Probiotic-Based Vaccines May Provide Effective Protection against COVID-19 Acute Respiratory Disease
Abstract
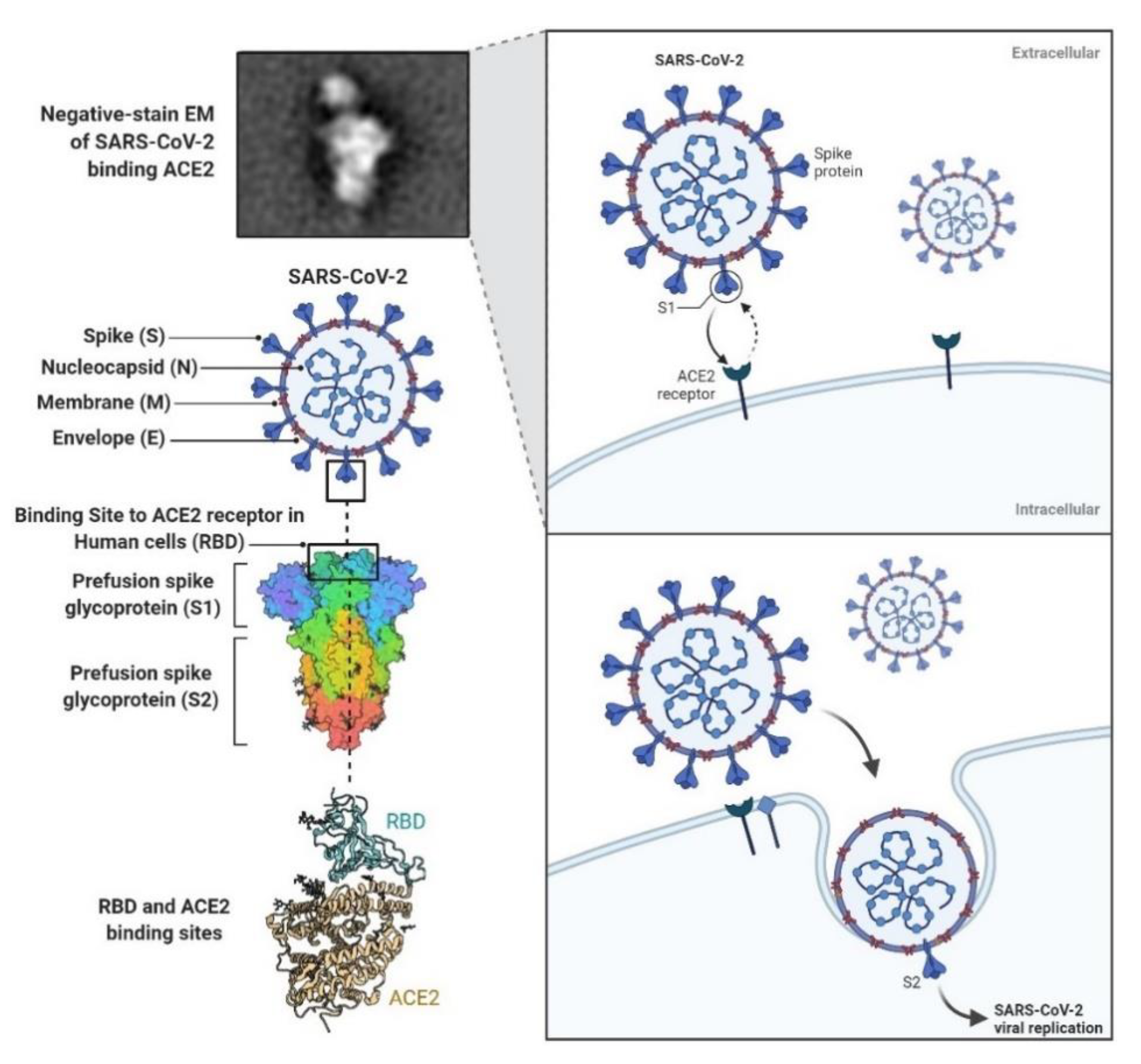
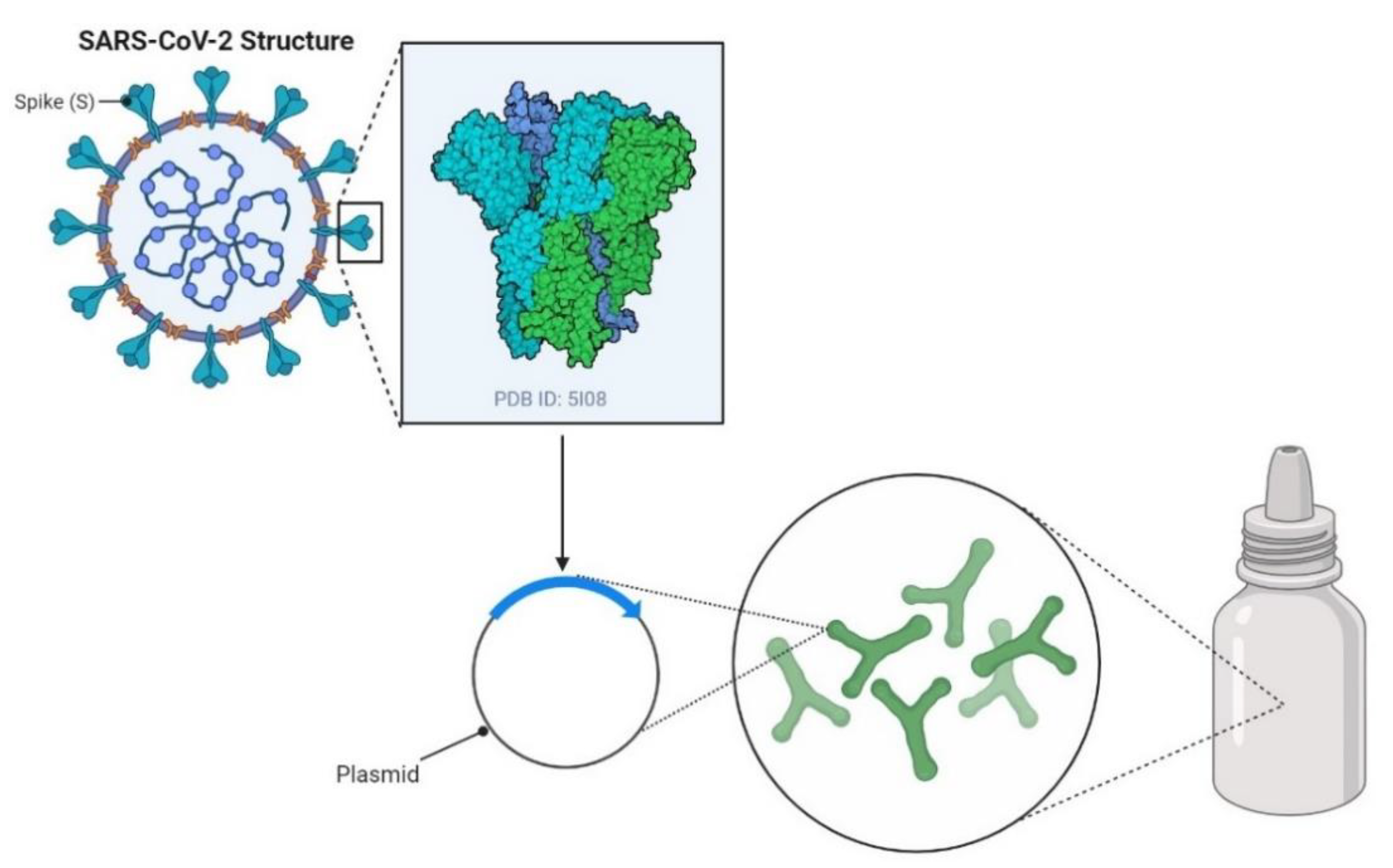
1. Introduction
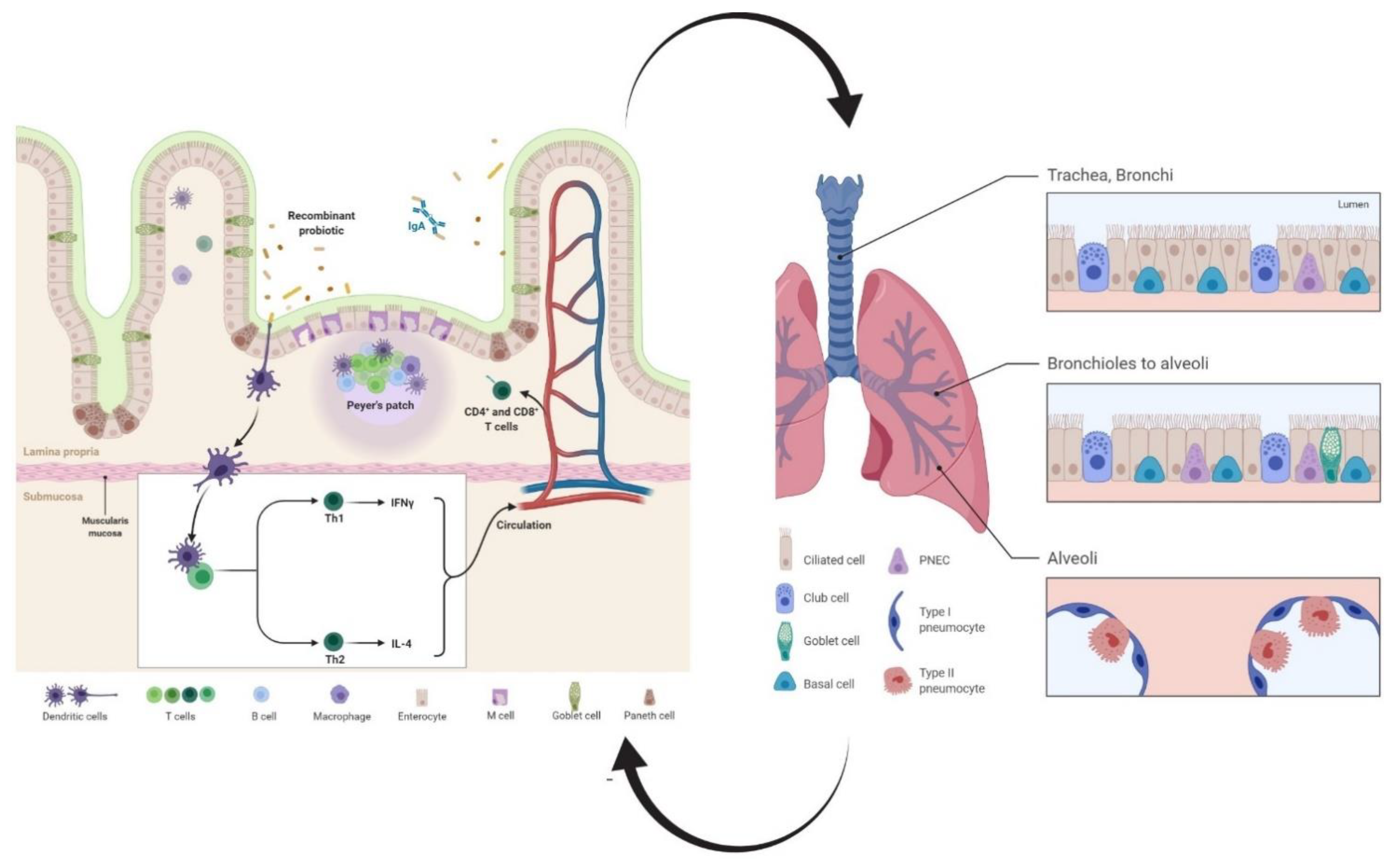
2. Recombinant Probiotics as Inducers of Humoral Immune Responses
3. Recombinant Probiotics as Inducers of Cell-Mediated Immune Responses
3.1. T Helper
3.2. T Killer
3.3. Dendritic Cells (DCs)
4. Optimization of the Immune Response Induced by Recombinant Probiotic-Based Vaccines
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Availability of Data and Material
Code Availability
References
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Chen, L.; Liu, W.; Zhang, Q.; Xu, K.; Ye, G.; Wu, W.; Sun, Z.; Liu, F.; Wu, K.; Zhong, B.; et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg. Microbes Infect. 2020, 9, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, A.H.; Taghinezhad, S.S.; Xu, Z.; Fu, X. Body fluids may contribute to human-to-human transmission of severe acute respiratory syndrome coronavirus 2: Evidence and practical experience. Chin. Med. 2020, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents. 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Tsang, O.T.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.; Cai, J.P.; Chan, J.M.; Chik, T.S.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- Yu, J.; Tostanoski, L.H.; Peter, L.; Mercado, N.B.; McMahan, K.; Mahrokhian, S.H.; Nkolola, J.P.; Liu, J.; Li, Z.; Chandrashekar, A.; et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020, 369, 806–811. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y.; et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, 586, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, A.H.; Taghinezhad-Saroukalaei, S.; Su, B.; Wang, F. Inferring MHC interacting SARS-CoV-2 epitopes recognized by TCRs towards designing T cell-based vaccines. bioRxiv 2020. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Sun, J.; Zhuang, Z.; Zheng, J.; Li, K.; Wong, R.L.; Liu, D.; Huang, J.; He, J.; Zhu, A.; Zhao, J.; et al. Generation of a Broadly Useful Model for COVID-19 Pathogenesis, Vaccination, and Treatment. Cell 2020, 182, 734–743. [Google Scholar] [CrossRef]
- Landete, J.M. A review of food-grade vectors in lactic acid bacteria: From the laboratory to their application. Crit. Rev. Biotechnol. 2017, 37, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, P.P.; Muriana, P.M. A Microplate Growth Inhibition Assay for Screening Bacteriocins against Listeria monocytogenes to Differentiate Their Mode-of-Action. Biomolecules 2015, 5, 1178–1194. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Girones, R.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; Robertson, L.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 7: Suitability of taxonomic units notified to EFSA until September 2017. EFSA J. 2018, 16, e05131. [Google Scholar]
- Mohseni, A.H.; Casolaro, V.; Bermúdez-Humarán, L.G.; Keyvani, H.; Taghinezhad, S.S. Modulation of the PI3K/Akt/mTOR signaling pathway by probiotics as a fruitful target for orchestrating the immune response. Gut. Microbes 2021, 13, 1–17. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Aubry, C.; Cortes-Perez, N.G.; de Moreno de LeBlanc, A.; Vergnolle, N.; Langella, P.; Azevedo, V.; Chatel, J.M.; Miyoshi, A.; Bermúdez-Humarán, L.G. Mucosal targeting of therapeutic molecules using genetically modified lactic acid bacteria: An update. FEMS. Microbiol. Lett. 2013, 344, 1–9. [Google Scholar] [CrossRef]
- Walter, J. Ecological role of lactobacilli in the gastrointestinal tract: Implications for fundamental and biomedical research. Appl. Environ. Microbiol. 2008, 74, 4985–4996. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, Q.; Gao, J.; Yang, Q. Mucosal and systemic immune responses induced by recombinant Lactobacillus spp. expressing the hemagglutinin of the avian influenza virus H5N1. Clin. Vaccine Immunol. 2012, 19, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Li, G.; Wang, X.; Li, X.; Liu, M.; Li, Y. Recombinant porcine rotavirus VP4 and VP4-LTB expressed in Lactobacillus casei induced mucosal and systemic antibody responses in mice. BMC Microbiol. 2009, 9, 1471–2180. [Google Scholar] [CrossRef] [PubMed]
- Engchanil, C. Construction of the Recombinant Probiotic Lactobacillus casei and Lactobacillus fermentum Expressing the Codon-Optimized M2e: HBc Fusion Gene. J. Med. Assoc. Thail. 2016, 99, S9–S18. [Google Scholar]
- Peirotén, Á.; Landete, J.M. Natural and engineered promoters for gene expression in Lactobacillus species. Appl. Microbiol. Biotechnol. 2020, 104, 3797–3805. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.L.; Liu, M.; Ge, J.W.; Qiao, X.Y.; Li, Y.J.; Liu, D.Q. Expression of infectious pancreatic necrosis virus (IPNV) VP2-VP3 fusion protein in Lactobacillus casei and immunogenicity in rainbow trouts. Vaccine 2012, 30, 1823–1829. [Google Scholar]
- Mohseni, A.H.; Taghinezhad, S.S.; Keyvani, H. The First Clinical Use of a Recombinant Lactococcus lactis Expressing Human Papillomavirus Type 16 E7 Oncogene Oral Vaccine: A Phase I Safety and Immunogenicity Trial in Healthy Women Volunteers. Mol. Cancer Ther. 2020, 19, 717–727. [Google Scholar] [CrossRef]
