Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination
Abstract
1. Introduction
2. Methods
3. Results
3.1. RMD Cases
3.2. Non-RMD
3.3. Laboratory Findings
3.4. Therapy
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonaccorsi, G.; Pierri, F.; Cinelli, M.; Flori, A.; Galeazzi, A.; Porcelli, F.; Schmidt, A.L.; Valensise, C.M.; Scala, A.; Quattrociocchi, W.; et al. Economic and social consequences of human mobility restrictions under COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 15530–15535. [Google Scholar] [CrossRef] [PubMed]
- Neerukonda, S.N.; Katneni, U. A Review on SARS-CoV-2 Virology, Pathophysiology, Animal Models, and Anti-Viral Interventions. Pathogens 2020, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Onyeaka, H.; Al-Sharify, Z.T.; Ghadhban, M.Y.; Al-Najjar, S.Z. A review on the advancements in the development of vaccines to combat coronavirus disease 2019. Clin. Exp. Vaccine Res. 2021, 10, 6–12. [Google Scholar] [CrossRef]
- Golob, J.L.; Lugogo, N.; Lauring, A.S.; Lok, A.S. SARS-CoV-2 vaccines: A triumph of science and collaboration. JCI Insight 2021. [Google Scholar] [CrossRef]
- Li, Y.D.; Chi, W.Y.; Su, J.H.; Ferrall, L.; Hung, C.F.; Wu, T.C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. The lightning-fast quest for COVID vaccines—And what it means for other diseases. Nature 2021, 589, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar]
- Wise, J. Covid-19: Rare immune response may cause clots after AstraZeneca vaccine, say researchers. BMJ 2021, 373, n954. [Google Scholar] [CrossRef]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021, 20, 102792. [Google Scholar] [CrossRef]
- Wucherpfennig, K.W. Mechanisms for the induction of autoimmunity by infectious agents. J. Clin. Invest. 2001, 108, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Torres-Aguilar, H.; Sosa-Luis, S.A.; Aguilar-Ruiz, S.R. Infections as triggers of flares in systemic autoimmune diseases: Novel innate immunity mechanisms. Curr. Opin. Rheumatol. 2019, 31, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L.; Watad, A.; Sharif, K.; Adawi, M.; Aljadeff, G.; Amital, H.; Shoenfeld, Y. Advances in our understanding of immunisation and vaccines for patients with systemic lupus erythematosus. Expert Rev. Clin. Immunol. 2017, 13, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Toussirot, É.; Bereau, M. Vaccination and Induction of Autoimmune Diseases. Inflamm. Allergy Drug Targets 2015, 14, 94–98. [Google Scholar] [CrossRef]
- Vera-Lastra, O.; Medina, G.; Cruz-Dominguez, M.D.P.; Jara, L.J.; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by adjuvants (Shoenfeld’s syndrome): Clinical and immunological spectrum. Expert Rev. Clin. Immunol. 2013, 9, 361–373. [Google Scholar]
- Pellegrino, P.; Clementi, E.; Radice, S. On vaccine’s adjuvants and autoimmunity: Current evidence and future perspectives. Autoimmun. Rev. 2015, 14, 880–888. [Google Scholar] [CrossRef]
- Gherardi, R.K.; Aouizerate, J.; Cadusseau, J.; Yara, S.; Authier, F.J. Aluminum adjuvants of vaccines injected into the muscle: Normal fate, pathology and associated disease. Morphologie 2016, 100, 85–94. [Google Scholar] [CrossRef]
- Gherardi, R.K. Myofasciite à macrophages et hydroxyde d’aluminium: Vers la définition d’un syndrome des adjuvants [Lessons from macrophagic myofasciitis: Towards definition of a vaccine adjuvant-related syndrome]. Rev. Neurol. 2003, 159, 162–164. (In French) [Google Scholar]
- Shoenfeld, Y.; Agmon-Levin, N. ’ASIA’—autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011, 36, 4–8. [Google Scholar] [CrossRef]
- Ameratunga, R.; Gillis, D.; Gold, M.; Linneberg, A.; Elwood, J.M. Evidence Refuting the Existence of Autoimmune/Autoinflammatory Syndrome Induced by Adjuvants (ASIA). J. Allergy Clin. Immunol. Pract. 2017, 5, 1551–1555.e1. [Google Scholar] [CrossRef]
- Blasco, L.M. Refutation is a strong word for partial evidence in ASIA. J. Allergy Clin. Immunol. Pract. 2018, 6, 707–708. [Google Scholar] [CrossRef]
- Tatematsu, M.; Funami, K.; Seya, T.; Matsumoto, M. Extracellular RNA Sensing by Pattern Recognition Receptors. J. Innate Immun. 2018, 10, 398–406. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]
- Rodero, M.P.; Crow, Y.J. Type I interferon-mediated monogenic autoinflammation: The type I interferonopathies, a conceptual overview. J. Exp. Med. 2016, 213, 2527–2538. [Google Scholar] [CrossRef]
- Krug, A. Nucleic acid recognition receptors in autoimmunity. Handb. Exp. Pharmacol. 2008, 129–151. [Google Scholar] [CrossRef]
- El-Gabalawy, H.; Guenther, L.C.; Bernstein, C.N. Epidemiology of immune-mediated inflammatory diseases: Incidence, prevalence, natural history, and comorbidities. J. Rheumatol. Suppl. 2010, 85, 2–10. [Google Scholar] [CrossRef]
- Chandler, R.E. Optimizing safety surveillance for COVID-19 vaccines. Nat. Rev. Immunol. 2020, 20, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Danova, J.; Kocourkova, A.; Celko, A.M. Active surveillance study of adverse events following immunisation of children in the Czech Republic. BMC Public Health 2017, 17, 167. [Google Scholar] [CrossRef] [PubMed]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; CARE Group. The CARE guidelines: Consensus-based clinical case reporting guideline development. J. Med. Case Rep. 2013, 7, 223. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Manual on Surveillance of Adverse Events Following Immunization, 2016 Update. World Health Organization. 2014. Available online: https://apps.who.int/iris/handle/10665/206144 (accessed on 27 April 2021).
