Adjuvant HPV Vaccination to Prevent Recurrent Cervical Dysplasia after Surgical Treatment: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
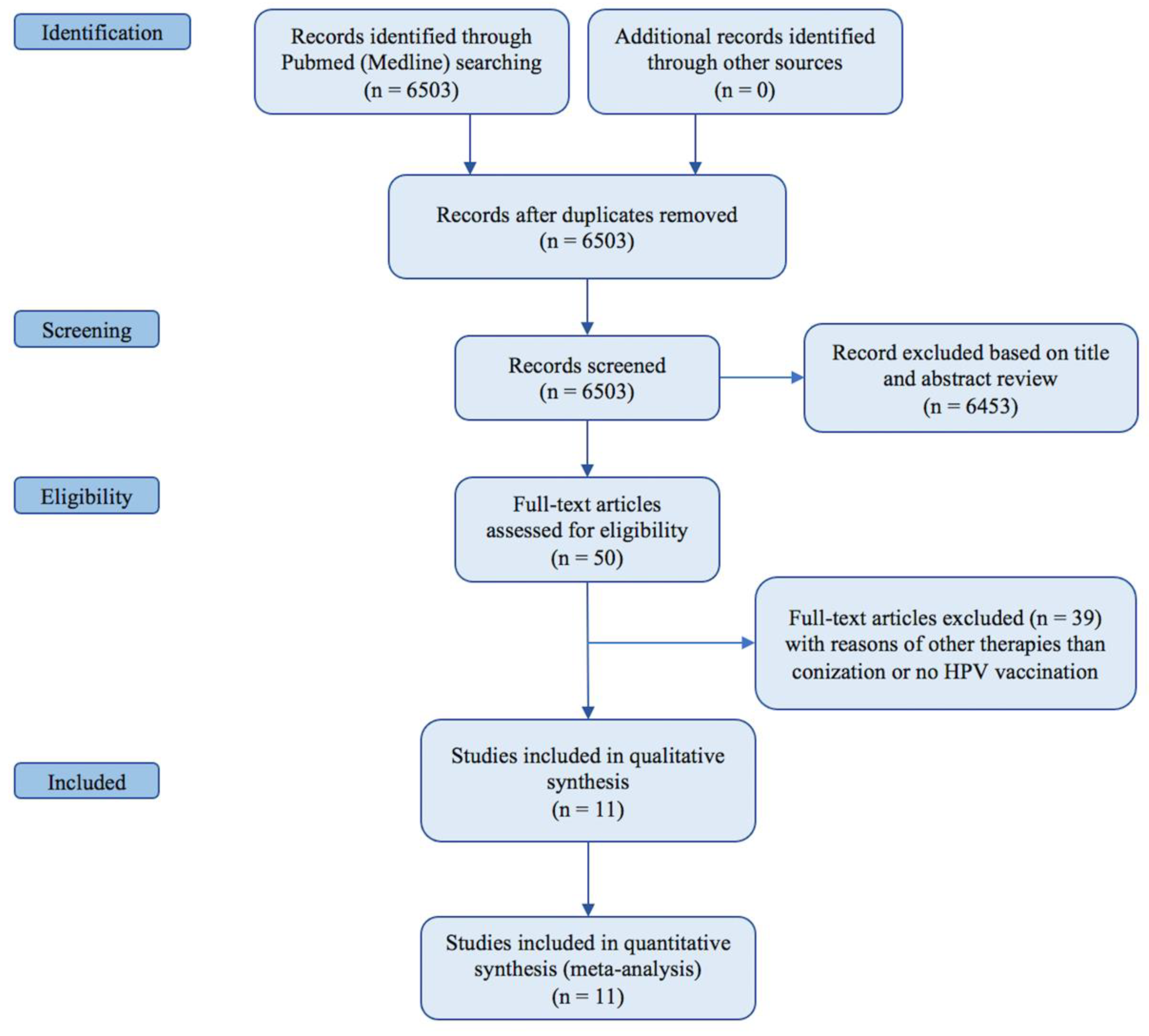
2.2. Selection of Studies and Methodologic Quality Assessment
2.3. Outcomes
- (1)
- Primary outcomes
- 1.1
- CIN 2+ recurrence: recurrences of CIN 2+ (irrespective of HPV type and HPV 16/18-related) have been extrapolated from the studies.
