Designing a SARS-CoV-2 T-Cell-Inducing Vaccine for High-Risk Patient Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. SARS-CoV-2 Peptide Prediction and Selection
2.2. Self-Administered Vaccination
2.3. Ethical and Scientific Considerations
2.4. Immunomonitoring
3. Results
3.1. Clinical Aspect of the Vaccination Sites
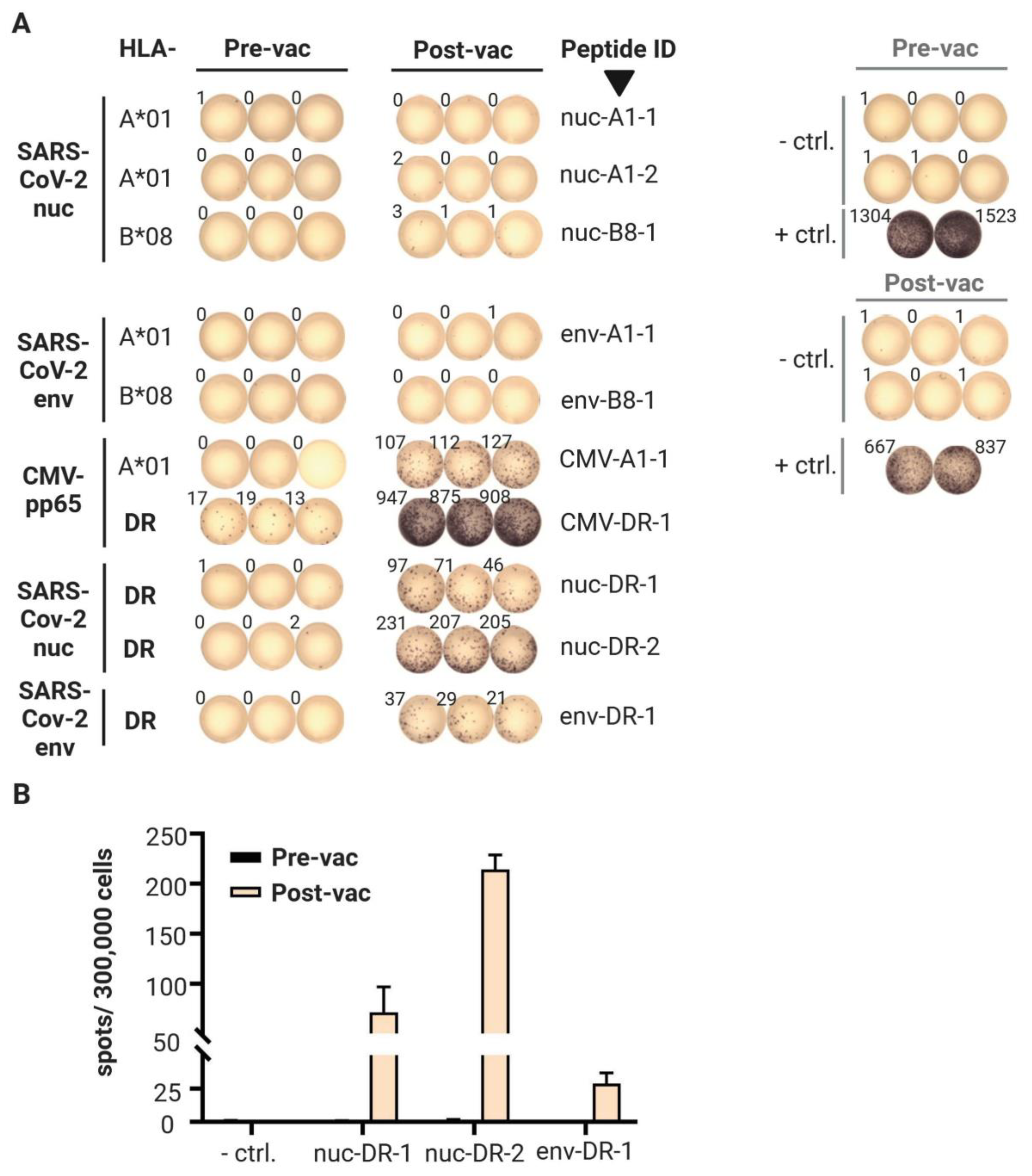
3.2. T Cell Responses
3.3. Antibody Responses
4. Discussion
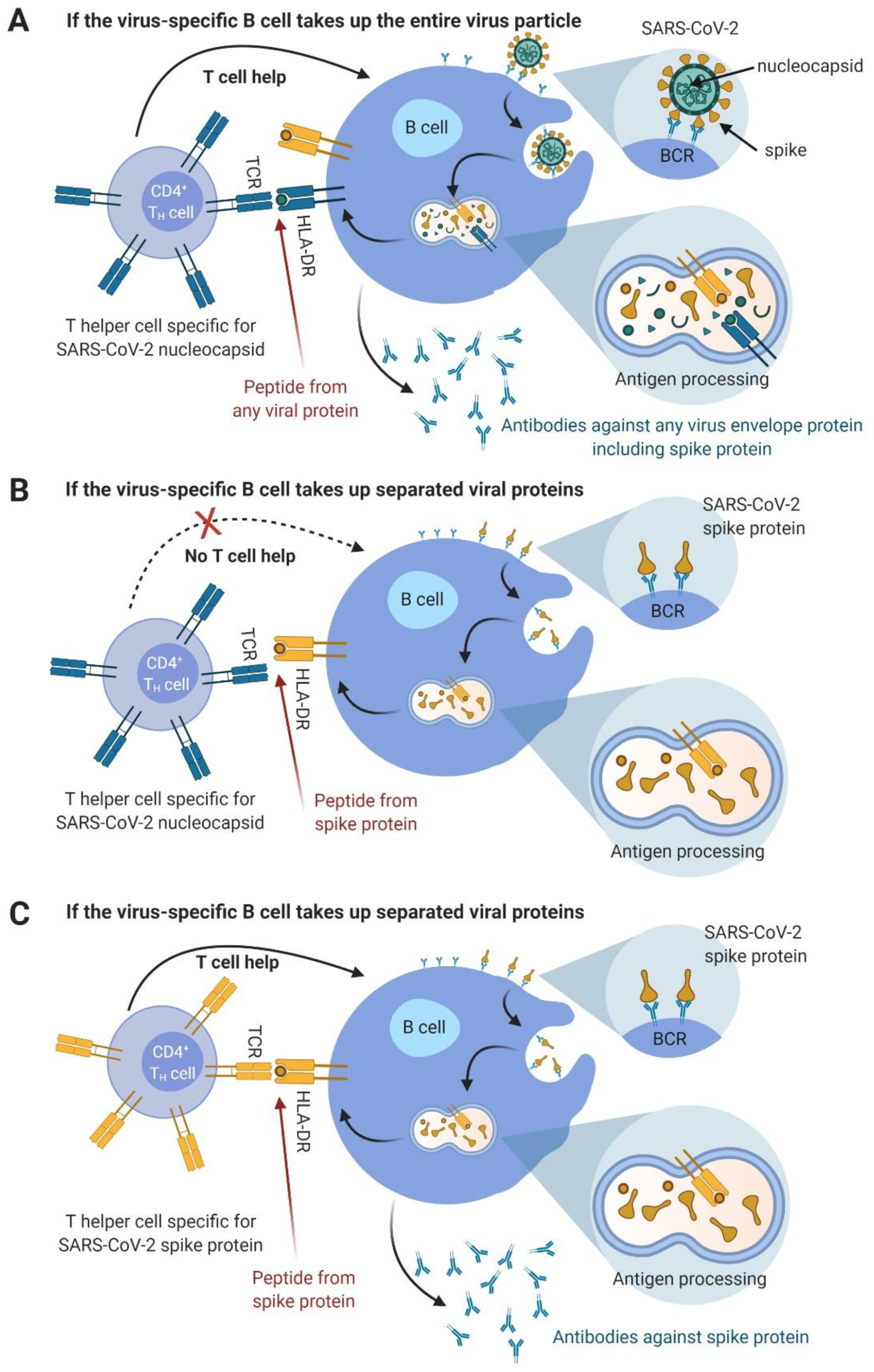
- CD4 Th1 cells should vigorously activate virus antigen-experienced B cells that should already pre-exist in most COVID-19-patients. An illustration for this assumption is provided in Figure 1. These CD4 T cells would be expected to directly contribute to virus clearance and deliver strong T helper signals to the CD8 T cells already primed during natural infection. The resulting enhanced activity could lead to more rapid virus clearance or transiently increased lung damage.