- Bellavite, P. Causality assessment of adverse events following immunization: The problem of multifactorial pathology. F1000Research 2020, 9, 170. [Google Scholar] [CrossRef]
- Lee, H.; Kang, H.Y.; Cho, S.; Park, S.; Kim, A.Y.; Jung, S.Y.; Seong, B.L.; Lee, Y.M. Causality Assessment Guidelines for Adverse Events Following Immunization with a Focus on Guillain-Barré Syndrome. Vaccines 2020, 8, 101. [Google Scholar] [CrossRef]
- Koenig, H.C.; Sutherland, A.; Izurieta, H.S.; McGonagle, D. Application of the immunological disease continuum to study autoimmune and other inflammatory events after vaccination. Vaccine 2011, 29, 913–919. [Google Scholar] [CrossRef]
- McGonagle, D.; McDermott, M.F. A proposed classification of the immunological diseases. PLoS Med. 2006, 3, e297. [Google Scholar] [CrossRef]
- López-González, M.D.; Peral-Garrido, M.L.; Calabuig, I.; Tovar-Sugrañes, E.; Jovani, V.; Bernabeu, P.; García-Sevila, R.; León-Ramírez, J.M.; Moreno-Perez, O.; Boix, V.; et al. Case series of acute arthritis during COVID-19 admission. Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef]
- Segal, Y.; Shoenfeld, Y. Vaccine-induced autoimmunity: The role of molecular mimicry and immune cross-reaction. Cell. Mol. Immunol. 2018, 15, 586–594. [Google Scholar] [CrossRef]
- Hussein, H.M.; Rahal, E.A. The role of viral infections in the development of autoimmune diseases. Crit. Rev. Microbiol. 2019, 45, 394–412. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef]
- Duthie, M.S.; Windish, H.P.; Fox, C.B.; Reed, S.G. Use of defined TLR ligands as adjuvants within human vaccines. Immunol. Rev. 2011, 239, 178–196. [Google Scholar] [CrossRef]
- McGonagle, D.; Bridgewood, C.; Ramanan, A.V.; Meaney, J.F.M.; Watad, A. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol. 2021. [Google Scholar] [CrossRef]
- Saad, M.A.; Alfishawy, M.; Nassar, M.; Mohamed, M.; Esene, I.N.; Elbendary, A. Covid-19 and Autoimmune Diseases: A Systematic Review of Reported Cases. Curr. Rheumatol. Rev. 2021, 33, 150. [Google Scholar]
- Liu, Y.; Sawalha, A.H.; Lu, Q. COVID-19 and autoimmune diseases. Curr. Opin. Rheumatol. 2021, 33, 155–162. [Google Scholar]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. Covid-19 and autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar]
- Castells, M.C.; Phillips, E.J. Maintaining Safety with SARS-CoV-2 Vaccines. N. Engl. J. Med. 2021, 384, 643–649. [Google Scholar] [CrossRef]
- Benucci, M.; Infantino, M.; Marotto, D.; Ardizzone, S.; Manfredi, M.; Sarzi-Puttini, P. Vaccination against SARS-CoV-2 in patients with rheumatic diseases: Doubts and perspectives. Clin. Exp. Rheumatol. 2021, 39, 196–202. [Google Scholar]
- Cirillo, N. Reported orofacial adverse effects of COVID-19 vaccines: The knowns and the unknowns. J. Oral Pathol. Med. 2021. [Google Scholar] [CrossRef]
