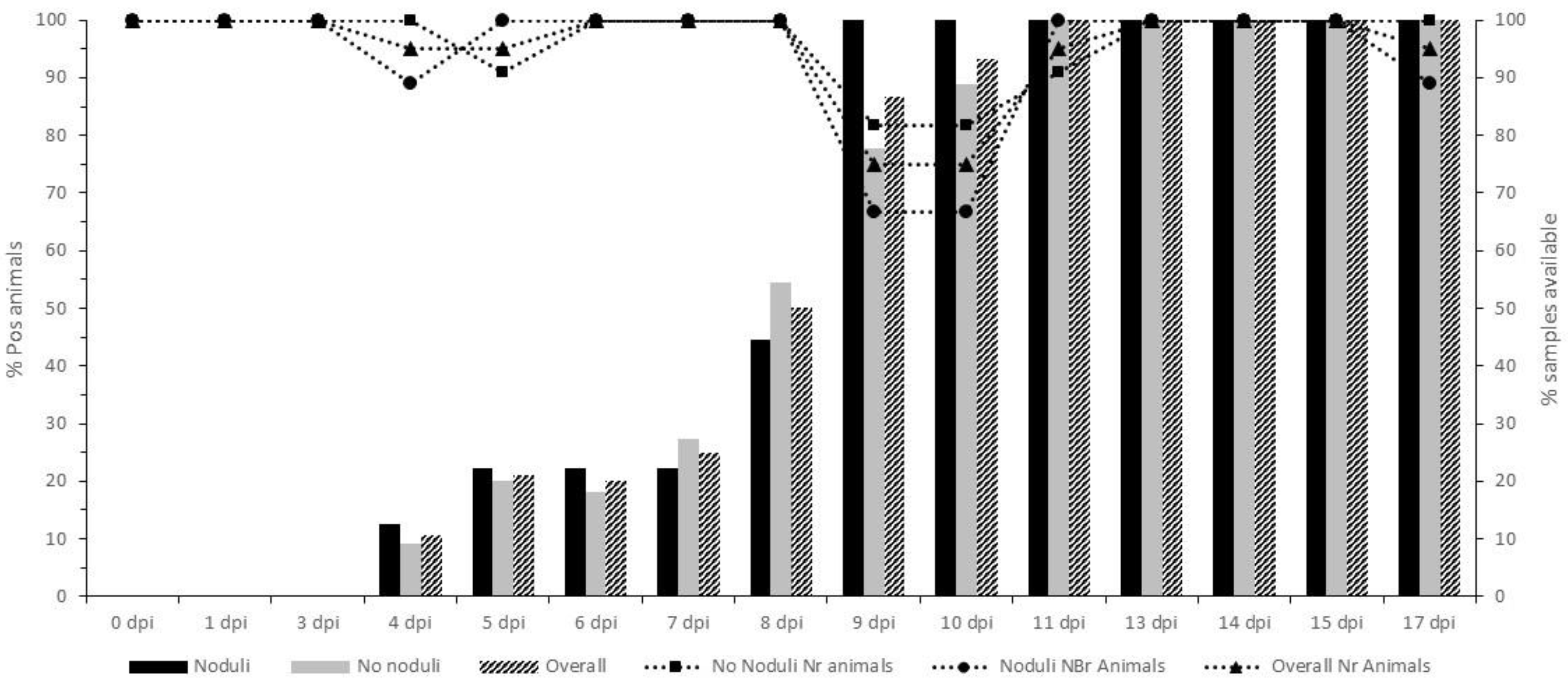
3.4. Vaccinated Groups: Virology
3.5. Vaccinated Groups: Serology
3.6. Vaccinated Groups: Cellular Immunity (IFNg Assay)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tulman, E.R.; Afonso, C.L.; Lu, Z.; Zsak, L.; Sur, J.H.; Sandybaev, N.T.; Kerembekova, U.Z.; Zaitsev, V.L.; Kutish, G.F.; Rock, D.L. The Genomes of Sheeppox and Goatpox Viruses. J. Virol. 2002, 76, 6054–6061. [Google Scholar] [CrossRef] [PubMed]
- Greth, A.; Gourreau, J.M.; Vassart, M.; Wyers, M.; Lefevre, P.C. Capripoxvirus Disease in an Arabian Oryx (Oryx leucoryx) from Saudi Arabia. J. Wildl. Dis. 1992, 28, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Tageldin, M.H.; Wallace, D.B.; Gerdes, G.H.; Putterill, J.F.; Greyling, R.R.; Phosiwa, M.N.; Al Busaidy, R.M.; Al Ismaaily, S.I. Lumpy Skin Disease of Cattle: An Emerging Problem in the Sultanate of Oman. Trop. Anim. Health Prod. 2014, 46, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, G.; Zewde, G.; Admassu, B. Risk Assessments of Lumpy Skin Diseases in Borena Bull Market Chain and its Implication for Livelihoods and International Trade. Trop. Anim. Health Prod. 2013, 45, 1153–1159. [Google Scholar] [CrossRef]
- Gari, G.; Bonnet, P.; Roger, F.; Waret-Szkuta, A. Epidemiological Aspects and Financial Impact of Lumpy Skin Disease in Ethiopia. Prev Vet Med. 2011, 102, 274–283. [Google Scholar] [CrossRef]
- Green, H.F. Lumpy Skin Disease: Its Effect on Hides and Leather and a Comparison on this Respect with some other Skin Diseases. Bull. Epizoot. Dis. Afr. 1959, 7, 63–74. [Google Scholar]
- Abera, Z.; Degefu, H.; Gari, G.; Ayana, Z. Review on Epidemiology and Economic Importance of Lumpy Skin Disease. Int. J. Basic Appl. Virol. 2015, 4, 8–21. [Google Scholar] [CrossRef]
- Ramaswamy, N. Draught Animals and Welfare. Rev. Sci. Technol. O.I.E. 1994, 13, 195–216. [Google Scholar] [CrossRef]
- Molla, W.; de Jong, M.C.M.; Gari, G.; Frankena, K. Economic Impact of Lumpy Skin Disease and Cost Effectiveness of Vaccination for the Control of Outbreaks in Ethiopia. Prev. Vet. Med. 2017, 147, 100–107. [Google Scholar] [CrossRef]
- Ali, A.; Esmat, M.; Attia, A.; Abdel-Hamid, Y. Clinical and Pathological Studies on Lumpy Skin Disease in Egypt. Vet. Rec. 1990, 127, 549–550. [Google Scholar]
- Yeruham, I.; Nir, O.; Braverman, Y.; Davidson, M.; Grinstein, H.; Zamir, O. Spread of Lumpy Skin Disease in Israel Dairy Herds. Vet. Rec. 1995, 137, 91–93. [Google Scholar] [CrossRef]
- Şevik, M.; Doğan, M. Epidemiological and Molecular Studies on Lumpy Skin Disease Outbreaks in Turkey during 2014–2015. Transbound. Emerg. Dis. 2017, 64, 1268–1279. [Google Scholar] [CrossRef]