- Vaccine-induced CD8 T cells against peptides embedded in the longer peptides do appear later, as preliminary data from the majority of subjects in our presently running study suggest (https://clinicaltrials.gov, accessed on 20 April 2021, Identifier: NCT04546841). In this study, six immunodominant 15-mer peptides reported in [16] are used in a vaccine with Montanide and XS15. Once activated, such CD8 T cells should also contribute to faster virus clearance.
- Vaccine-induced antibodies against the viral peptides tested in this study might also appear much later, if at all.
- Since we found IFNγ-producing T cells, we conclude that Th1 CD4 T cells were induced. Therefore, there should be no disease enhancing-effects related to the induction of Th2-bias, as described for other coronaviruses [21].
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Prendecki, M.; Clarke, C.; Brown, J.; Cox, A.; Gleeson, S.; Guckian, M.; Randell, P.; Pria, A.D.; Lightstone, L.; Xu, X.N.; et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet 2021. [Google Scholar] [CrossRef]
- Panagiotou, O.A.; Kosar, C.M.; White, E.M.; Bantis, L.E.; Yang, X.; Santostefano, C.M.; Feifer, R.A.; Blackman, C.; Rudolph, J.L.; Gravenstein, S.; et al. Risk Factors Associated With All-Cause 30-Day Mortality in Nursing Home Residents With COVID-19. JAMA Intern. Med. 2021. [Google Scholar] [CrossRef]
- Guan, W.J.; Liang, W.H.; Zhao, Y.; Liang, H.R.; Chen, Z.S.; Li, Y.M.; Liu, X.Q.; Chen, R.C.; Tang, C.L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef] [PubMed]
- Erdal, G.S.; Polat, O.; Erdem, G.U.; Korkusuz, R.; Hindilerden, F.; Yilmaz, M.; Yasar, K.K.; Isiksacan, N.; Tural, D. The mortality rate of COVID-19 was high in cancer patients: A retrospective single-center study. Int. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hanley, B.P.; Bains, W.; Church, G. Review of Scientific Self-Experimentation: Ethics History, Regulation, Scenarios, and Views Among Ethics Committees and Prominent Scientists. Rejuvenation Res. 2019, 22, 31–42. [Google Scholar] [CrossRef]
- Rammensee, H.G.; Wiesmüller, K.H.; Chandran, P.A.; Zelba, H.; Rusch, E.; Gouttefangeas, C.; Kowalewski, D.J.; Di Marco, M.; Haen, S.P.; Walz, J.S.; et al. A new synthetic toll-like receptor 1/2 ligand is an efficient adjuvant for peptide vaccination in a human volunteer. J. Immunother. Cancer 2019, 7, 307. [Google Scholar] [CrossRef]
- Manriquez Roa, T.; Biller-Andorno, N. Going first: The ethics of vaccine self-experimentation in coronavirus times. Swiss Med. Wkly. 2020, 150, w20415. [Google Scholar] [CrossRef] [PubMed]
- Rammensee, H.G.; Stevanović, S.; Gouttefangeas, C.; Heidu, S.; Klein, R.; Preuß, B.; Walz, J.S.; Nelde, A.; Haen, S.P.; Reth, M.; et al. Designing a therapeutic SARS-CoV-2 T-cell-inducing vaccine for high-risk patient groups. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Avalos, A.M.; Ploegh, H.L. Early BCR Events and Antigen Capture, Processing, and Loading on MHC Class II on B Cells. Front. Immunol. 