- 1.2
- CIN 1+ recurrence: recurrences of CIN 1+ (irrespective of HPV type and HPV 16/18-related) have been extrapolated from the studies.
- (2)
- Secondary outcomes
- 2.1
- HPV persistence: the detection of positive HPV test at the first clinical follow-up after surgical treatment.
- 2.2
- CIN 3 recurrence: recurrences of CIN 3 (irrespective of HPV type and HPV 16/18-related) have been extrapolated from the studies.
- 2.3
- Persistence of abnormal cervical cytology: the detection of abnormal cervical cytology at the first clinical follow-up after surgical treatment.
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics
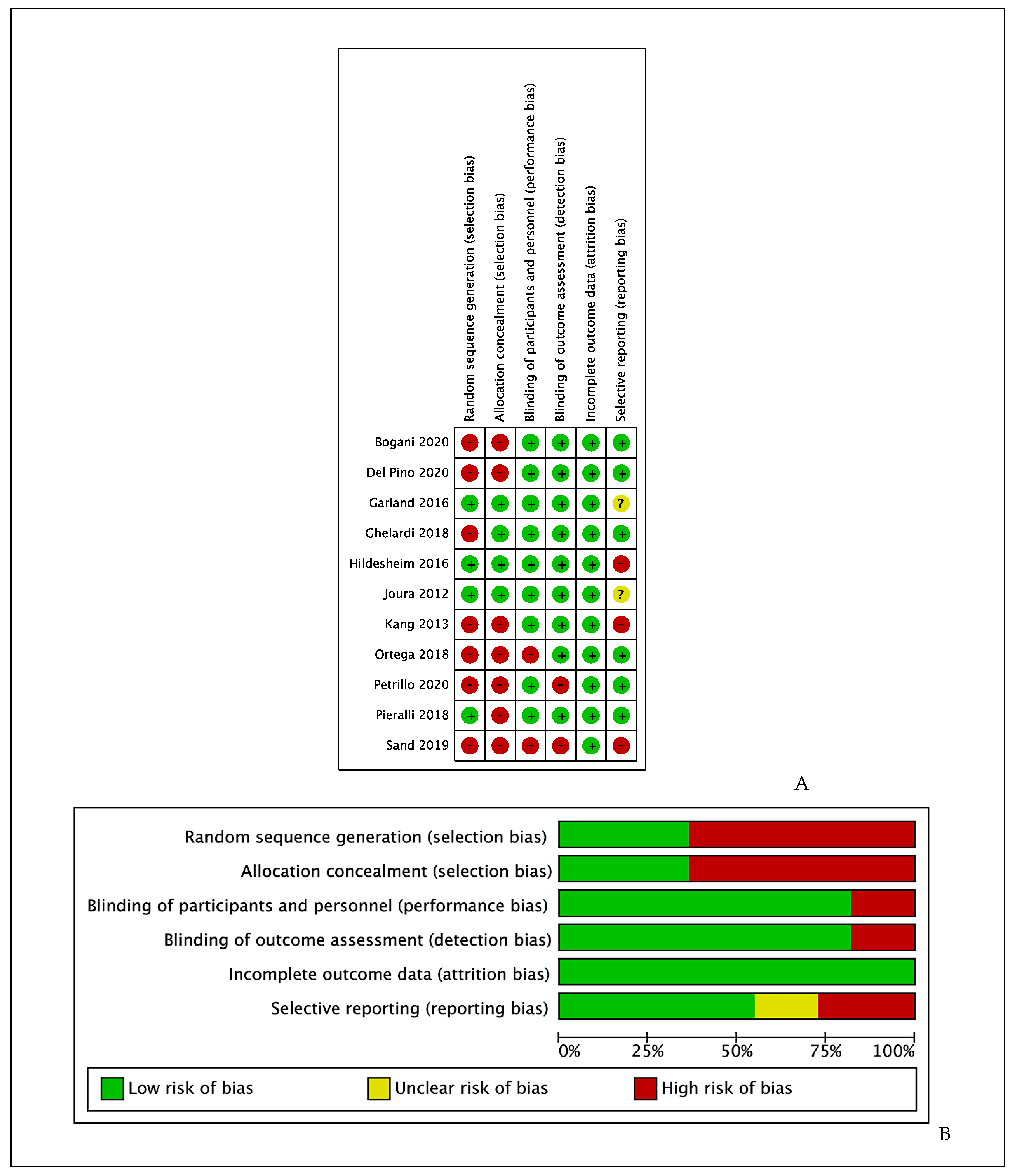
3.2. Risk of Bias
- -
- -
- -
- -
- -
- Incomplete outcome data (attrition bias): All studies reported complete outcome data, thus we rated all them as having low risk of bias for this item.