- Tasioudi, K.E.; Antoniou, S.E.; Iliadou, P.; Sachpatzidis, A.; Plevraki, E.; Agianniotaki, E.I.; Fouki, C.; Mangana-Vougiouka, O.; Chondrokouki, E.; Dile, C. Emergence of Lumpy Skin Disease in Greece, 2015. Transbound. Emerg. Dis. 2016, 63, 260–265. [Google Scholar] [CrossRef]
- FAO. Sustainable Prevention, Control and Elimination of Lumpy Skin Disease—Eastern Europe and the Balkans; FAO Animal Production and Health Position Paper: Rome, Italy, 2017; No. 2; p. 26. ISBN 978-92-5-109937-7. [Google Scholar]
- FAO. Introduction and Spread of Lumpy Skin Disease in South, East and Southeast Asia—Qualitative Risk Assessment and Management; FAO Animal Production and Health: Rome, Italy, 2020; p. 183. [Google Scholar] [CrossRef]
- Ben-Gera, J.; Klement, E.; Khinich, E.; Stram, Y.; Shpigel, N.Y. Comparison of the Efficacy of Neethling Lumpy Skin Disease Virus and x10RM65 Sheep-Pox Live Attenuated Vaccines for the Prevention of Lumpy Skin Disease—The Results of a Randomized Controlled Field Study. Vaccine 2015, 33, 4837–4842. [Google Scholar] [CrossRef]
- Tuppurainen, E.S.M.; Venter, E.H.; Shisler, J.L.; Gari, G.; Mekonnen, G.A.; Juleff, N.; Lyons, N.A.; De Clercq, K.; Upton, C.; Bowden, T.R.; et al. Review: Capripoxvirus Diseases: Current Status and Opportunities for Control. Transbound. Emerg. Dis. 2017, 64, 729–745. [Google Scholar] [CrossRef]
- Klement, E.; Broglia, A.; Antoniou, S.E.; Tsiamadis, V.; Plevraki, E.; Petrović, T.; Polaček, V.; Debeljak, Z.; Miteva, A.; Alexandrov, T.; et al. Neethling Vaccine Proved Highly Effective in Controlling Lumpy Skin Disease Epidemics in the Balkans. Prev. Vet. Med. 2020, 181, 104595. [Google Scholar] [CrossRef]
- Abutarbush, S.M.; Hananeh, W.M.; Ramadan, W.; Al Sheyab, O.M.; Alnajjar, A.R.; Al Zoubi, I.G.; Knowles, N.J.; Bachanek-Bankowska, K.; Tuppurainen, E.S. Adverse Reactions to Field Vaccination Against Lumpy Skin Disease in Jordan. Transbound. Emerg. Dis. 2016, 63, e213–e219. [Google Scholar] [CrossRef]
- Ayelet, G.; Abate, Y.; Sisay, T.; Nigussie, H.; Gelaye, E.; Jemberie, S.; Asmare, K. Lumpy Skin Disease: Preliminary Vaccine Efficacy Assessment and Overview on Outbreak Impact in Dairy Cattle at Debre Zeit, central Ethiopia. Antiviral. Res. 2013, 98, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Brenner, J.; Bellaiche, M.; Gross, E.; Elad, D.; Oved, Z.; Haimovitz, M.; Wasserman, A.; Friedgut, O.; Stram, Y.; Bumbarov, V.; et al. Appearance of Skin Lesions in Cattle Populations Vaccinated against Lumpy Skin Disease: Statutory Challenge. Vaccine 2009, 27, 1500–1503. [Google Scholar] [CrossRef] [PubMed]