- Shi, S.H.; Yang, W.T.; Yang, G.L.; Cong, Y.L.; Huang, H.B.; Wang, Q.; Cai, R.P.; Ye, L.P.; Hu, J.T.; Zhou, J.Y.; et al. Immunoprotection against influenza virus H9N2 by the oral administration of recombinant Lactobacillus plantarumNC8 expressing hemagglutinin in BALB/c mice. Virology 2014, 465, 166–176. [Google Scholar] [CrossRef]
- Jee, P.F.; Tiong, V.; Shu, M.H.; Khoo, J.J.; Wong, W.F.; Abdul Rahim, R.; AbuBakar, S.; Chang, L.Y. Oral immunization of a non-recombinant Lactococcus lactis surface displaying influenza hemagglutinin 1 (HA1) induces mucosal immunity in mice. PLoS ONE 2017, 12, e0187718. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Poo, H.; Han, D.P.; Hong, S.P.; Kim, K.; Cho, M.W.; Kim, E.; Sung, M.H.; Kim, C.J. Mucosal immunization with surface-displayed severe acute respiratory syndrome coronavirus spike protein on Lactobacillus casei induces neutralizing antibodies in mice. J. Virol. 2006, 80, 4079–4087. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, J.; Guo, Y.; Yang, W.; Ye, L.; Shi, C.; Liu, Y.; Yang, G.; Wang, C. Construction and immunological evaluation of recombinant Lactobacillus plantarum expressing HN of Newcastle disease virus and DC-targeting peptide fusion protein. J. Biotechnol. 2015, 216, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, A.H.; Razavilar, V.; Keyvani, H.; Razavi, M.R.; Khavari-Nejad, R.A. Oral immunization with recombinant Lactococcus lactis NZ9000 expressing human papillomavirus type 16 E7 antigen and evaluation of its immune effects in female C57BL/6 mice. J. Med. Virol. 2019, 91, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Yang, G.L.; Wang, Q.; Huang, H.B.; Jiang, Y.L.; Shi, C.W.; Wang, J.Z.; Huang, K.Y.; Jin, Y.B.; Wang, C.F. Protective efficacy of Fc targeting conserved influenza virus M2e antigen expressed by Lactobacillus plantarum. Antivir. Res. 2017, 138, 9–21. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yang, W.T.; Shi, S.H.; Li, Y.J.; Zhao, L.; Shi, C.W.; Zhou, F.Y.; Jiang, Y.L.; Hu, J.T.; Gu, W.; et al. Immunogenicity of recombinant Lactobacillus plantarum NC8 expressing goose parvovirus VP2 gene in BALB/c mice. J. Vet. Sci. 2017, 18, 159–167. [Google Scholar] [CrossRef]
- Yang, W.T.; Yang, G.L.; Zhao, L.; Jin, Y.B.; Jiang, Y.L.; Huang, H.B.; Shi, C.W.; Wang, J.Z.; Wang, G.; Kang, Y.H.; et al. Lactobacillus plantarum displaying conserved M2e and HA2 fusion antigens induces protection against influenza virus challenge. Appl. Microbiol. Biotechnol. 2018, 102, 5077–5088. [Google Scholar] [CrossRef]
- Yang, W.T.; Shi, S.H.; Yang, G.L.; Jiang, Y.L.; Zhao, L.; Li, Y.; Wang, C.F. Cross-protective efficacy of dendritic cells targeting conserved influenza virus antigen expressed by Lactobacillus plantarum. Sci. Rep. 2016, 6, 39665. [Google Scholar] [CrossRef]
- Chowdhury, M.Y.; Li, R.; Kim, J.H.; Park, M.E.; Kim, T.H.; Pathinayake, P.; Weeratunga, P.; Song, M.K.; Son, H.Y.; Hong, S.P.; et al. Mucosal vaccination with recombinant Lactobacillus casei-displayed CTA1-conjugated consensus matrix protein-2 (sM2) induces broad protection against divergent influenza subtypes in BALB/c mice. PLoS ONE 2014, 9, e94051. [Google Scholar] [CrossRef]
- Li, R.; Chowdhury, M.Y.; Kim, J.H.; Kim, T.H.; Pathinayake, P.; Koo, W.S.; Park, M.E.; Yoon, J.E.; Roh, J.B.; Hong, S.P.; et al. Mucosally administered Lactobacillus surface-displayed influenza antigens (sM2 and HA2) with cholera toxin subunit A1 (CTA1) Induce broadly protective immune responses against divergent influenza subtypes. Vet. Microbiol. 2015, 179, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Hou, X.L.; Yu, L.Y.; Liu, J.K.; Wei, C.H. Studies on Mucosal Immunity Induced by Transmissible Gastroenteritis Virus Nucleocapsid Protein Recombinant Lactobacillus casei in Mice and Sow. Agric. Sci. China 2009, 8, 231–237. [Google Scholar] [CrossRef]
- Yoon, S.W.; Lee, T.Y.; Kim, S.J.; Lee, I.H.; Sung, M.H.; Park, J.S.; Poo, H. Oral administration of HPV-16 L2 displayed on Lactobacillus casei induces systematic and mucosal cross-neutralizing effects in Balb/c mice. Vaccine 2012, 30, 3286–3294. [Google Scholar] [CrossRef]
- Jiang, X.; Hou, X.; Tang, L.; Jiang, Y.; Ma, G.; Li, Y. A phase trial of the oral Lactobacillus casei vaccine polarizes Th2 cell immunity against transmissible gastroenteritis coronavirus infection. Appl. Microbiol. Biotechnol. 2016, 100, 7457–7469. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, L.; Tian, C.; Zhang, G.; Huo, G.; Tang, L.; Li, Y. Immunogenicity of recombinant classic swine fever virus CD8(+) T lymphocyte epitope and porcine parvovirus VP2 antigen coexpressed by Lactobacillus casei in swine via oral vaccination. Clin. Vaccine Immunol. 2011, 18, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.; Hua, X.; Wang, Y.; Chen, Y.; Shi, W.; Tang, L.; Li, Y.; Liu, M. Oral immunization with a recombinant Lactobacillus expressing CK6 fused with VP2 protein against IPNV in rainbow trout (Oncorhynchus mykiss). Fish Shellfish. Immunol. 2018, 83, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Ouh, Y.T.; Sung, M.H.; Park, H.G.; Kim, T.J.; Cho, C.H.; Park, J.S.; Lee, J.K. A phase 1/2a, dose-escalation, safety and preliminary efficacy study of oral therapeutic vaccine in subjects with cervical intraepithelial neoplasia 3. J. Gynecol. Oncol. 2019, 30, e88. [Google Scholar] [CrossRef]
