Association between Influenza Vaccination and Positive SARS-CoV-2 IgG and IgM Tests in the General Population of Katowice Region, Poland
Abstract
1. Introduction
2. Materials and Methods
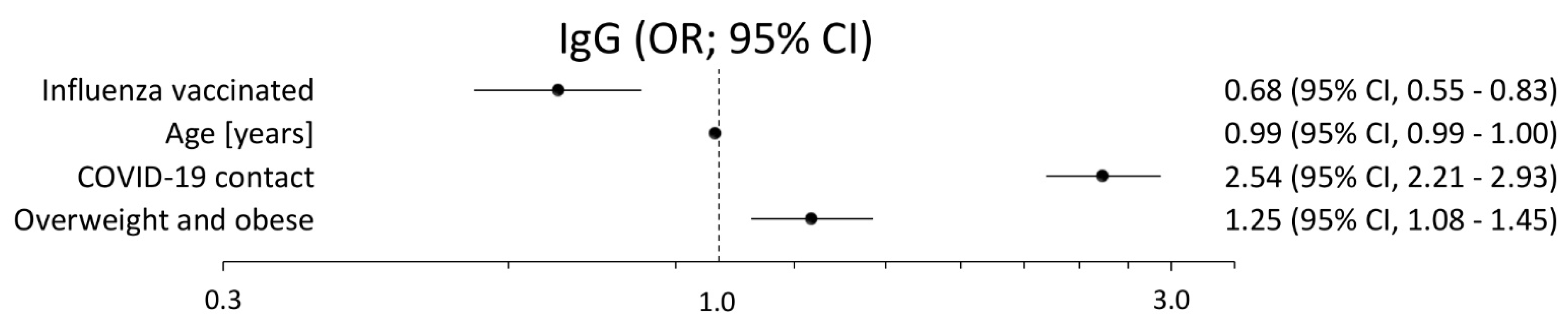
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maltezou, H.C.; Theodoridou, K.; Poland, G. Influenza immunization and COVID-19. Vaccine 2020, 38, 6078–6079. [Google Scholar] [CrossRef] [PubMed]
- Manzanares-Meza, L.D.; Medina-Contreras, O. SARS-CoV-2 and influenza: A comparative overview and treatment implications. Bol. Med. Hosp. Infant. Mex. 2020, 77, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Debisarun, P.A.; Struycken, P.; Domínguez-Andrés, J.; Moorlag, S.J.C.F.M.; Taks, E.; Gössling, K.L.; Ostermann, P.N.; Müller, L.; Schaal, H.; ten Oever, J.; et al. The effect of influenza vaccination on trained immunity: Impact on COVID-19. BMJ Yale 2020. [Google Scholar] [CrossRef]
- Salem, M.L.; El-Hennawy, D. The possible beneficial adjuvant effect of influenza vaccine to minimize the severity of COVID-19. Med. Hypotheses 2020, 140, 10975. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. SARS-CoV-2 immunity: Review and applications to phase 3 vaccine candidates. Lancet 2020, 396, 1595–1606. [Google Scholar] [CrossRef]
- Altenburg, A.F.; Kreijtz, J.H.C.M.; de Vries, R.D.; Song, F.; Fux, R.; Rimmelzwaan, G.F.; Sutter, G.; Asisa, V. Modified Vaccinia Virus Ankara (MVA) as Production Platform for Vaccines against Influenza and Other Viral Respiratory Diseases. Viruses 2014, 6, 2735–2761. [Google Scholar] [CrossRef]
- Amato, M.; Werba, J.P.; Frigerio, B.; Coggi, D.; Sansaro, D.; Ravani, A.; Ferrante, P.; Veglia, F.; Tremoli, E.; Baldassarre, D. Relationship between Influenza Vaccination Coverage Rate and COVID-19 Outbreak: An Italian Ecological Study. Vaccines 2020, 8, 535. [Google Scholar] [CrossRef] [PubMed]
- Marín-Hernández, D.; Schwartz, R.E.; Nixon, D.F. Epidemiological evidence for association between higher influenza vaccine uptake in the elderly and lower COVID-19 deaths in Italy. J. Med. Virol. 2020, 1–2. [Google Scholar] [CrossRef]
- Zanettini, C.; Omar, M.; Dinalankara, W.; Imada, E.L.; Colantuoni, E.; Parmigiani, G.; Marchionni, L. Influenza Vaccination and COVID19 Mortality in the USA. medRxiv 2020. [Google Scholar] [CrossRef]
- Sultana, J.; Mazzaglia, G.; Luxi, N.; Cancellieri, A.; Capuano, A.; Ferrajolo, C.; de Waure, C.; Ferlazzo, G.; Trifiró, G. Potential effects of vaccinations on the prevention of COVID-19: Rationale, clinical evidence, risks, and public health considerations. Expert Rev. Vaccines 2020, 19, 919–936. [Google Scholar] [CrossRef]
- Del Riccio, M.; Lorini, L.; Bonaccorsi, G.; Paget, J.; Caini, S. The Association between Influenza Vaccination and the Risk of SARS-CoV-2 Infection, Severe Illness, and Death: A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 7870. [Google Scholar] [CrossRef]
- Martínez-Baz, I.; Trobajo-Sanmartín, C.; Arregui, I.; Navascués, A.; Adelantado, M.; Indurain, J.; Fresán, U.; Ezpeleta, C.; Castilla, J. Influenza Vaccination and Risk of SARS-CoV-2 Infection in a Cohort of Health Workers. Vaccines 2020, 15, 611. [Google Scholar] [CrossRef] [PubMed]