- Gari, G.; Abie, G.; Gizaw, D.; Wubete, A.; Kidane, M.; Asgedom, H.; Bayissa, B.; Ayelet, G.; Oura, C.A.; Roger, F.; et al. Evaluation of the Safety, Immunogenicity and Efficacy of Three Capripoxvirus Vaccine Strains against Lumpy Skin Disease Virus. Vaccine 2015, 33, 3256–3261. [Google Scholar] [CrossRef] [PubMed]
- Katsoulos, P.D.; Chaintoutis, S.C.; Dovas, C.I.; Polizopoulou, Z.S.; Brellou, G.D.; Agianniotaki, E.I.; Tasioudi, K.E.; Chondrokouki, E.; Papadopoulos, O.; Karatzias, H.; et al. Investigation on the Incidence of Adverse Reactions, Viraemia and Haematological Changes Following Field Immunization of Cattle Using a Live Attenuated Vaccine Against Lumpy Skin Disease. Transbound. Emerg. Dis. 2018, 65, 174–185. [Google Scholar] [CrossRef]
- Bedeković, T.; Šimić, I.; Krešić, N.; Lojkić, I. Detection of Lumpy Skin Disease Virus in Skin Lesions, Blood, Nasal Swabs and Milk Following Preventive Vaccination. Transbound. Emerg. Dis. 2018, 65, 491–496. [Google Scholar] [CrossRef]
- Sprygin, A.; Pestova, Y.; Prutnikov, P.; Kononov, A. Detection of Vaccine-Like Lumpy Skin Disease Virus in Cattle and Musca Domestica L. Flies in an Outbreak of Lumpy Skin Disease in Russia in 2017. Transbound. Emerg. Dis. 2018, 65, 1137–1144. [Google Scholar] [CrossRef]
- Tilahun, Z.; Berecha, B.; Simenew, K.; Reta, D. Towards Effective Vaccine Production, A Controlled Field Trial on the Immunological Response of Three Lumpy Skin Disease Vaccine Strains in Dairy Farms. Acad. J. Anim. Dis. 2014, 3, 17–26. [Google Scholar] [CrossRef]
- Davies, F.G. Lumpy skin disease of cattle: A Growing Problem in Africa and the Near East. World Anim. Rev. 1991, 68, 37–42. [Google Scholar]
- Babiuk, S.; Parkyn, G.; Copps, J.; Larence, J.E.; Sabara, M.I.; Bowden, T.R.; Boyle, D.B.; Kitching, R.P. Evaluation of an Ovine Testis Cell Line (OA3.Ts) for Propagation of Capripoxvirus Isolates and Development of an Immunostaining Technique for Viral Plaque Visualization. J. Vet. Diagn. Invest. 2007, 19, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Haegeman, A.; De Leeuw, I.; Mostin, L.; Van Campe, W.; Aerts, L.; Vastag, M.; De Clercq, K. An Immunoperoxidase Monolayer Assay (IPMA) for the Detection of Lumpy Skin Disease Antibodies. J. Virol. Methods 2020, 277. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Percent Endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- World Organization for Animal Health (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Chapter 3.4.12: Lumpy Skin Disease. 2019. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.04.12_LSD.pdf (accessed on 31 March 2021).