- Taghinezhad, S.S.; Mohseni, A.H.; Keyvani, H.; Razavi, M.R. Phase 1 Safety and Immunogenicity Trial of Recombinant Lactococcus lactis Expressing Human Papillomavirus Type 16 E6 Oncoprotein Vaccine. Mol. Ther. Methods Clin. Dev. 2019, 15, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, A.; Zhang, L.; Long, J.; Nordone, S.; Stoeker, L.; LaVoy, A.; Bumgardner, S.; Klaenhammer, T.; Dean, G. Construction and immunological evaluation of dual cell surface display of HIV-1 gag and Salmonella enterica serovar Typhimurium FliC in Lactobacillus acidophilus for vaccine delivery. Clin. Vaccine Immunol. 2012, 19, 1374–1381. [Google Scholar] [CrossRef]
- Hanniffy, S.B.; Carter, A.T.; Hitchin, E.; Wells, J.M. Mucosal delivery of a pneumococcal vaccine using Lactococcus lactis affords protection against respiratory infection. J. Infect. Dis. 2007, 195, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Q.; Ge, J.W.; Qiao, X.Y.; Jiang, Y.P.; Liu, S.M.; Li, Y.J. High-level mucosal and systemic immune responses induced by oral administration with Lactobacillus-expressed porcine epidemic diarrhea virus (PEDV) S1 region combined with Lactobacillus-expressed N protein. Appl. Microbiol. Biotechnol. 2012, 93, 2437–2446. [Google Scholar]
- Wang, Z.; Gao, J.; Yu, Q.; Yang, Q. Oral immunization with recombinant Lactococcus lactis expressing the hemagglutinin of the avian influenza virus induces mucosal and systemic immune responses. Future Microbiol. 2012, 7, 1003–1010. [Google Scholar] [CrossRef]
- Lei, H.; Sheng, Z.; Ding, Q.; Chen, J.; Wei, X.; Lam, D.M.; Xu, Y. Evaluation of oral immunization with recombinant avian influenza virus HA1 displayed on the Lactococcus lactis surface and combined with the mucosal adjuvant cholera toxin subunit B. Clin. Vaccine Immunol. 2011, 18, 1046–1051. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, G.; Wang, Q.; Wang, Z.; Yang, W.; Gu, W.; Shi, C.; Wang, J.; Huang, H.; Wang, C. Molecular mechanisms underlying protection against H9N2 influenza virus challenge in mice by recombinant Lactobacillus plantarum with surface displayed HA2-LTB. J. Biotechnol. 2017, 259, 6–14. [Google Scholar] [CrossRef]
- Gao, S.; Li, D.; Liu, Y.; Zha, E.; Zhou, T.; Yue, X. Oral immunization with recombinant hepatitis E virus antigen displayed on the Lactococcus lactis surface enhances ORF2-specific mucosal and systemic immune responses in mice. Int. Immunopharmacol. 2015, 24, 140–145. [Google Scholar] [CrossRef]
- Taghinezhad, S.S.; Mohseni, A.H.; Keyvani, H.; Razavilar, V. Protection against human papillomavirus type 16-induced tumors in C57BL/6 mice by mucosal vaccination with Lactococcus lactis NZ9000 expressing E6 oncoprotein. Microb. Pathog. 2019, 126, 149–156. [Google Scholar] [CrossRef]
- Kawana, K.; Adachi, K.; Kojima, S.; Taguchi, A.; Tomio, K.; Yamashita, A.; Nishida, H.; Nagasaka, K.; Arimoto, T.; Yokoyama, T.; et al. Oral vaccination against HPV E7 for treatment of cervical intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific mucosal immunity in the cervix of CIN3 patients. Vaccine 2014, 32, 6233–6239. [Google Scholar] [CrossRef]
- Sim, A.C.; Lin, W.; Tan, G.K.; Sim, M.S.; Chow, V.T.; Alonso, S. Induction of neutralizing antibodies against dengue virus type 2 upon mucosal administration of a recombinant Lactococcus lactis strain expressing envelope domain III antigen. Vaccine 2008, 26, 1145–1154. [Google Scholar] [CrossRef]
- Xin, K.Q.; Hoshino, Y.; Toda, Y.; Igimi, S.; Kojima, Y.; Jounai, N.; Ohba, K.; Kushiro, A.; Kiwaki, M.; Hamajima, K.; et al. Immunogenicity and protective efficacy of orally administered recombinant Lactococcus lactis expressing surface-bound HIV Env. Blood 2003, 102, 223–228. [Google Scholar] [CrossRef]
- Wang, M.; Fu, T.; Hao, J.; Li, L.; Tian, M.; Jin, N.; Ren, L.; Li, C. A recombinant Lactobacillus plantarum strain expressing the spike protein of SARS-CoV-2. Int. J. Biol. Macromol. 2020, 160, 736–740. [Google Scholar] [CrossRef]
- Shi, S.H.; Yang, W.T.; Yang, G.L.; Zhang, X.K.; Liu, Y.Y.; Zhang, L.J.; Ye, L.P.; Hu, J.T.; Xing, X.; Qi, C.; et al. Lactobacillus plantarum vaccine vector expressing hemagglutinin provides protection against H9N2 challenge infection. Virus. Res. 2016, 211, 46–57. [Google Scholar] [CrossRef]
- Komatsu, A.; Igimi, S.; Kawana, K. Optimization of human papillomavirus (HPV) type 16 E7-expressing lactobacillus-based vaccine for induction of mucosal E7-specific IFNγ-producing cells. Vaccine 2018, 36, 3423–3426. [Google Scholar] [CrossRef]
- Temprana, C.F.; Argüelles, M.H.; Gutierrez, N.M.; Barril, P.A.; Esteban, L.E.; Silvestre, D.; Mandile, M.G.; Glikmann, G.; Castello, A.A. Rotavirus VP6 protein mucosally delivered by cell wall-derived particles from Lactococcus lactis induces protection against infection in a murine model. PLoS ONE 2018, 13, e0203700. [Google Scholar] [CrossRef] [PubMed]