- Terracina, K.A.; Tan, F.K. Flare of rheumatoid arthritis after COVID-19 vaccination. Lancet Rheumatol. 2021. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Robinson, L.B.; Camargo CAJr Shenoy, E.S.; Banerji, A.; Landman, A.B.; Wickner, P. Acute Allergic Reactions to mRNA COVID-19 Vaccines. JAMA 2021, e213976. [Google Scholar] [CrossRef]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [CrossRef]
- Klimek, L.; Eckrich, J.; Hagemann, J.; Casper, I.; Huppertz, J. Allergische Reaktionen auf COVID-19-Impfstoffe—Evidenz und praxisorientiertes Vorgehen [Allergic reactions to COVID-19 vaccines: Evidence and practice-oriented approach]. Internist 2021, 62, 326–332. (In German) [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine—United States, December 14-23, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 46–51. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| Sex Female Male | 15 (55.6%) 12 (44.4%) |
| Age (mean ± standard deviation; median) | 54.44 ± 19.20; 55 |
| Pre-vaccine autoimmune/rheumatic history None Rheumatoid Arthritis Behçet’s disease Systemic lupus erythematosus (SLE)/SLE-like Pericarditis Others | 6 (22.2%) 6 (22.2%) 4 (14.8%) 3 (11.1%) 2 (7.4%) 6 (22.2%) |
| Vaccine BNT-162b2 mRNA-1273 ChAdOx1 | 23 (85.2%) 2 (7.4%) 2 (7.4%) |
| 2nd Vaccine Administered Not administered yet | 12 (44.4%) 15 (55.6%) |
| Days from vaccine to flare/new-onset After 1st dose After 2nd dose | 21/27 (77.8%); median 4 days [1–25 days] 8/12 (66.7%); median 4 days [1–7 days] |
| Flare New-onset | 17 (63.0%) 10 (37.0%) |
| Severity Mild Moderate Severe | 6 (22.2%) 14 (51.9%) 7 (25.9%) |
| Therapy Glucocorticoids Colchicine NSAIDs Plasma exchange Hydroxychloroquine Rituximab Local steroids Pyridostigmine Combination of drugs No additional treatment | 21 (77.8%) 4 (14.8%) 3 (11.1%) 2 (7.4%) 1 (3.7%) 1 (3.7%) 1 (3.7%) 1 (3.7%) 7 (25.9%) 1 (3.7%) |
| Response Rapid response Spontaneous resolution Slow response Intubation | 21 (77.8%) 2 (7.4%) 2 (7.4%) 1 (3.7%) |
| ID | Sex | Age | Pre-Vaccine History of Immune Mediated Disease + Medication | Date 1st Vaccine Dose | Date 2nd Vaccine Dose | Days from Vaccine to Flare/New-Onset (Number) | Immune Mediated Disease Complication | Flare (F)/New-Onset (N) | Severity | Relevant Lab Tests | Therapy | Comments on Therapy and Response | Country | Can the reaction be Explained Based on Drug Received by the Patient? (Yes/No) | Can the Reaction be Explained Based on the Underlying Co-Morbidity of the Patient? (Yes/No) | Do you Feel the Timing of the Side-Effect Compatible with the Time from Vaccine to Flare? (Yes/No) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 83 |
Polymyalgia rheumatica since 2005 (clinical diagnosis) Hypothyroidism |
January 2021 BNT162b2 vaccine | Not given yet | 7 days (after dose 1) |
Severe Seronegative Polyarthritis with RS3PE pattern (clinical assessment) | N | Severe (articular swelling and substantial limitation of range of motion) |