- Obernier, J.A.; Baldwin, R. Establishing an Appropriate Period of Acclimatization Following Transportation of Laboratory Animals. ILAR J. 2006, 47, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Haegeman, A.; Zro, K.; Vandenbussche, F.; Demeestere, L.; Van Campe, W.; Ennaji, M.M.; De Clercq, K. Development and Validation of Three Capripoxvirus Real-Time PCRs for Parallel Testing. J. Virol. Methods 2013, 193, 446–451. [Google Scholar] [CrossRef]
- Agianniotaki, E.I.; Chaintoutis, S.C.; Haegeman, A.; Tasioudi, K.E.; De Leeuw, I.; Katsoulos, P.D.; Sachpatzidis, A.; De Clercq, K.; Alexandropoulos, T.; Polizopoulou, Z.S.; et al. Development and Validation of a TaqMan Probe-Based Real-Time PCR Method for the Differentiation of Wild Type Lumpy Skin Disease Virus from Vaccine Virus Strains. J. Virol. Methods 2017, 249, 48–57. [Google Scholar] [CrossRef]
- Parida, S.; Oh, Y.; Reid, S.M.; Cox, S.J.; Statham, R.J.; Mahapatra, M.; Anderson, J.; Barnett, P.V.; Charleston, B.; Paton, D.J. Interferon-Gamma Production in Vitro from Whole Blood of Foot-and-Mouth Disease Virus (FMDV) Vaccinated and Infected Cattle after Incubation with Inactivated FMDV. Vaccine 2006, 24, 964–969. [Google Scholar] [CrossRef]
- Petrie, A.; Watson, P. Statistics for Veterinary and Animal Science; John Wiley & Sons, Ltd.: West Sussex, UK, 2013; p. 414. [Google Scholar]
- Howell, D.C. Méthodes Statistiques en Sciences Humaines; De Boeck Université: Paris, France, 1998; p. 762. [Google Scholar]
- Tuppurainen, E.S.; Pearson, C.R.; Bachanek-Bankowska, K.; Knowles, N.J.; Amareen, S.; Frost, L.; Henstock, M.R.; Lamien, C.E.; Diallo, A.; Mertens, P.P. Characterization of Sheep Pox Virus Vaccine for Cattle Against Lumpy Skin Disease Virus. Antivir. Res. 2014, 109, 1–6. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Mathijs, E.; Haegeman, A.; Al-Majali, A.; Van Borm, S.; De Clercq, K. Complete Genome Sequence of Capripoxvirus Strain KSGP 0240 from a Commercial Live Attenuated Vaccine. Genome Announc. 2016, 4, e01114-16. [Google Scholar] [CrossRef] [PubMed]
- Prozesky, L.; Barnard, B.J. A Study of the Pathology of Lumpy Skin Disease in Cattle. Onderstepoort. J. Vet. Res. 1982, 49, 167–175. [Google Scholar] [PubMed]