2014, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Rammensee, H.G.; Bachmann, J.; Emmerich, N.P.; Bachor, O.A.; Stevanovic, S. SYFPEITHI: Database for MHC ligands and peptide motifs. Immunogenetics 1999, 50, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Nelde, A.; Bilich, T.; Heitmann, J.S.; Maringer, Y.; Salih, H.R.; Roerden, M.; Lubke, M.; Bauer, J.; Rieth, J.; Wacker, M.; et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 2021, 22, 74–85. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. The beginnings of cardiac catheterization and the resulting impact on pulmonary medicine. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L651–L658. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P. In defense of case reports and case series. Ann. Intern. Med. 2001, 134, 330–334. [Google Scholar] [CrossRef]
- Widenmeyer, M.; Griesemann, H.; Stevanovic, S.; Feyerabend, S.; Klein, R.; Attig, S.; Hennenlotter, J.; Wernet, D.; Kuprash, D.V.; Sazykin, A.Y.; et al. Promiscuous survivin peptide induces robust CD4+ T-cell responses in the majority of vaccinated cancer patients. Int. J. Cancer 2012, 131, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Löffler, M.W.; Chandran, P.A.; Laske, K.; Schroeder, C.; Bonzheim, I.; Walzer, M.; Hilke, F.J.; Trautwein, N.; Kowalewski, D.J.; Schuster, H.; et al. Personalized peptide vaccine-induced immune response associated with long-term survival of a metastatic cholangiocarcinoma patient. J. Hepatol. 2016, 65, 849–855. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Barnard, D.; Ong, C.H.; Peng, B.H.; Tseng, C.T.; Petrovsky, N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J. Virol. 2015, 89, 2995–3007. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Kawakami, C.; Yamada, S.; Satoh, R.; Hohdatsu, T. Antibody-dependent enhancement occurs upon re-infection with the identical serotype virus in feline infectious peritonitis virus infection. J. Vet. Med. Sci. 2008, 70, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Tetro, J.A. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020, 22, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Werner, H.C.; Kong, W.P.; Leung, K.; Traggiai, E.; Lanzavecchia, A.; Nabel, G.J. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc. Natl. Acad. Sci. USA 2005, 102, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Zhao, J.; Perlman, S. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 2014, 59, 118–128. [Google Scholar] [CrossRef]
- Markovic-Plese, S.; Hemmer, B.; Zhao, Y.; Simon, R.; Pinilla, C.; Martin, R. High level of cross-reactivity in influenza virus hemagglutinin-specific CD4+ T-cell response: Implications for the initiation of autoimmune response in multiple sclerosis. J. Neuroimmunol. 2005, 169, 31–38. [Google Scholar] [CrossRef] [PubMed]


| AA Sequence | Predicted HLA Restriction | Source Protein | Peptide ID | AA Position | Peptide Length | Administered Amount (μg) |
|---|---|---|---|---|---|---|
| SPDDQIGYY | A*01 | SARS-CoV-2 nuc | nuc-A1-1 | 79–87 | 9 | 240 |
| MKDLSPRWY | A*01 | nuc-A1-2 | 101–109 | 9 | 240 | |
| LLLDRLNQL | B*08 | nuc-B8-1 | 222–230 | 9 | 240 | |
| IGYYRRATRRIRGGD | DR | nuc-DR-1 | 84–98 | 15 | 240 | |
| ASAFFGMSRIGMEVT | DR | nuc-DR-2 | 311–325 | 15 | 240 | |
| VSLVKPSFY | A*01 | SARS-CoV-2 env | env-A1-1 | 49–57 | 9 | 720 |
| YVYSRVKNL | B*08 | env-B8-1 | 57–65 | 9 | 720 | |