CRP 74mg/L; ANA screening (including anti-dsDNA) negative; Anti-CCP negative; RF negative | Prednisolone 15mgs day | Rapid response to therapy with quick symptom improvement | UK | Yes | No | Yes |
| 2 | F | 42 | Transient synovitis (one episode in 2015, clinical diagnosis) |
January 2021 BNT162b2 vaccine | Not given | 4 days (after dose 1) | Migratory arthritis small joints with erythema and hemorrhagic rash on toes (clinical assessment) | F | Moderate (articular swelling and moderate limitation of range of motion) |
CRP < 4 mg/L ANA screening (including anti-dsDNA) negative; RF negative | Prednisolone 10 mgs day | Rapid response to therapy with low grade swelling of 4 joints without tenderness after 1 week | UK | Yes | No | Yes |
| 3 | M | 43 | Neurosarcoidos is and small fiber neuropathy, diagnosed 3 years prior to vaccination. treated with infliximab for the last 2.5 years and asymptomatic under treatment |
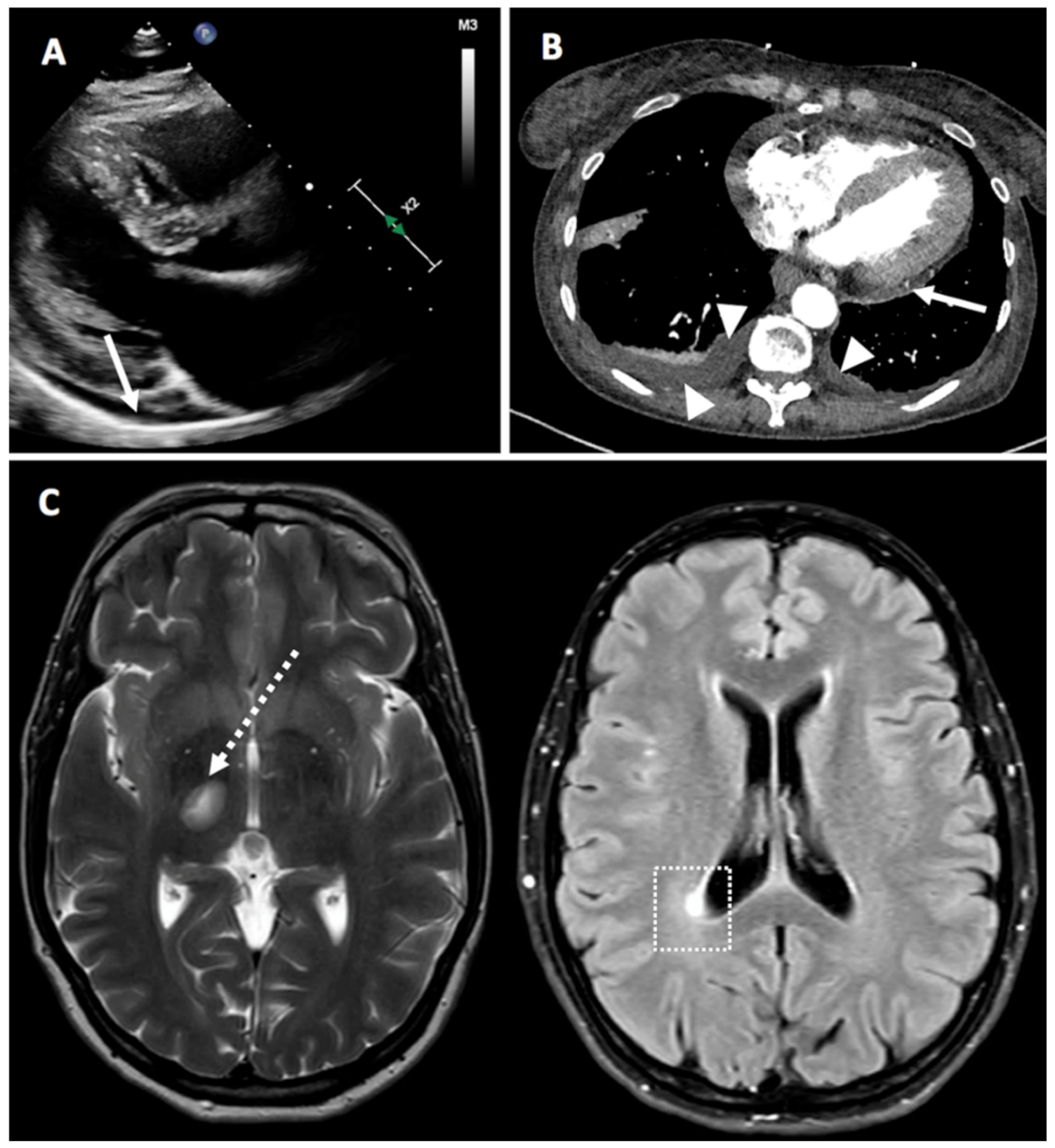
February 2021 BNT162b2 vaccine | February 2021 | 3 (after first dose) | Flare in neurosarcoidosis and neuropathy | F | Moderate | MRI of the spine did not show relapse of myelitis | No additional treatment | Flare spontaneously improved after 2 weeks, the patient received 2nd dose of vaccine with no additional side effects | Israel | No | Yes | Yes |
| 4 | M | 38 | Hypertension; dyslipidemia, and hypertrophic cardiomyopathy, treated with beta-blockers, ACEi, and atorvastatin |
January 2021 BNT162b2 vaccine |
January 2021 BNT162b2 vaccine | Onset (4 days after 1st dose) and flare (4 days after 2nd dose) | Pericarditis | N | Moderate | CRP 45, ESR 60. Negative for ANA | Treated with NSAIDs and colchicine. (1st event) and 20 mg prednisone (2nd event) | Rapid response to therapy with quick symptom improvement | Israel | No | No | Yes |
| 5 | M | 28 | Ulcerative Colitis (since 10 years) + hypereosinophilic syndrome (since 5 years) (Vedoliziumab 300 mg every 8 week and cyclosporin 150 mg BID) well controlled for a year |
January 2021 BNT162b2 vaccine | Not given | 4 days after 1st dose | Flare of hypereosinophilic syndrome and UC with vesicular skin rash, oral aphthosis and hemorrhagic diarrhea | F | Severe | IgE 41400, 9000 eosinophils, CRP 35 | 1 gr of sulomedrol daily for 3 days, then prednisone 60 mg/day tapering dose (decrease 20 mg every 2 weeks) | Slow response, eosinophils of 1200 after 2 weeks of therapy, significant and partial improvement in diarrhea and skin rash, respectively. | Israel | No | Yes | Yes |
| 6 | M | 70 | No medical history | December 2020 BNT162b2 vaccine | Not given | 3 days after 1st dose | Polymyalgia rheumatica; severe stiffness and pain in shoulders and hips, fever and fatigue | N | Severe | CRP 175, ESR 90 Anti-CCP, RF and ANA negative | Prednisone dose 40 mg once daily | Rapid response to therapy with quick symptom improvement | Israel | No | No | Yes |