- Babiuk, S.; Bowden, T.R.; Parkyn, G.; Dalman, B.; Manning, L.; Neufeld, J.; Embury-Hyatt, C.; Copps, J.; Boyle, D.B. Quantification of Lumpy Skin Disease Virus Following Experimental Infection in Cattle. Transbound. Emerg. Dis. 2008, 55, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Möller, J.; Moritz, T.; Schlottau, K.; Krstevski, K.; Hoffmann, D.; Beer, M.; Bernd Hoffmann, B. Experimental lumpy skin disease virus infection of cattle: Comparison of a Field Strain and a Vaccine Strain. Arch. Virol. 2019, 164, 2931–2941. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Bernardo, B.; Haga, I.R.; Wijesiriwardana, N.; Hawes, P.C.; Simpson, J.; Morrison, L.R.; MacIntyre, N.; Brocchi, E.; Atkinson, J.; Haegeman, A.; et al. Lumpy Skin Disease Is Characterized by Severe Multifocal Dermatitis With Necrotizing Fibrinoid Vasculitis Following Experimental Infection. Vet. Path. 2020, 57, 388–396. [Google Scholar] [CrossRef]
- Tuppurainen, E.S.; Lubinga, J.C.; Stoltsz, W.H.; Troskie, M.; Carpenter, S.T.; Coetzer, J.A.; Venter, E.H.; Oura, C.A. Mechanical Transmission of Lumpy Skin Disease Virus by Rhipicephalus Appendiculatus Male Ticks. Epidemiol Infect. 2013, 141, 425–430. [Google Scholar] [CrossRef]
- Tuppurainen, E.S.M.; Antoniou, S.-E.; Tsiamadis, E.; Topkaridou, M.; Labus, T.; Debeljak, Z.; Plavšić, B.; Miteva, A.; Alexandrov, T.; Pite, L.; et al. Field Observations and Experiences Gained from the Implementation of Control Measures Against Lumpy Skin Disease in South-East Europe between 2015 and 2017. J. Prev. Vet. Med. 2020, 181, 104600. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Lumpy Skin Disease: I. Data Collection and Analysis. EFSA J. 2017, 15, 54. [Google Scholar] [CrossRef]
- Osuagwuh, U.I.; Bagla, V.; Venter, E.H.; Annandale, C.H.; Irons, P.C. Absence of Lumpy Skin Disease Virus in Semen of Vaccinated Bulls Following Vaccination and Subsequent Experimental Infection. Vaccine 2007, 25, 2238–2243. [Google Scholar] [CrossRef] [PubMed]
- Mathijs, E.; Vandenbussche, F.; Haegeman, A.; King, A.; Nthangeni, B.; Potgieter, C.; Maartens, L.; Van Borm, S.; De Clercq, K. Complete Genome Sequence of the Neethling-like Lumpy Skin Disease Virus Strains Obtained Directly from Three Commercial Live Attenuated Vaccines. Genome Anounc. 2016, 4, e01255-16. [Google Scholar] [CrossRef]
- Douglass, N.; Van Der Walt, A.; Omar, R.; Munyanduki, H.; Williamson, A.L. The Complete Genome Sequence of the Lumpy Skin Disease Virus Vaccine Herbivac LS Reveals a Mutation in the Superoxide Dismutase Gene Homolog. Arch Virol. 2019, 164, 3107–3109. [Google Scholar] [CrossRef]
- Munyanduki, H.; Douglass, N.; Offerman, K.; Carulei, O.; Williamson, A.L. Influence of the Lumpy Skin Disease Virus (LSDV) Superoxide Dismutase Homologue on Host Transcriptional Activity, Apoptosis and Histopathology. J. Gen. Virol. 2020, 101, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Salib, F.A.; Osman, A.H. Incidence of Lumpy Skin Disease Among Egyptian Cattle in Giza Governorate, Egypt. Vet. World 2011, 4, 162–167. [Google Scholar] [CrossRef]