- Joan, S.S.; Pui-Fong, J.; Song, A.A.; Chang, L.Y.; Yusoff, K.; AbuBakar, S.; Rahim, R.A. Oral vaccine of Lactococcus lactis harbouring pandemic H1N1 2009 haemagglutinin1 and nisP anchor fusion protein elevates anti-HA1 sIgA levels in mice. Biotechnol. Lett. 2016, 38, 793–799. [Google Scholar] [CrossRef]
- Tang, L.; Li, Y. Oral immunization of mice with recombinant Lactococcus lactis expressing porcine transmissible gastroenteritis virus spike glycoprotein. Virus Genes 2009, 39, 238–245. [Google Scholar] [CrossRef]
- Cui, L.C.; Guan, X.T.; Liu, Z.M.; Tian, C.Y.; Xu, Y.G. Recombinant lactobacillus expressing G protein of spring viremia of carp virus (SVCV) combined with ORF81 protein of koi herpesvirus (KHV): A promising way to induce protective immunity against SVCV and KHV infection in cyprinid fish via oral vaccination. Vaccine 2015, 33, 3092–3099. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Hultberg, A.; Zhao, Y.; Svensson, L.; Pan-Hammarstrom, Q.; Johansen, K.; Pouwels, P.H.; Ruggeri, F.M.; Hermans, P.; Frenken, L.; et al. Lactobacilli expressing variable domain of llama heavy-chain antibody fragments (lactobodies) confer protection against rotavirus-induced diarrhea. J. Infect. Dis. 2006, 194, 1580–1588. [Google Scholar] [CrossRef]
- Li, Y.J.; Ma, G.P.; Li, G.W.; Qiao, X.Y.; Ge, J.W.; Tang, L.J.; Liu, M.; Liu, L.W. Oral vaccination with the porcine rotavirus VP4 outer capsid protein expressed by Lactococcus lactis induces specific antibody production. J. Biomed. Biotechnol. 2010, 6, 708460. [Google Scholar]
- Li, Y.; Li, X.; Liu, H.; Zhuang, S.; Yang, J.; Zhang, F. Intranasal immunization with recombinant Lactococci carrying human papillomavirus E7 protein and mouse interleukin-12 DNA induces E7-specific antitumor effects in C57BL/6 mice. Oncol. Lett. 2014, 7, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Reese, K.A.; Lupfer, C.; Johnson, R.C.; Mitev, G.M.; Mullen, V.M.; Geller, B.L.; Pastey, M. A Novel Lactococcal Vaccine Expressing a Peptide from the M2 Antigen of H5N2 Highly Pathogenic Avian Influenza A Virus Prolongs Survival of Vaccinated Chickens. Vet. Med. Int. 2013, 22, 316926. [Google Scholar] [CrossRef]
- Di-Qiu, L.; Xin-Yuan, Q.; Jun-Wei, G.; Li-Jie, T.; Yan-Ping, J.; Yi-Jing, L. Construction and characterization of Lactobacillus pentosus expressing the D antigenic site of the spike protein of Transmissible gastroenteritis virus. Can. J. Microbiol. 2011, 57, 392–397. [Google Scholar] [CrossRef]
- Asahi-Ozaki, Y.; Yoshikawa, T.; Iwakura, Y.; Suzuki, Y.; Tamura, S.; Kurata, T.; Sata, T. Secretory IgA antibodies provide cross-protection against infection with different strains of influenza B virus. J. Med. Virol. 2004, 74, 328–335. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Kunitoh-Asari, A.; Hayakawa, K.; Imai, S.; Kasuya, K.; Abe, K.; Adachi, Y.; Fukudome, S.; Takahashi, Y.; Hachimura, S. Oral administration of Lactobacillus plantarum strain AYA enhances IgA secretion and provides survival protection against influenza virus infection in mice. PLoS ONE 2014, 9, e86416. [Google Scholar] [CrossRef]
- Renegar, K.B.; Small, P.A., Jr.; Boykins, L.G.; Wright, P.F. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J. Immunol. 2004, 173, 1978–1986. [Google Scholar] [CrossRef]
- LeCureux, J.S.; Dean, G.A. Lactobacillus Mucosal Vaccine Vectors: Immune Responses against Bacterial and Viral Antigens. mSphere 2018, 3, e00061-18. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Yang, Q.; Deng, H.; Liu, Y.; Zhou, P.; Xu, H.; Chen, D.; Feng, D.; Zhang, H.; et al. Critical Role of Intestinal Microbiota in ATF3-Mediated Gut Immune Homeostasis. J. Immunol. 2020, 205, 842–852. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Kharrat, P.; Chatel, J.M.; Langella, P. Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA vaccines. Microb. Cell Fact. 2011, 1, 1475–2859. [Google Scholar] [CrossRef]
- Xu, R.; Jia, H.; Terkawi, M.A.; Xuan, X.; Zhang, H. Immunogenicity of orally administrated recombinant Lactobacillus casei Zhang expressing Cryptosporidium parvum surface adhesion protein P23 in mice. Curr. Microbiol. 2011, 62, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Ohkouchi, K.; Kawamoto, S.; Tatsugawa, K.; Yoshikawa, N.; Takaoka, Y.; Miyauchi, S.; Aki, T.; Yamashita, M.; Murooka, Y.; Ono, K. Prophylactic effect of Lactobacillus oral vaccine expressing a Japanese cedar pollen allergen. J. Biosci. Bioeng. 2012, 113, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Peng, X.; Jiao, H.; Zhao, D.; Ouyang, J. Broadly protective immunity against divergent influenza viruses by oral co-administration of Lactococcus lactis expressing nucleoprotein adjuvanted with cholera toxin B subunit in mice. Microb. Cell. Fact. 2015, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Peng, X.; Zhao, D.; Ouyang, J.; Jiao, H.; Shu, H.; Ge, X. Lactococcus lactis displayed neuraminidase confers cross protective immunity against influenza A viruses in mice. Virology 2015, 476, 189–195. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Taghinezhad, S.S.; Keyvani, H.; Razavilar, V. Extracellular overproduction of E7 oncoprotein of Iranian human papillomavirus type 16 by genetically engineered Lactococcus lactis. BMC Biotechnol. 2019, 19, 019–0499. [Google Scholar] [CrossRef] [PubMed]