| FYVYSRVKNLNSSRV | DR | env-DR-1 | 56–70 | 15 | 240 | |
| YSEHPTFTSQY | A*01 | CMV-pp65 | CMV-A1-1 | 363–373 | 11 | 720 |
| YQEFFWDANDIYRIF | DR | CMV-DR-1 | 510–524 | 15 | 240 |
| AA Sequence | Source Protein | Peptide ID | AA Position | Peptide Length |
|---|---|---|---|---|
| ASVYAWNRKRISN | SARS-CoV-2 spike | spi-DR-1 | 348–360 | 13 |
| VADYSVLYNSASFST | spi-DR-2 | 362–376 | 15 | |
| IGYYRRATRRIRGGD | SARS-CoV-2 nuc | nuc-DR-1 | 84–98 | 15 |
| ASAFFGMSRIGMEVT | nuc-DR-2 | 311–325 | 15 | |
| RWYFYYLGTGPEAGL | nuc-DR-5 | 107–121 | 15 | |
| ASWFTALTQHGKEDL | nuc-DR-6 | 50–64 | 15 | |
| LLLLDRLNQLESKMS | nuc-DR-7 | 221–235 | 15 | |
| AADLDDFSKQLQQSM | nuc-DR-8 | 397–411 | 15 | |
| AIVLQLPQGTTLPKG | nuc-DR-9 | 156–170 | 15 | |
| YKHWPQIAQFAPSAS | nuc-DR-10 | 298–312 | 15 |
| AA Sequence | Source Protein | Peptide ID | IFNγ | IL-2 | CD154 | TNF |
|---|---|---|---|---|---|---|
| % (Fold Increase) | ||||||
| IGYYRRATRRIRGGD | nuc | nuc-DR-1 | 4.2 (199) | 1.6 (35) | 16.0 (60) | 14.2 (191) |
| ASAFFGMSRIGMEVT | nuc | nuc-DR-2 | 12.0 (571) | 3.2 (67) | 31.7 (118) | 24.0 (321) |
| ASVYAWNRKRISN | spike | spi-DR-1 | 8.9 (424) | 4.6 (76) | 34.0 (122) | 22.0 (201) |
| VADYSVLYNSASFST | spike | spi-DR-2 | 0.0 | 0.0 | −0.3 | −0.1 |
| RWYFYYLGTGPEAGL | nuc | nuc-DR-5 | 0.1 (4) | 0.3 (6) | 1.3 (4) | 1.2 (12) |
| ASWFTALTQHGKEDL | nuc | nuc-DR-6 | 0.0 | 0.0 | 0.1 | 0.1 |
| LLLLDRLNQLESKMS | nuc | nuc-DR-7 | 16.0 (696) | 3.3 (62) | 37.1 (99) | 31.1 (312) |
| AADLDDFSKQLQQSM | nuc | nuc-DR-8 | 0.0 | 0.0 | −0.1 | 0.0 |
| AIVLQLPQGTTLPKG | nuc | nuc-DR-9 | 0.0 | 0.0 | 0.0 | −0.1 |
| YKHWPQIAQFAPSAS | nuc | nuc-DR-10 | 0.0 | 0.0 | −0.1 | 0.0 |
| Embedded AA sequence | ||||||
| VYAWNRKRI * | spike | - | 1.6 (45) | 1.0 (13) | 15.8 (13) | 8.0 (22) |
| SVLYNSASF * | spike | - | 4.6 (128) | 3.9 (49) | 17.6 (15) | 15.1 (41) |
| LLDRLNQL * | nuc | nuc-B8-1 | 0.0 | 0.2 (3) | 0.2 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rammensee, H.-G.; Gouttefangeas, C.; Heidu, S.; Klein, R.; Preuß, B.; Walz, J.S.; Nelde, A.; Haen, S.P.; Reth, M.; Yang, J.; et al. Designing a SARS-CoV-2 T-Cell-Inducing Vaccine for High-Risk Patient Groups. Vaccines 2021, 9, 428. https://doi.org/10.3390/vaccines9050428
Rammensee H-G, Gouttefangeas C, Heidu S, Klein R, Preuß B, Walz JS, Nelde A, Haen SP, Reth M, Yang J, et al. Designing a SARS-CoV-2 T-Cell-Inducing Vaccine for High-Risk Patient Groups. Vaccines. 2021; 9(5):428. https://doi.org/10.3390/vaccines9050428
Chicago/Turabian StyleRammensee, Hans-Georg, Cécile Gouttefangeas, Sonja Heidu, Reinhild Klein, Beate Preuß, Juliane Sarah Walz, Annika Nelde, Sebastian P. Haen, Michael Reth, Jianying Yang, and et al. 2021. "Designing a SARS-CoV-2 T-Cell-Inducing Vaccine for High-Risk Patient Groups" Vaccines 9, no. 5: 428. https://doi.org/10.3390/vaccines9050428
APA StyleRammensee, H.-G., Gouttefangeas, C., Heidu, S., Klein, R., Preuß, B., Walz, J. S., Nelde, A., Haen, S. P., Reth, M., Yang, J., Tabatabai, G., Bösmüller, H., Hoffmann, H., Schindler, M., Planz, O., Wiesmüller, K.-H., & Löffler, M. W. (2021). Designing a SARS-CoV-2 T-Cell-Inducing Vaccine for High-Risk Patient Groups. Vaccines, 9(5), 428. https://doi.org/10.3390/vaccines9050428