| 7 | F | 45 | Hypothyroidism controlled with Eltroxin 50 mcg daily | December 2020 BNT162b2 vaccine | Not given | 7 after the 1st dose | Multiple sclerosis with left leg weakness disequilibrium, and lower limbs distal numbness | N | Moderate | Normal CBC and biochemistry Multiple Peri-ventricular white matter changes on MRI. CSF-oligoclonal bands. | 1 g of sulomedrol daily for 5 days, then prednisone 60 mg daily with tapering down dose | Rapid response to therapy with quick symptom improvement | Israel | No | No | No |
| 8 | F | 66 | Idiopathic pericarditis, anemia normocytic normochromic, left femoro-popliteal DVT treated with rivaroxaban 20 mg 12.2020, spontaneous abortion of 1st trimester Idiopathic pericarditis |
January 2021 BNT162b2 vaccine | Not given | 2 days after the 1st dose | Pericarditis with fever, typical pleuritic chest pain, elevated inflammation markers No RA articular manifestation. | F | Mild (moderate chest pain, no symptoms of heart failure, no limitation of function) | Positive ACPA 5.4, RF 44, CRP 40, ESR 70 | Continued Prednisone 15 mg prednisone. On 19 January 2021, dose was increased to 30 mg and 1 mg of colchicine. by physician. 15 mg of Methotrexate was started together with 5 mg of folic acid | Rapid response to therapy with quick symptom improvement. | Israel | No | No | Yes |
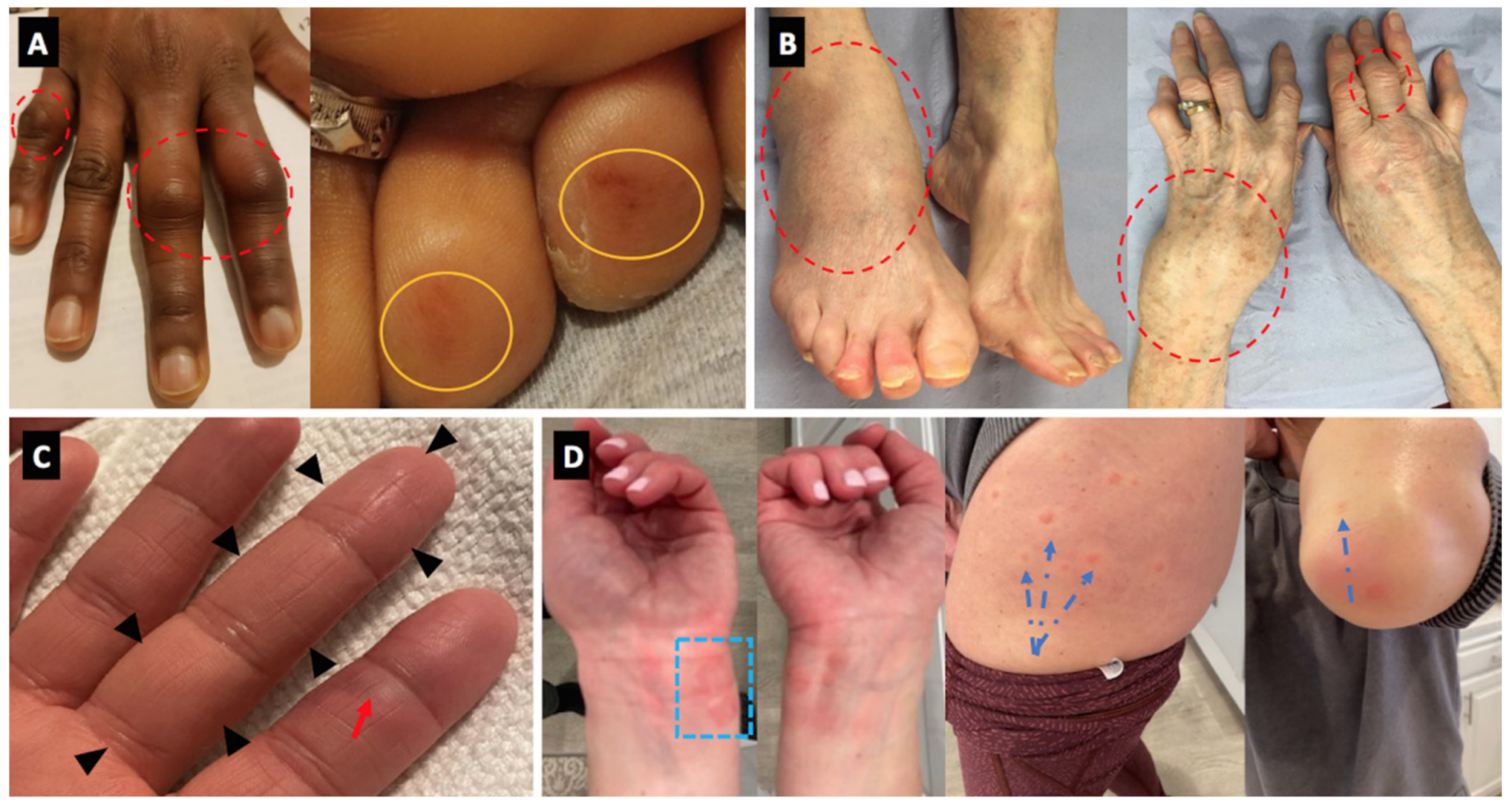
| 9 | F | 36 | Psoriasis since childhood, mild | December 2020 MRNA-1273 | January 2021 | 10 days after 1st dose | Dactylitis of RT 3rd finger Onset of R 2nd and 3rd finger joint pain, stiffness and tightness. Also developed painful erythematous macules over palmar surface of several fingers on both hands Pain chilblains like lesions on fingers (painful) | N | Mild | Normal CBC, chemistry, ESR and CRP. Musculoskeletal US without synovitis or tenosynovitis (done 1/27, symptoms had nearly resolved) | Ibuprofen 800 mg | Rapid response to therapy with quick symptom improvement. | USA | No | Yes | Yes |
| 10 | F | 48 | Not relevant | January 2021 MRNA-1273 | February 2021 | 10 days after 1st dose | Chilblains like lesions on fingers (painful) Urticarial-type lesions (resembled urticarial multiforme). 10 days after first vaccine developed itchy urticarial lesions over volar aspect of both wrists and feet, lesions evolved over thighs (symptomless) and extensor surfaces of both elbows, painful macular/nodular lesions over palmar surface both hands. Resolved in 7 days | N | Moderate | No work up | Hydrocortisone 0.5% Naproxen 500 mg | Rapid response to therapy with quick symptom improvement. | USA | No | No | Yes |
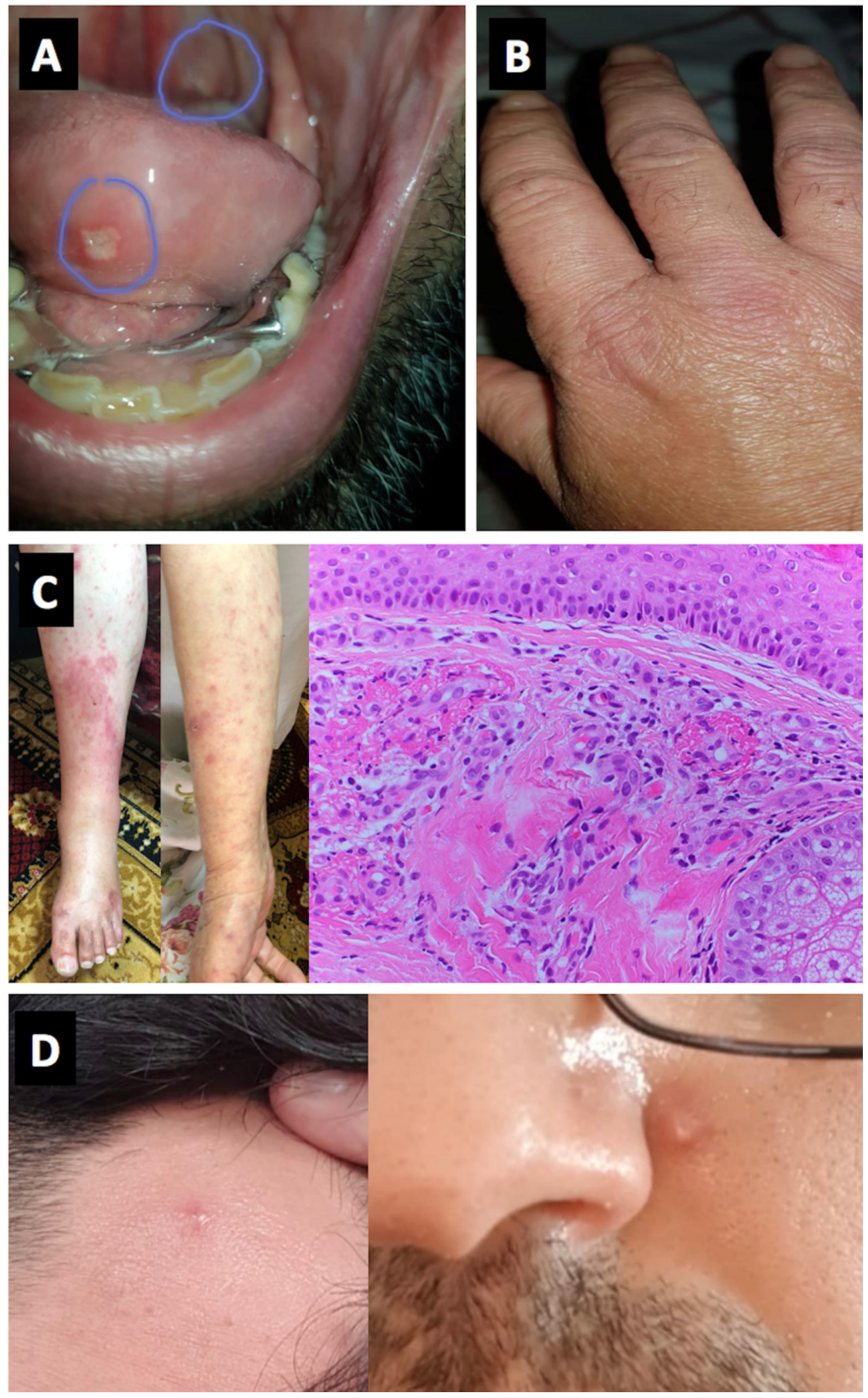
| 11 | M | 21 | Behcet’s disease (known for 3 years) treated with colchicine 1.5 mg daily | January 2021 BNT162b2 vaccine | Not given | 5 days after 1st dose | Oral aphthous ulcers | F | Moderate | No work up | 20 mg of prednisone for 1 week | Rapid response to therapy with quick symptom improvement | Israel | No | No | Yes |