- Taghinezhad-Saroukalaei, S.; Keyvani, H.; Bermúdez-Humarán, L.G.; Donders, G.G.; Fu, X.; Mohseni, A.H. Twenty years of research on HPV vaccines based on genetically modified lactic acid bacteria: An overview on the gut-vagina axis. Cell Mol. Life Sci. 2020, 78, 1191–1206. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Rowland, I.; Yaqoob, P. Comparative effects of six probiotic strains on immune function in vitro. Br. J. Nutr. 2012, 108, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, K.; Dhodapkar, K.M.; Dhodapkar, M.V. Targeting human dendritic cells in situ to improve vaccines. Immunol. Lett. 2014, 162, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.B.; Yang, W.T.; Shi, C.W.; Feng, B.; Huang, K.Y.; Zhao, G.X.; Li, Q.Y.; Xie, J.; Huang, H.B.; Jiang, Y.L.; et al. Immune responses induced by recombinant Lactobacillus plantarum expressing the spike protein derived from transmissible gastroenteritis virus in piglets. Appl. Microbiol. Biotechnol. 2018, 102, 8403–8417. [Google Scholar] [CrossRef] [PubMed]
- Kathania, M.; Zadeh, M.; Lightfoot, Y.L.; Roman, R.M.; Sahay, B.; Abbott, J.R.; Mohamadzadeh, M. Colonic immune stimulation by targeted oral vaccine. PLoS ONE 2013, 8, e55143. [Google Scholar] [CrossRef]
- Mierau, I.; Kleerebezem, M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl. Microbiol. Biotechnol. 2005, 68, 705–717. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; Angulo, C.; Meza, B. Food-Grade Organisms as Vaccine Biofactories and Oral Delivery Vehicles. Trends Biotechnol. 2016, 34, 124–136. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Razavilar, V.; Keyvani, H.; Razavi, M.R.; Khavari-Nejad, R.A. Efficient production and optimization of E7 oncoprotein from Iranian human papillomavirus type 16 in Lactococcus lactis using nisin-controlled gene expression (NICE) system. Microb. Pathog. 2017, 110, 554–560. [Google Scholar] [CrossRef]
- Taghinezhad-Saroukalaei, S.; Razavilar, V.; Keyvani, H.; Razavi, M.R.; Nejadsattari, T. Extracellular overproduction of recombinant Iranian HPV-16 E6 oncoprotein in Lactococcus lactis using the NICE system. Future Virol. 2018, 13, 697–710. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Razavilar, V.; Keyvani, H.; Razavi, M.R.; Khavari Nejad, R.A. Codon Usage Optimization and Construction of Plasmid Encoding Iranian Human Papillomavirus Type 16 E7 Oncogene for Lactococcus Lactis Subsp. Cremoris MG1363. Asian. Pac. J. Cancer Prev. 2017, 18, 783–788. [Google Scholar]
- Suebwongsa, N.; Panya, M.; Namwat, W.; Sookprasert, S.; Redruello, B.; Mayo, B.; Alvarez, M.A.; Lulitanond, V. Cloning and expression of a codon-optimized gene encoding the influenza A virus nucleocapsid protein in Lactobacillus casei. Int. Microbiol. 2013, 16, 93–101. [Google Scholar]
- Taghinezhad-Saroukalaei, S.; Razavilar, V.; Keyvani, H.; Razavi, M.R.; Nejadsattari, T. Codon optimization of Iranian human papillomavirus Type 16 E6 oncogene for Lactococcus lactis subsp. cremoris MG1363. Future Virol. 2017, 12, 499–511. [Google Scholar] [CrossRef]
- Fuglsang, A. Lactic acid bacteria as prime candidates for codon optimization. Biochem. Biophys. Res. Commun. 2003, 312, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Kawana, K.; Yokoyama, T.; Adachi, K.; Yamashita, A.; Tomio, K.; Kojima, S.; Oda, K.; Fujii, T.; Kozuma, S. Adjuvant effect of Japanese herbal medicines on the mucosal type 1 immune responses to human papillomavirus (HPV) E7 in mice immunized orally with Lactobacillus-based therapeutic HPV vaccine in a synergistic manner. Vaccine 2012, 30, 5368–5372. [Google Scholar] [CrossRef]
- van Roosmalen, M.L.; Kanninga, R.; El Khattabi, M.; Neef, J.; Audouy, S.; Bosma, T.; Kuipers, A.; Post, E.; Steen, A.; Kok, J.; et al. Mucosal vaccine delivery of antigens tightly bound to an adjuvant particle made from food-grade bacteria. Methods 2006, 38, 144–149. [Google Scholar] [CrossRef]
- Ramirez, K.; Ditamo, Y.; Rodriguez, L.; Picking, W.L.; van Roosmalen, M.L.; Leenhouts, K.; Pasetti, M.F. Neonatal mucosal immunization with a non-living, non-genetically modified Lactococcus lactis vaccine carrier induces systemic and local Th1-type immunity and protects against lethal bacterial infection. Mucosal. Immunol. 2010, 3, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Mohamadzadeh, M.; Duong, T.; Sandwick, S.J.; Hoover, T.; Klaenhammer, T.R. Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proc. Natl. Acad. Sci. USA 2009, 106, 4331–4336. [Google Scholar] [CrossRef] [PubMed]
- Zhen-guo, W.; Ning-yi, J.; Ming-xiao, M.; Dong-liang, F.; Min, Z.; Ge-fen, Y.; Lei-li, J.; Kuo-shi, J.; Zhi-ping, X.; Ming-lan, J. Immunogenicity of Recombinant Fowl-pox Vaccines coexpressing HA of AIV H5N1, H7N1 and chicken IL-18. Virol. Sin. 2005, 20, 607–612. [Google Scholar]
- Seegers, J.F. Lactobacilli as live vaccine delivery vectors: Progress and prospects. Trends Biotechnol. 2002, 20, 508–515. [Google Scholar] [CrossRef]
- Cai, R.; Jiang, Y.; Yang, W.; Shi, S.; Shi, C.; Hu, J.; Gu, W.; Ye, L.; Zhou, F.; Gong, Q.; et al. Surface-Displayed IL-10 by Recombinant Lactobacillus plantarum Reduces Th1 Responses of RAW264.7 Cells Stimulated with Poly(I:C) or LPS. J. Microbiol. Biotechnol. 2016, 26, 421–431. [Google Scholar] [CrossRef]