- Noale, M.; Trevisan, C.; Maggi, S.; Incalzi, R.A.; Pedone, C.; Di Bari, M.; Adorni, F.; Jesuthasan, N.; Sojic, A.; Galli, M.; et al. The Association between Influenza and Pneumococcal Vaccinations and SARS-Cov-2 Infection: Data from the EPICOVID19 Web-Based Survey. Vaccines 2020, 8, 471. [Google Scholar] [CrossRef]
- Grech, V.; Borg, M. Influenza vaccination in the COVID-19 era. Early Hum. Dev. 2020, 148, 105116. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov Identifier: NCT04627623. Available online: https://clinicaltrials.gov/ct2/show/NCT04627623 (accessed on 12 November 2020).
- Zejda, J.E.; Brożek, G.M.; Kowalska, M.; Barański, K.; Kaleta-Pilarska, A.; Nowakowski, A.; Xia, Y.; Buszman, P. Seroprevalence of Anti-SARS-CoV-2 Antibodies in a Random Sample of Inhabitants of the Katowice Region, Poland. Int. J. Environ. Res. Public Health 2021, 18, 3188. [Google Scholar] [CrossRef] [PubMed]
- WHO. Population-Based Age-Stratified Seroepidemiological Investigation Protocol for COVID-19 Virus Infection. March 2020. Available online: https://apps.who.int/iris/handle/10665/331656 (accessed on 20 March 2020).
- Yue, H.; Zhang, M.; Xing, L.; Wang, K.; Rao, X.; Liu, H.; Tian, J.; Zhou, P.; Deng, Y.; Shang, J. The epidemiology and clinical characteristics of coinfection of SARS-CoV-2 and influenza viruses in patients during COVID-19 outbreak. J. Med. Virol. 2020, 92, 2870–2873. [Google Scholar] [CrossRef]
- Richarson, S.; Hirsch, J.S.; Narasimbam, N. Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City are. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Kim, D.; Quinn, J.; Pinsky, B.; Shah, N.H.; Brown, I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA 2020, 323, 2085–2086. [Google Scholar] [CrossRef]
- Belongia, E.; Osterholm, M. COVID-19 and flu, a perfect storm. Science 2020, 368, 1163. [Google Scholar] [CrossRef]
- Lacobucci, G. Covid-19: Risk of death more than doubled in people who also has flu, English data show. Br. Med. J. 2020, 370, 3720. [Google Scholar] [CrossRef]
- Bai, L.; Zhao, Y.; Dong, J.; Liang, S.; Guo, M.; Liu, X.; Wang, X.; Huang, Z.; Sun, X.; Zhang, Z.; et al. Coinfection with influenza A virus enhances SARS-CoV-2 infectivity. Cell Res. 2021, 1–9. [Google Scholar] [CrossRef]
- Reina, J. Influenza vaccination in the time of SARS-CoV-2. Med. Clin. 2021, 156, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.; Ohler, A. The Flu Vaccination May Have a Protective Effect on the Course of COVID-19 in the Pediatric Population: When Does Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Meet Influenza? Cureus 2021, 13, 12533. [Google Scholar] [CrossRef]
- Colon, A.; Ashur, C.; Washer, L.; Eagle, K.M.; Hofmann Bowman, M.A. Impact of the influenza vaccine on COVID-19 infection rates and severity. Am. J. Infect. Control. 2021, 1–7. [Google Scholar] [CrossRef]
- Szmyd, B.; Karuga, F.F.; Bartoszek, A.; Staniecka, K.; Siwecka, N.; Bartoszek, A.; Błaszczyk, M.; Radek, M. Attitude and Behaviors towards SARS-CoV-2 Vaccination among Healthcare Workers: A Cross-Sectional Study from Poland. Vaccines 2021, 9, 218. [Google Scholar] [CrossRef]