| 12 | M | 55 | Behcet’s disease known for 20 years and well controlled treated with apremilast 30 mg BID. Also CLL (known for 5 years, controlled with no therapy) | December 2020 BNT162b2 vaccine | January 2021 BNT162b2 vaccine | 7 days after 2nd dose | Oral aphthous ulcers and synovitis of small joints (7 days after the second dose, synovitis in MCPS and PIPS both hands, and a few days later ulcers on the tongue) | F | Moderate | No work up | Colchicine 0.5 mg twice daily and 5 mg prednisone | Rapid response to therapy with quick symptom improvement | Israel | No | No | Yes |
| 13 | M | 20 | Behcet’s disease (known for 2 years) treated with colchicine 1 mg daily | December 2020 BNT162b2 vaccine | Not given | 3 days after 1st dose | Oral aphthous ulcers | F | Moderate | No work up | Increased dosage of colchicine to 2 mg daily | Rapid response to therapy with quick symptom improvement | Israel | No | No | Yes |
| 14 | F | 62 | Patient with DM treated with MTX and PLQ 200 mg BID | January 2021 BNT162b2 vaccine | February 2021 BNT162b2 vaccine | 7 days after 1st dose | Skin rash similar to the rash when diagnosed with DM | F | Mild | No work up | Local steroid cream | Flare spontaneously improved after a week, the patient received 2nd dose of vaccine with no additional side effects | Israel | No | No | Yes |
| 15 | F | 70 | RA, treated with MTX 15 mg wk and Certolizumab 200 mg every other week (on remission for the last 18 months). Medical history included hypertension | December 2020 BNT162b2 vaccine | January 2021 BNT162b2 vaccine | 7 days after 2nd dose | Polyarthritis of small joints | F | Moderate | High CRP | NSAIDs | Rapid response to therapy with quick symptom improvement | Israel | No | No | Yes |
| 16 | F | 78 | Laboratory SLE with no clinical manifestation | January 2021 BNT162b2 vaccine | Not given | 2 days after 1st dose | Fever, erythematous rash (generalized acute cutaneous lupus), purpura, oral aphthous ulcers and arthritis | N | Mild | +ANA, high CRP and ESR, Skin biopsy from purpura was compatible with leukocytoclastic vasculitis | Started on Hydroxychloroquine | Rapid response to therapy with quick symptom improvement | Israel | Yes | No | Yes |
| 17 | F | 50 | SLE with arthritis, mucosal ulcers and hemolysis (2019 EULAR/ACR SLE criteria) | January 2021 ChAdOx1 nCoV-19 vaccine | Not given | 14 | Severe hemolysis, oral and nasal ulceration, arthralgia | F | Severe (clinical assessment) | Hb 53, reticulocytes 42%, bilirubin 48, LDH 864 | High dose glucocorticoids (Prednisolone 60 mg daily) and rituximab | Slow response | UK | Yes | No | Yes |
| 18 | M | 34 | Behcet’s disease (known for 10 years, included arthritis, uveitis, skin rash, oral aphthosis controlled for the last 12 months, treated with colchicine and Humira), no other medical history | January 2021 BNT162b2 vaccine | Not given | 5 days after 1st dose | Pustular skin lesions -developed skin lesions as a part of Behcet’s disease for the first time | F | Moderate | No work up | Short course of NSAIDs and increase of colchicine dose | Rapid response to therapy with quick symptom improvement | Israel | No | No | Yes |