- Mbow, M.L.; De Gregorio, E.; Valiante, N.M.; Rappuoli, R. New adjuvants for human vaccines. Curr. Opin. Immunol. 2010, 22, 411–416. [Google Scholar] [CrossRef]
- Davidson, L.E.; Fiorino, A.M.; Snydman, D.R.; Hibberd, P.L. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: A randomized double-blind placebo-controlled trial. Eur. J. Clin. Nutr. 2011, 65, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Plant, L.J.; Conway, P.L. Adjuvant properties and colonization potential of adhering and non-adhering Lactobacillus spp following oral administration to mice. FEMS Immunol. Med. Microbiol. 2002, 34, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Petrarca, C.; Carpiniello, F.; DiGioacchino, M. Recombinant Probiotics for Allergen Immunotherapy. J. Vaccines Vaccin. 2015, 1. [Google Scholar] [CrossRef]
- Enomoto, M.; Noguchi, S.; Hattori, M.; Sugiyama, H.; Suzuki, Y.; Hanaoka, A.; Okada, S.; Yoshida, T. Oral administration of Lactobacillus plantarum NRIC0380 suppresses IgE production and induces CD4(+)CD25(+)Foxp3(+) cells in vivo. Biosci. Biotechnol. Biochem. 2009, 73, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Immunization without Needles. Nat. Rev. Immunol. 2005, 5, 905–916. [Google Scholar] [CrossRef]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef]
- Ellebedy, A.H.; Ducatez, M.F.; Duan, S.; Stigger-Rosser, E.; Rubrum, A.M.; Govorkova, E.A.; Webster, R.G.; Webby, R.J. Impact of prior seasonal influenza vaccination and infection on pandemic A (H1N1) influenza virus replication in ferrets. Vaccine 2011, 29, 3335–3339. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.A.; Zielen, S.; Baumann, U. Mucosal immunity and nasal influenza vaccination. Expert Rev. Vaccines 2012, 11, 595–607. [Google Scholar] [CrossRef]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef]
- Sundararaman, A.; Ray, M.; Ravindra, P.V.; Halami, P.M. Role of probiotics to combat viral infections with emphasis on COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 8089–8104. [Google Scholar] [CrossRef]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Vitiñi, E.; Alvarez, S.; Medina, M.; Medici, M.; de Budeguer, M.V.; Perdigón, G. Gut mucosal immunostimulation by lactic acid bacteria. Biocell 2000, 24, 223–232. [Google Scholar] [PubMed]
- Dieye, Y.; Usai, S.; Clier, F.; Gruss, A.; Piard, J.C. Design of a protein-targeting system for lactic acid bacteria. J. Bacteriol. 2001, 183, 4157–4166. [Google Scholar] [CrossRef] [PubMed]
- Michon, C.; Langella, P.; Eijsink, V.G.; Mathiesen, G.; Chatel, J.M. Display of recombinant proteins at the surface of lactic acid bacteria: Strategies and applications. Microb. Cell Fact. 2016, 15, 70. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Cortes-Perez, N.G.; Lefèvre, F.; Guimarães, V.; Rabot, S.; Alcocer-Gonzalez, J.M.; Gratadoux, J.J.; Rodriguez-Padilla, C.; Tamez-Guerra, R.S.; Corthier, G.; et al. A novel mucosal vaccine based on live Lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J. Immunol. 2005, 175, 7297–7302. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.; Roussel, Y.; Kleerebezem, M.; Pot, B. Recombinant lactic acid bacteria as mucosal biotherapeutic agents. Trends Biotechnol. 2011, 29, 499–508. [Google Scholar] [CrossRef]
- Dicks, L.M.; Botes, M. Probiotic lactic acid bacteria in the gastro-intestinal tract: Health benefits, safety and mode of action. Benef. Microbes. 2010, 1, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Hugentobler, F.; Yam, K.K.; Gillard, J.; Mahbuba, R.; Olivier, M.; Cousineau, B. Immunization against Leishmania major infection using LACK- and IL-12-expressing Lactococcus lactis induces delay in footpad swelling. PLoS ONE 2012, 7, e30945. [Google Scholar] [CrossRef][Green Version]
- Bahey-El-Din, M. Lactococcus lactis-based vaccines from laboratory bench to human use: An overview. Vaccine 2012, 30, 685–690. [Google Scholar] [CrossRef] [PubMed]
- del Rio, B.; Dattwyler, R.J.; Aroso, M.; Neves, V.; Meirelles, L.; Seegers, J.F.; Gomes-Solecki, M. Oral immunization with recombinant lactobacillus plantarum induces a protective immune response in mice with Lyme disease. Clin. Vaccine Immunol. 2008, 15, 1429–1435. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adachi, K.; Kawana, K.; Yokoyama, T.; Fujii, T.; Tomio, A.; Miura, S.; Tomio, K.; Kojima, S.; Oda, K.; Sewaki, T.; et al. Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine 2010, 28, 2810–2817. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-N.; Youn, H.-N.; Kwon, J.-H.; Lee, D.-H.; Park, J.-K.; Yuk, S.-S.; Erdene-Ochir, T.-O.; Kim, K.-T.; Lee, J.-B.; Park, S.-Y.; et al. Sublingual administration of Lactobacillus rhamnosus affects respiratory immune responses and facilitates protection against influenza virus infection in mice. Antivir. Res. 2013, 98, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Azegami, T.; Yuki, Y.; Hayashi, K.; Hishikawa, A.; Sawada, S.I.; Ishige, K.; Akiyoshi, K.; Kiyono, H.; Itoh, H. Intranasal vaccination against angiotensin II type 1 receptor and pneumococcal surface protein A attenuates hypertension and pneumococcal infection in rodents. J. Hypertens. 2018, 36, 387–394. [Google Scholar] [CrossRef] [PubMed]