- -
3.3. Effects of Interventions
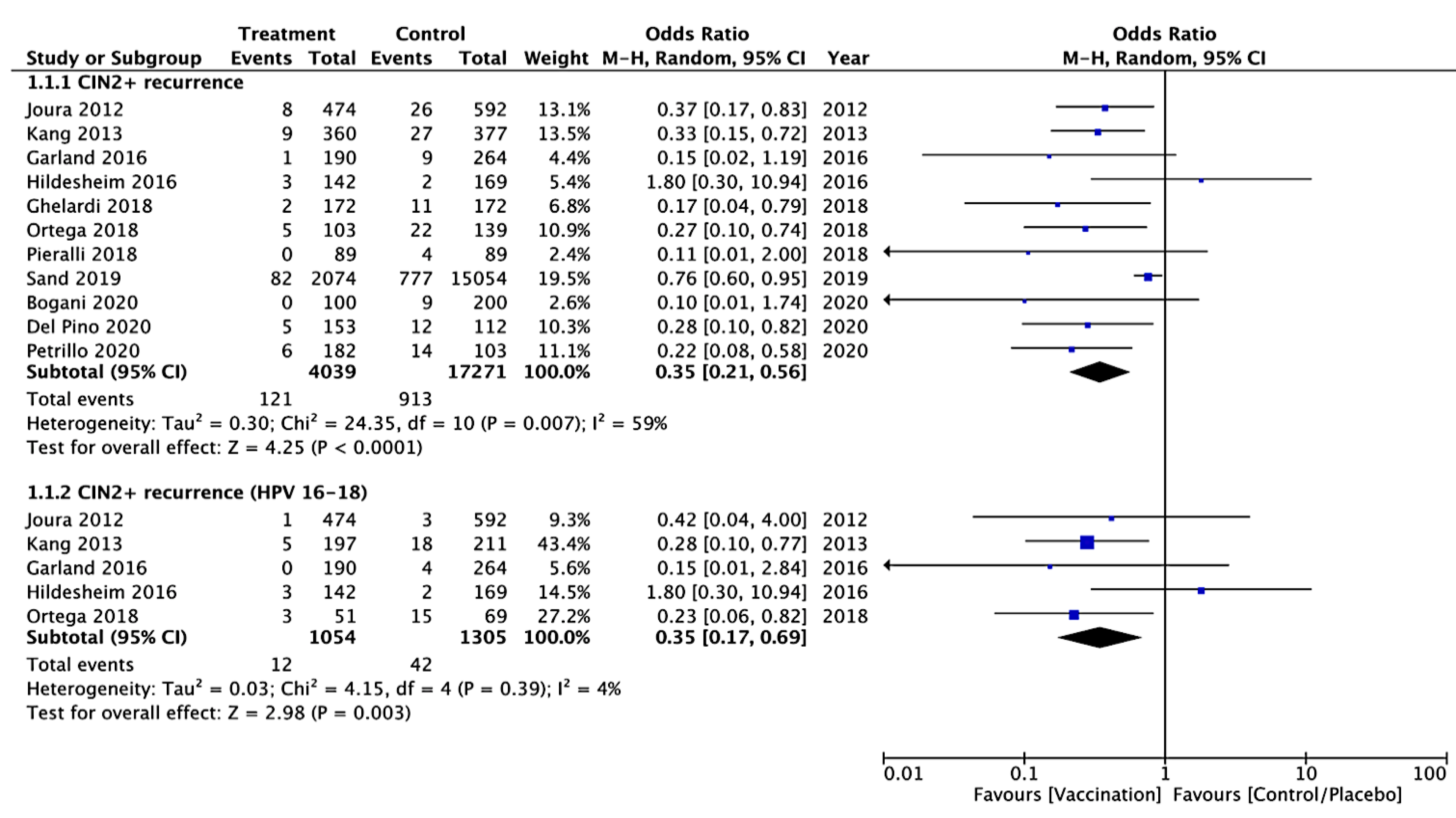