| 19 | M | 72 | Recurrent. pericarditis - treated with colchicine in the past | January 2021 BNT162b2 vaccine | January 2021 BNT162b2 vaccine | 1 day after 2nd dose | Myasthenia Gravis | N | Moderate | EMG-decrement of 28–46% on facial and shoulder muscles | Plasma exchange + Prednisone 60 mg | Rapid response to therapy with quick symptom improvement | Israel | No | No | Yes |
| 20 | M | 73 | Not relevant | Dec 2020 BNT162b2 vaccine | January 2021 BNT162b2 vaccine | 7 days after 2nd dose | Myasthenia Gravis (firstly ocular signs then appearance of bulbar signs and respiratory symptoms) | N | Severe | EMG- borderline decrement, markedly pathologic jitter | Plasma Exchange, Pyridostigmin and prednisone | Patient intubated due to respiratory symptoms | Israel | No | No | Yes |
| 21 | F | 68 | RA seropositive-well controlled with Actemra | January 2021 BNT162b2 vaccine | February 2021 BNT162b2 vaccine | 3 days after 2nd dose | Flare of synovitis of small joints CDAI = 41 compared to CDAI = 4 in 19 January 2021 | F | Severe | ESR=12 after depomedrol, CRP = 0.06 before flare, CRP = 0.28 after flare, RF = 1782 before flare, RF = 2300 after flare, ANA negative after vaccine | IM Depomedrol 160 mg | Rapid response to therapy with quick symptom improvement | Israel | No | No | Yes |
| 22 | M | 53 | Not relevant | January 2021 BNT162b2 vaccine | Not given | 3 days after first dose | Palpable purpura, mild abdominal pain and arthralgia w/o arthritis, no renal involvement. Diagnosed with Henoch Schlonein Pupura with Leukocytoclastic cutaneous vasculitis among skin bipsy | N | Mild-moderate | Skin biopsy revealed IgA and C3 deposits in the vessel walls Mild elevation in CRP to 0.8 mg/dL, mild elevation in IgA to 412 mg%, mild leucocytosis to 11.5 k/ul | Dexamethasone 10 mg, prednisone 60 mg thereafter | Rapid response to therapy with quick symptom improvement | Israel | No | No | Yes |
| 23 | F | 80 | Seronegative RA–2007 (ACR/EULAR 2010 class. Crit.) stable on Methotrexate 15mgs week | January 2021 BNT162b2 vaccine | Not given | 25 days flare with progressive worsening | Severe Right ankle synovitis, severe wrist synovitis and 5 small joint synovitis | F | Severe (articular swelling and substantial limitation of range of motion) | ESR 71 CRP 27 | Prednisolone 15 mgs day tapering and increase methotrexate dose | Rapid response to therapy with quick symptom improvement | UK | No | No | Yes |