| Probiotic | Virus | Host/Inoculation Route | Pathways of Immune System Induction | Number | Dosage | Reference |
|---|---|---|---|---|---|---|
| L. acidophilus | Avian influenza virus H5N1 | Mouse/Oral | Induction of anti-HA1 IgA antibody, anti-HA1 IgG, lymphocyte proliferative reaction, and IL-4 | 6 times | 1 × 1010 CFU/mL | [22] |
| L. delbrueckiisubsp. lactis | Avian influenza virus H5N1 | Mouse/Oral | Induction of anti-HA1 IgA antibody, anti-HA1 IgG, lymphocyte proliferative reaction, and IL-4 | 6 times | 1 × 1010 CFU/mL | |
| L. casei | Porcine rotavirus | Mouse/Oral | Induction of serum IgG and mucosal IgA | 9 times | 1 × 109 CFU/mL | [23] |
| Infectious pancreatic necrosis virus (IPNV) | Rainbow trouts/Oral | Induction of specific IgM anti-pIPNV, and reduction of viral loads | 2 times | 5 × 108 pfu/200 µL | [26] | |
| L. lactis | Human papillomavirus type 16 (HPV-16) | Healthy women/Oral | Induction of E7-specific IgG and SIgA antibody and, E7-specific IFN-γ-secreting CD8+ T cell immune response | 20 times | 1 × 109, 5 × 109, and 1 × 1010 CFU/mL | [27] |
| L. plantarum | Influenza virus H9N2 | Mouse/Oral | Induction of IgG, sIgA, HI antibodies, and CD8+ T cell immune response | 7 times | 1 × 109 CFU/mouse | [28] |
| L. lactis | Influenza virus H1N1 | Mouse/Oral | Induction of specific serum IgG and IgA, and sIgA | 9 times | 1 × 1010 and 5 × 1010 CFU/mL | [29] |
| L. casei | Severe acute respiratory syndrome (SARS) | Mouse/Oral and nasal | Induction of serum IgG and mucosal IgA | For oral: 20 times For nasal: 8 times | For oral: 5 × 109 cells/100 µL For nasal: 2 × 109 cells/20 µL | [30] |
| L. plantarum | Newcastle disease virus (NDV) | Chicken/Oral | Induction of sIgA, CD3+CD4+T, T lymphocytes proliferation and increasing survival rates | 9 times | 109 CFU/0.2 mL | [31] |
| L. lactis | Human papillomavirus type 16 (HPV-16) | Mouse/Oral | Induction of E7-specific antibody and E7-specific CD4+ Th and CD8+ T cell precursors, specific IL-2- and IFN-γ-secreting T cells | 9 times | 1 × 108, 1 × 109, and 1 × 1010 CFU/mL | [32] |
| L. plantarum | Influenza A virus H1N1 | Mouse/Oral | Induction of Peyer’s patch (PP) DC, PP B220+IgA+, sIgA, growth centers (GCs) in PPs, T immune response, CD8+IFN-γ+ cells, and reduction viral load | 6 times | - | [33] |
| Goose parvovirus (GPV) | Mouse/Oral | Induction of CD11c+, CD3+CD4+, CD3+CD8+, IFN-γ+ and TNF-α, and sIgA | 14 times | 2 × 109 CFU/mL | [34] | |
| Avian influenza virus | Chicks/Oral | Induction of specific humoral, mucosal, and T cell-mediated immune responses, and reduction viral load | 6 times | 2 × 109 CFU/300 μL | [35] | |
| Avian influenza virus H9N2 | Mouse/Oral | Induction of specific mucosal antibody responses and B and T cell responses, specific CD8 T cells, and antigen specific cytotoxicity | 6 times | 1 × 109 CFU/mouse | [36] | |
| L. casei | Mouse/Oral and nasal | Induction of serum IgG, mucosal IgA, and cell-mediated immune response | For oral: 10 times For nasal: 8 times | For oral: 1 × 1010 CFU/100 µL For nasal: 1 × 109 CFU/20 µL | [37] | |
| Influenza A viruses | Mouse/Oral and nasal | Induction of serum IgG and their isotypes (IgG1 & IgG2a), mucosal IgA, sM2- or HA2-specific cell-mediated immunity, IFN-g, and IL-4 | For oral: 8 times For nasal: 6 times | For oral: 1 × 1010 CFU/100 µL For nasal: 1 × 109 CFU/20 µL | [38] | |
| Transmissible gastroenteritis virus (TGEV) | Mouse and pregnant sow/Oral and nasal | Induction of IgG and sIgA | For oral :20 times For nasal: 8 times | For oral: 5 × 109 CFU/mL For nasal: 2 × 109 CFU/mL | [39] | |
| Human papillomavirus type 16 (HPV-16) | Mouse/Oral | Induction of L2-specific serum IgG and vaginal IgG, and IgA | 30 times | 5 × 109 cells/mL | [40] | |
| Transmissible gastroenteritis coronavirus (TGEV) | Oral/Piglet | Induction of systemic and mucosal immune responses, cellular immunity, switching from Th1 to Th2-based immune responses | 1–48 h | 1 × 1010 CFU/mL | [41] | |
| Classical swine fever virus (CSFV) and porcine parvovirus (PPV) | Pig/Oral | Induction of mucosal and systemic CSFV-specific CD8 CTL responses, anti-PPV-VP2 serum IgG, and mucosal IgA | 6 times | 1 × 1010 CFU/mL | [42] | |
| Infectious pancreatic necrosis virus (IPNV) | Juvenile rainbow trouts/Oral | Induction of IgM and IgT, IL-1β, IL-8, CK6, MHC-II, β-defensin, TNF-1α, and reduction in viral load. | 2 times | 1 × 109 CFU/mL | [43] | |
| Human papillomavirus type 16 (HPV-16) | Human/Oral | Induction of E7-specific humoral, cellular, and mucosal immune response | 20 times | 500, 1000, and 1500 mg/day | [44] | |
| L. lactis | Human papillomavirus type 16 (HPV-16) | Healthy women/Oral | Induction of E6-specific IgG and SIgA antibody and, E6-specific IFN-γ-secreting CD8+ T cell immune response | 20 times | 1 × 109, 5 × 109, and 1 × 1010 CFU/mL | [45] |
| L. acidophilus | Human immunodeficiency virus 1 (HIV-1) | Mouse/Oral | TLR5-stimulating activity, maturation and cytokine responses of DCs, induction of gamma interferon-producing cells, and Gag-specific IgA-secreting cells | Three daily doses on weeks 0, 2, and 4 | 2 × 109 CFU/mL | [46] |