- Gumbe, A.A.F. Review on Lumpy Skin Disease and its Economic Impacts in Ethiopia. J. Dairy. Vet. Anim. Res. 2018, 7, 39–46. [Google Scholar] [CrossRef]
- Sameea Yousefi, P.; Mardani, K.; Dalir-Naghadeh, B.; Jalilzadeh-Amin, G. Epidemiological Study of Lumpy Skin Disease Outbreaks in North-western Iran. Transbound. Emerg. Dis. 2017, 64, 1782–1789. [Google Scholar] [CrossRef]
- Abera, Z.; Degefu, H.; Gari, G.; Kidane, M. Sero-Prevalence of Lumpy Skin Disease in Selected Districts of West Wollega Zone, Ethiopia. BMC Vet. Res. 2015, 11, 135. [Google Scholar] [CrossRef]
- Molla, W.; Frankena, K.; Gari, G.; Kidane, M.; Shegu, D.; de Jong, M.C.M. Seroprevalence and Risk Factors of Lumpy Skin Disease in Ethiopia. Prev. Vet. Med. 2018, 160, 99–104. [Google Scholar] [CrossRef]
- Elhaig, M.M.; Selim, A.; Mahmoud, M. Lumpy Skin Disease in Cattle: Frequency of Occurrence in a Dairy Farm and a Preliminary Assessment of its Possible Impact on Egyptian Buffaloes. Onderstepoort. J. Vet. Res. 2017, 84, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Ochwo, S.; VanderWaal, K.; Munsey, A.; Nkamwesiga, J.; Ndekezi, C.; Auma, E.; Mwiine, F.N. Seroprevalence and Risk Factors for Lumpy Skin Disease Virus Seropositivity in Cattle in Uganda. BMC Vet. Res. 2019, 15, 236. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.E. Lumpy Skin Disease. Virol. Monogr. 1968, 3, 112–282. [Google Scholar]




| Body Temperature | Swelling at the Site of Challenge (Intradermal) | |||||
|---|---|---|---|---|---|---|
| 1 Day | Consecutive Days | Diameter (a) | Points | |||
| °C | points | °C | points | Slightly enlarged | <2 cm | 0.5 |
| Up to 39.3 | 0.5 | Up to 39.1 | 0 | Moderately enlarged | 2–4 cm | 1 |
| 39.4 to 39.7 | 39.2 to 39.5 | 0.5 | Large | 4–7 cm | 1.5 | |
| >39.8 | 1 | 39.6 to 40 | 1 | Very large | >7 cm | 2 |
| >40 | 1.5 | General health status (b) | ||||
| Prescapular lymph node swelling | Normal | 0 | ||||
| Enlarged | 0.5 | Mild illness/Unwell | 1 | |||
| Food intake | Severe illness/Severely unwell | 2 | ||||
| Normal feeding | 0 | Number of noduli | ||||
| Slight decreased feeding | 0.5 | No nodules | 0 | |||
| Decreased feeding | 1 | Less than 10 | 1 | |||
| Does not eat | 1.5 | More than 10 | 2 | |||
| Nodule dissemination | ||||||
| No noduli | 0 | |||||
| Local | 1 | |||||
| Generalized | 2 | |||||