| 24 | M | 70 | Gout (known for many years) well controlled in the last 2 years), hypertension and dyslipidaemia | Dec 2020 BNT162b2 vaccine | January 2021 BNT162b2 vaccine | 1 day after first dose and another flare 1 day after the second dose | Monoarthritis at Rt elbow (after 1st dose) and at mid foot (after 2nd dose) | F | Moderate | CRP 44 mg/L. | Prednisone 40 mg for 7 day | Rapid response to therapy with quick symptom improvement | Israel | No | No | Yes |
| 25 | F | 78 | Seropositive RA (not active and with no therapy for many years) | January 2021 BNT162b2 vaccine | Not given | 2 days after first dose | Polyarthritis involving shoulders, wrists, MCPs and PIPs | F | Moderate | ANA negative, RF 60, ESR 52, CRP 2.1 mg/dl | Prednisone 20 mg daily with tapering down 5 mg every week was prescribed. | Rapid response to therapy with quick symptom improvement | Israel | No | No | Yes |
| 26 | F | 27 | Seronegative RA stable on Adalimumab every 2 weeks | January 2021 BNT162b2 vaccine | January 2021 BNT162b2 vaccine | 4 days after second dose developed Rt knee monoarthritis | Monoarthritis of Rt knee | F | Moderate | ANA negative, RF negative, CRP 1.3 mg/dl | Joint aspiration and injection of 80mg depomedrol | Rapid response to therapy with quick symptom improvement | Israel | No | No | Yes |
| 27 | F | 60 | SLE (never previously referred to rheumatology): Discoid lupus since 2001, severe raynauds, alopecia, pancytopenia (since September 2020), proteinuria (Developed Sept 2020, diagnosed as class IV lupus nephritis March 2021), hypocomplementaemia, ANA positive, dsDNA, anti-chromatin and anti-ribosomal antibody positive), 2019 EULAR/ACR SLE criteria) Baseline Disease modifying therapy: Prednisolone 8mg OD | February 2021 ChAdOx1 vaccine | Not given yet | 4 days | Pericardial and pleural effusions, possible pericarditis | F | Mild | Central intermittent pleuritic chest pain, had CTPA and bedside echo. Trop normal CRP 17.8 | Prednisone increased from 8mg to 15mg for 5 days, colchicine 500 mcg BD | Rapid response to therapy with quick symptom improvement | UK | Yes | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. https://doi.org/10.3390/vaccines9050435
Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, Haddad A, Elias M, Zisman D, Naffaa ME, et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines. 2021; 9(5):435. https://doi.org/10.3390/vaccines9050435
Chicago/Turabian StyleWatad, Abdulla, Gabriele De Marco, Hussein Mahajna, Amit Druyan, Mailam Eltity, Nizar Hijazi, Amir Haddad, Muna Elias, Devy Zisman, Mohammad E. Naffaa, and et al. 2021. "Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination" Vaccines 9, no. 5: 435. https://doi.org/10.3390/vaccines9050435
APA StyleWatad, A., De Marco, G., Mahajna, H., Druyan, A., Eltity, M., Hijazi, N., Haddad, A., Elias, M., Zisman, D., Naffaa, M. E., Brodavka, M., Cohen, Y., Abu-Much, A., Abu Elhija, M., Bridgewood, C., Langevitz, P., McLorinan, J., Bragazzi, N. L., Marzo-Ortega, H., ... McGonagle, D. (2021). Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines, 9(5), 435. https://doi.org/10.3390/vaccines9050435