| L. lactis | Streptococcus pneumoniae | Mouse/Nasal | Induction of PspA-specific IgG and IgA antibodies, and Th1-mediated immune response | 3 times | 1 × 109 CFU/mL | [47] |
| L. casei | Porcine epidemic diarrhea virus (PEDV) | Mouse/Oral | Induction of mucosal and systemic immune responses, IL-4, and IFN-γ | 9 times | 2 × 109 cell/0.1 mL | [48] |
| L. lactis | Avian influenza virus | Mouse/Oral | Induction of specific anti-HA1 IgA and IgG antibodies, IL-4, and IFN-γ | 6 times | 1 × 1010 CFU/mL | [49] |
| Avian Influenza (HA1) Virus | Mouse/Oral | Induction of HA-specific serum IgG and fecal IgA, CD8+ T cell proliferation, and IFN-γ+ | 13 times | 1 × 1010 CFU/mL | [50] | |
| L. plantarum | Influenza virus H9N2 | Mouse/Oral | Induction of CD3+CD4+IL-4+, CD3+CD4+IFN-γ+ and CD3+CD4+IL-17+ T cells, CD3+CD8+IFN-γ+ T cells, serum IFN-γ, IgA, sIgA, and increasing survival rate | 9 times | 109 CFU/0.1 mL | [51] |
| L. lactis | Hepatitis E virus (HEV) | Mouse/Oral | Induction of ORF2-specific mucosal IgA and serum IgG, and cellular immunity | 6 times | 1 × 1010 CFU/mL | [52] |
| Human papillomavirus type 16 (HPV-16) | Mouse/Oral | Induction of specific IgA and IgG, specific IL-2- and IFN-γ-secreting lymphocytes, and increasing survival rate | 9 times | 1 × 109 CFU/mL | [53] | |
| L. casei | Human papillomavirus type 16 (HPV-16) | Human/Oral | Induction of cellular and mucosal immune response | 1, 2, 4, or 6 capsules/day at weeks 1, 2, 4, and 8 | 250 mg/ capsule | [54] |
| L. lactis | Dengue (DEN) virus | Mouse/Oral and nasal | Induction of anti-EDIII antibody responses | 6 times | For oral: 1 × 1010 CFU/mL For nasal: 1 × 108 CFU/mL | [55] |
| Human immunodeficiency virus (HIV) | Mouse/Oral | Induction of HIV-specific serum IgG, fecal IgA, and Cell-mediated immune responses | 5 times | 1 × 108 CFU/mL | [56] | |
| L. plantarum | SARS-CoV-2 | - | - | - | - | [57] |
| Avian influenza virus H9N2 | Mouse and chicken/Oral | Induction of HI antibodies and T cell immune responses | 6 times | For mouse:1 × 108 CFU/200 μL For chicken: 5 × 108 CFU/500 μL | [58] | |
| L. casei | Human papillomavirus type 16 (HPV-16) | Oral/Mouse | Induction of E7-specific mucosal IFNγ-producing cells and mucosal Th1 immune response | 16 times | 1 × 105 cells/head | [59] |
| L. lactis | Rotavirus | Mouse/Oral and nasal | Induction of Anti-rotavirus IgG and IgA antibodies, and reduction viral load | For oral: 27 times For nasal: 3 times | 30 μg/dose | [60] |
| New influenza A H1N1 | Mouse/Oral | Induction of anti-HA1 sIgA antibodies and humoral response | 9 times | 1 × 1010 CFU/mL | [61] | |
| Porcine transmissible gastroenteritis virus (TGEV) | Mouse/Oral | Induction of IgG and IgA antibodies and local mucosal immune responses. | 9 times | 1 × 109 CFU/mL | [62] | |
| L. plantarum | Spring viremia of carp virus (SVCV) | Craps/Oral | Induction of IgM and reduction of viral loads | 27 times | 1 × 109 CFU/gr | [63] |
| L. paracasei | Rotavirus-induced diarrhea | Mouse/Oral | Reduction of infection in cell cultures, shortened disease duration, severity, and viral load | 4 times | 1 × 107, 1 × 108, and 1 × 109 CFU/mL | [64] |
| L. lactis | Rotavirus | Mouse/oral | Induction of sIgA and IgG | 9 times | 1 × 109 CFU/mL | [65] |
| Human papillomavirus type 16 (HPV-16) | Mouse/Nasal | Induction of E7-specific cytotoxic T-lymphocyte response, antigen-specific immune response, high survival rate | 3 times | 1 × 109 CFU/mL | [66] | |
| Avian influenza virus | Chicken/Nasal | Induction of specific serum IgG | 9 times | 4 × 1010 CFU/100 µL | [67] | |
| L. pentosus | Transmissible gastroenteritis virus (TGEV) | Mouse/Oral | Induction of serum IgG and mucosal IgA | 9 times | 2 × 109 CFU/100 µL | [68] |
| B. longum | SARS-CoV-2 | Human/Oral | Ongoing project; the final results will be made available on 28 February 2022. | Single dose | 1 × 109, 3 × 109, and 10 × 109 CFU | NCT number: NCT04334980 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghinezhad-S, S.; Mohseni, A.H.; Bermúdez-Humarán, L.G.; Casolaro, V.; Cortes-Perez, N.G.; Keyvani, H.; Simal-Gandara, J. Probiotic-Based Vaccines May Provide Effective Protection against COVID-19 Acute Respiratory Disease. Vaccines 2021, 9, 466. https://doi.org/10.3390/vaccines9050466
Taghinezhad-S S, Mohseni AH, Bermúdez-Humarán LG, Casolaro V, Cortes-Perez NG, Keyvani H, Simal-Gandara J. Probiotic-Based Vaccines May Provide Effective Protection against COVID-19 Acute Respiratory Disease. Vaccines. 2021; 9(5):466. https://doi.org/10.3390/vaccines9050466
Chicago/Turabian StyleTaghinezhad-S, Sedigheh, Amir Hossein Mohseni, Luis G. Bermúdez-Humarán, Vincenzo Casolaro, Naima G. Cortes-Perez, Hossein Keyvani, and Jesus Simal-Gandara. 2021. "Probiotic-Based Vaccines May Provide Effective Protection against COVID-19 Acute Respiratory Disease" Vaccines 9, no. 5: 466. https://doi.org/10.3390/vaccines9050466
APA StyleTaghinezhad-S, S., Mohseni, A. H., Bermúdez-Humarán, L. G., Casolaro, V., Cortes-Perez, N. G., Keyvani, H., & Simal-Gandara, J. (2021). Probiotic-Based Vaccines May Provide Effective Protection against COVID-19 Acute Respiratory Disease. Vaccines, 9(5), 466. https://doi.org/10.3390/vaccines9050466