| Organ/Tissue Type | Overall | Clinical | Non-Clinical | Difference |
|---|---|---|---|---|
| Inoculation site | 93.3 | 100 | 88.9 | 11.1 |
| Normal skin | 73.3 | 83.3 | 66.7 | 16.7 |
| Nasal mucosa | 53.3 | 100 | 22.2 | 77.8 |
| Skin lesions | 100 | 100 | / | / |
| Inguinal Lnn | 40 | 100 | 0 | 100 |
| Prescapular Lnn | 40 | 83.3 | 11.1 | 72.2 |
| Submandibular Lnn | 53.3 | 100 | 22.2 | 77.8 |
| Bronchial Lnn | 20 | 50 | 0 | 50 |
| Mesenteric Lnn | 20 | 50 | 0 | 50 |
| Mediastinal Lnn | 33.3 | 66.7 | 11.1 | 55.6 |
| Tonsils | 26.7 | 50 | 11.1 | 38.9 |
| Iliacal Lnn | 26.7 | 66.7 | 0 | 66.7 |
| Parotid | 60 | 100 | 0 | 100 |
| Lung | 33.3 | 66.7 | 11.1 | 55.6 |
| Liver | 13.3 | 16.7 | 11.1 | 5.6 |
| Spleen | 20 | 50 | 0 | 50 |
| Rumen | 53.3 | 83.3 | 33.3 | 50 |
| Kidney | 20 | 50 | 0 | 50 |
| Tongue | 40 | 83.3 | 11.1 | 72.2 |
| Testis | 26.7 | 66.7 | 0.0 | 66.7 |
| Epididymis | 40 | 66.7 | 22.2 | 44.4 |
| Masseter | 60 | 100 | 0 | 100 |
| Musculus trapezius | 60 | 100 | 0 | 100 |
| Musculus psoas major | 40 | 66.7 | 0 | 66.7 |
| Quadriceps | 60 | 100 | 0 | 100 |
| OBP | Lumpyvax | Kenyavac | Herbivac | MCI | |
|---|---|---|---|---|---|
| N | 6 | 7 | 5 | 4 | 4 |
| Tmax | 40.5 | 40.6 | 40.8 | 40 | 40.4 |
| Dmax | 5 | 7 | 7 | 4 | 6 |
| Damax | 2.5 | 4 | 4.4 | 2.25 | 3.25 |
| Ntdays | 25 (21) | 72 (42) | 34 (26) | 12 (12) | 15 (15) |
| Nadays | 4.2 (3) | 10.3 (6) | 6.8 (3.7) | 3 (3) | 3.8 (3.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haegeman, A.; De Leeuw, I.; Mostin, L.; Campe, W.V.; Aerts, L.; Venter, E.; Tuppurainen, E.; Saegerman, C.; De Clercq, K. Comparative Evaluation of Lumpy Skin Disease Virus-Based Live Attenuated Vaccines. Vaccines 2021, 9, 473. https://doi.org/10.3390/vaccines9050473
Haegeman A, De Leeuw I, Mostin L, Campe WV, Aerts L, Venter E, Tuppurainen E, Saegerman C, De Clercq K. Comparative Evaluation of Lumpy Skin Disease Virus-Based Live Attenuated Vaccines. Vaccines. 2021; 9(5):473. https://doi.org/10.3390/vaccines9050473
Chicago/Turabian StyleHaegeman, Andy, Ilse De Leeuw, Laurent Mostin, Willem Van Campe, Laetitia Aerts, Estelle Venter, Eeva Tuppurainen, Claude Saegerman, and Kris De Clercq. 2021. "Comparative Evaluation of Lumpy Skin Disease Virus-Based Live Attenuated Vaccines" Vaccines 9, no. 5: 473. https://doi.org/10.3390/vaccines9050473
APA StyleHaegeman, A., De Leeuw, I., Mostin, L., Campe, W. V., Aerts, L., Venter, E., Tuppurainen, E., Saegerman, C., & De Clercq, K. (2021). Comparative Evaluation of Lumpy Skin Disease Virus-Based Live Attenuated Vaccines. Vaccines, 9(5), 473. https://doi.org/10.3390/vaccines9050473