- Szmyd, B.; Bartoszek, A.; Karuga, F.F.; Staniecka, K.; Błaszczyk, M.; Radek, M. Medical Students and SARS-CoV-2 Vaccination: Attitude and Behaviors. Vaccines 2021, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Fink, G.; Orlova-Fink, N.; Schindler, T.; Grisi, S.; Ferrer, A.P.; Daubenberger, C.; Brentani, A. Inactivated trivalent influenza vaccine is associated with lower mortality among Covid-19 patients in Brazil. BMJ Evid. Based Med. 2020. [Google Scholar] [CrossRef]
- Bajgain, K.T.; Badal, S.; Bajgain, B.B.; Santana, M.J. Prevalence of comorbidities among individuals with COVID-19: A rapid review of current literature. Am. J. Infect. Control. 2021, 49, 238–246. [Google Scholar] [CrossRef]
- Davies, N.G.; Klepac, P.; Liu, Y.; Prem, K.; Jit, M.; CMMID COVID-19 Working Group; Eggo, R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020, 26, 1205–1211. [Google Scholar] [CrossRef]

| Influenza Vaccination in Last Year | Total 5479 | No 4576 (100) * | Yes 903 (100) * | p-Value | |
|---|---|---|---|---|---|
| Sex | Male | 2287 | 1895 (41.4) | 392 (43.4) | 0.2 1 |
| Female | 3192 | 2681 (58.6) | 511 (56.6) | ||
| Age (years) | 43.9 ± 16.8 | 43.1 ± 16.6 | 47.7 ± 17.6 | p < 0.0001 2 | |
| Body Mass Index Overweight and obese | No | 2382 | 2001 (47.4) | 381 (46.0) | 0.4 1 |
| Yes | 2669 | 2221 (52.6) | 448 (54.0) | ||
| Contact with COVID-19 or quarantine | No | 3618 | 3035 (66.3) | 583 (64.6) | 0.3 1 |
| Declared | 1861 | 1541 33.7) | 320 (35.4) | ||
| Hypertension | No | 4138 | 3510 (76.7) | 628 (69.5) | p < 0.0001 1 |
| Declared | 1341 | 1066 (23.3) | 275 (30.5) | ||
| Diabetes | No | 5126 | 4294 (93.8) | 832 (92.1) | 0.06 1 |
| Declared | 353 | 282 (6.2) | 71 (7.9) | ||
| Chronic allergy | No | 4851 | 4071 (89.0) | 780 (86.4) | 0.03 1 |
| Declared | 628 | 505 (11.0) | 123 (13.6) | ||
| Autoimmune diseases | No | 5112 | 4266 (93.2) | 846 (93.7) | 0.6 1 |
| Declared | 367 | 310 (6.8) | 57 (6.3) | ||
| Comorbidity (two or more coexisting diseases) | No | 4714 | 3960 (86.5) | 754 (83.5) | 0.02 1 |
| Declared | 765 | 616 (13.5) | 149 (16.5) | ||
| Influenza Vaccination in Last Year | Total Study Group (N = 5479) | |||||||
|---|---|---|---|---|---|---|---|---|
| Anti-SARS-CoV-2 Antibodies IgG N (%) | Anti-SARS-CoV-2 Antibodies IgM N (%) | |||||||
| Neg | Ques | Pos | p Value | Neg | Ques | Pos | p Value | |
| Yes 903 (100) | 753 (83.4) | 9 (1) | 141 (15.6) | <0.0001 | 842 (93.2) | 20 (2.2) | 41 (4.5) | 0.7 |
| No or I do not know 4576 (100) | 3473 (75.9) | 94 (2.1) | 1009 (22.0) | 4237 (92.6) | 105 (2.3) | 234 (5.1) | ||
| Male subjects (N = 2287) | ||||||||
| Yes 392 (100) | 328 (83.7) | 1 (0.3) | 63 (16.0) | 0.0007 | 369 (94.1) | 5 (1.3) | 18 (4.6) | 0.6 |
| No or I do not know 1895 (100) | 1449 (76.4) | 39 (2.1) | 407 (21.5) | 1774 (93.6) | 38 (2.0) | 83 (4.4) | ||
| Female subjects (N = 3192) | ||||||||
| Yes 511 (100) | 425 (83.2) | 8 (1.6) | 78 (15.2) | 0.0008 | 473 (92.5) | 15 (2.9) | 23 (4.5) | 0.5 |
| No or I do not know 2681 (100) | 2024 (75.5) | 55 (2.0) | 602 (22.5) | 2463 (91.9) | 67 (2.5) | 151 (5.6) | ||
| Subjects aged below 65 years (N = 4741) | ||||||||
| Yes 721 (100) | 588 (81.6) | 9 (1.2) | 124 (17.2) | 0.0006 | 674 (93.5) | 15 (2.1) | 32 (4.4) | 0.9 |
| No or I do not know 4020 (100) | 3016 (75) | 89 (2.2) | 915 (22.8) | 3739 (93) | 89 (2.2) | 192 (4.8) | ||
| Subjects aged 65+ years (N = 681) | ||||||||
| Yes 171 (100) | 155 (90.6) | 0 (0) | 16 (9.4) | 0.05 | 159 (93) | 5 (2.9) | 7 (4.1) | 0.2 |
| No or I do not know 510 (100) | 425 (83.3) | 3 (0.6) | 82 (16.1) | 458 (89.8) | 13 (2.5) | 39 (7.7) | ||
| Subjects with contact with COVID-19 or quarantine (N = 3618) | ||||||||
| Yes (100) | 510 (87.5) | 4 (0.7) | 69 (11.8) | 0.004 | 552 (94.7) | 12 (2.1) | 19 (3.2) | 0.5 |
| No or I do not know (100) | 2495 (82.2) | 57 (1.9) | 483 (15.9) | 2862 (94.3) | 50 (1.6) | 123 (4.1) | ||
| Subject without contact with COVID-19 or quarantine (N = 1861) | ||||||||
| Yes (100) | 243 (75.9) | 5 (1.6) | 72 (22.5) | p < 0.0001 | 290 (90.6) | 8 (2.5) | 22 (6.9) | 0.6 |
| No or I do not know (100) | 978 (63.5) | 37 (2.4) | 526 (34.1) | 1375 (89.2) | 55 (3.6) | 111 (7.2) | ||
| Classification Variable (Stratum) | Odds Ratio (95%CI) | |
|---|---|---|
| IgG Antibodies (Positive vs. Nonpositive 1) | IgM Antibodies (Positive vs. Nonpositive 1) | |
| Total population | 0.65 (0.54–0.79) | 0.88 (0.62–1.23) |
| Male | 0.70 (0.52–0.93) | 1.05 (0.60–1.73) |
| Female | 0.62 (0.48–0.80) | 0.79 (0.49–1.21) |
| Younger (<65 years) | 0.70 (0.57–0.86) | 0.93 (0.62–1.34) |
| Older (65+ years) | 0.54 (0.29–0.93) | 0.51 (0.21–1.11) |
| Overweight and obese | 0.73 (0.56–0.94) | 0.74 (0.45–1.16) |
| Declared previous contact with COVID-19 or quarantine | 0.56 (0.42–0.74) | 0.95 (0.58–1.50) |
| Declared hypertension | 0.63 (0.43–0.91) | 0.66 (0.34–1.16) |
| Declared diabetes | 0.34 (0.12–0.82) | 0.29 (0.02–1.52) |
| Declared chronic allergy | 0.47 (0.26–0.82) | 0.18 (0.02–1.35) |
| Declared autoimmune diseases | 0.19 (0.04–0.53) | 0.27 (0.01–1.36) |
| Declared comorbidity (two or more coexisting diseases) | 0.54 (0.31–0.90) | 0.43 (0.13–1.10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, M.; Niewiadomska, E.; Barański, K.; Kaleta-Pilarska, A.; Brożek, G.; Zejda, J.E. Association between Influenza Vaccination and Positive SARS-CoV-2 IgG and IgM Tests in the General Population of Katowice Region, Poland. Vaccines 2021, 9, 415. https://doi.org/10.3390/vaccines9050415
Kowalska M, Niewiadomska E, Barański K, Kaleta-Pilarska A, Brożek G, Zejda JE. Association between Influenza Vaccination and Positive SARS-CoV-2 IgG and IgM Tests in the General Population of Katowice Region, Poland. Vaccines. 2021; 9(5):415. https://doi.org/10.3390/vaccines9050415
Chicago/Turabian StyleKowalska, Małgorzata, Ewa Niewiadomska, Kamil Barański, Angelina Kaleta-Pilarska, Grzegorz Brożek, and Jan Eugeniusz Zejda. 2021. "Association between Influenza Vaccination and Positive SARS-CoV-2 IgG and IgM Tests in the General Population of Katowice Region, Poland" Vaccines 9, no. 5: 415. https://doi.org/10.3390/vaccines9050415
APA StyleKowalska, M., Niewiadomska, E., Barański, K., Kaleta-Pilarska, A., Brożek, G., & Zejda, J. E. (2021). Association between Influenza Vaccination and Positive SARS-CoV-2 IgG and IgM Tests in the General Population of Katowice Region, Poland. Vaccines, 9(5), 415. https://doi.org/10.3390/vaccines9050415