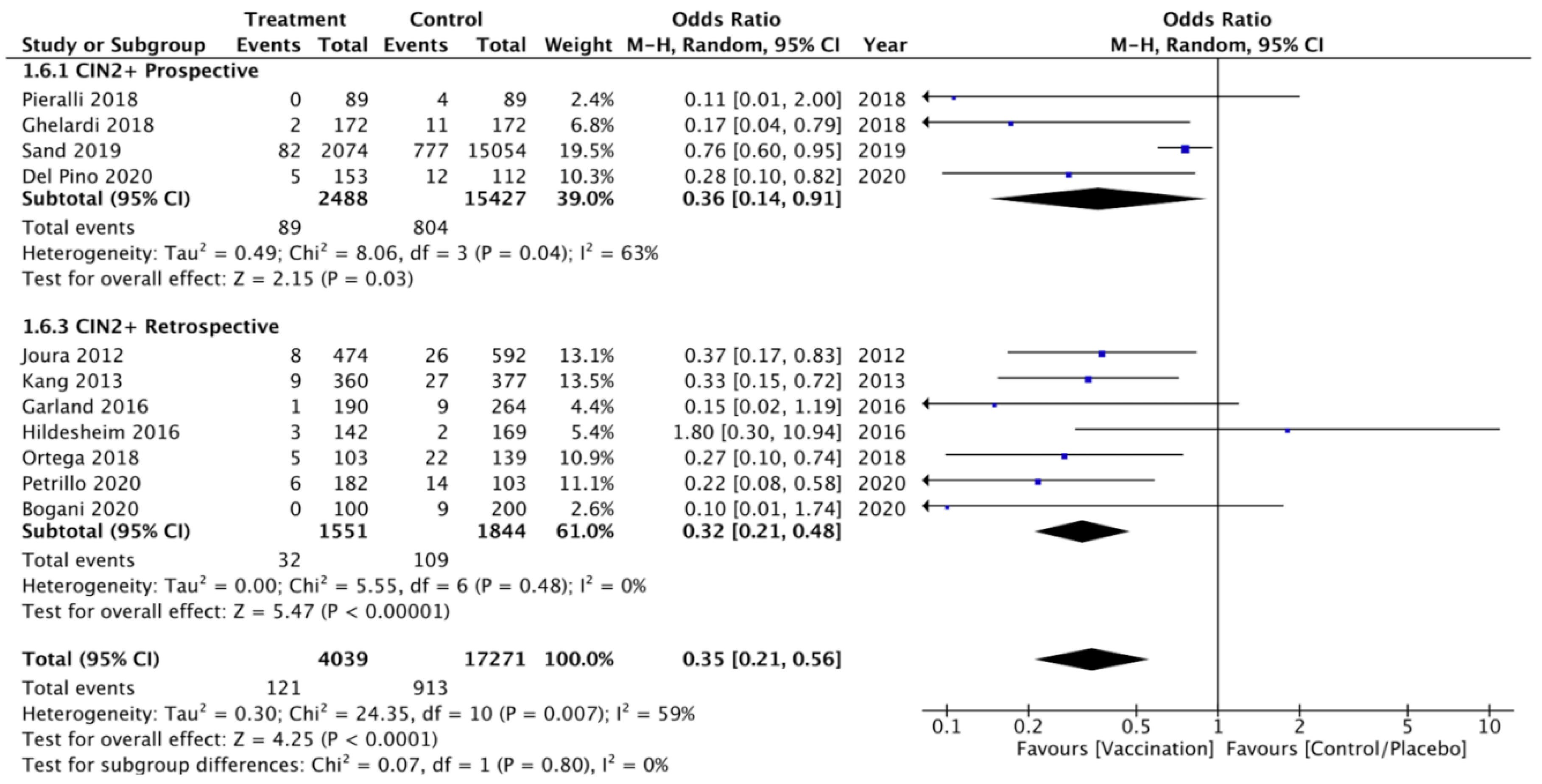
3.3.1. Primary Outcomes
CIN 2+ Recurrence
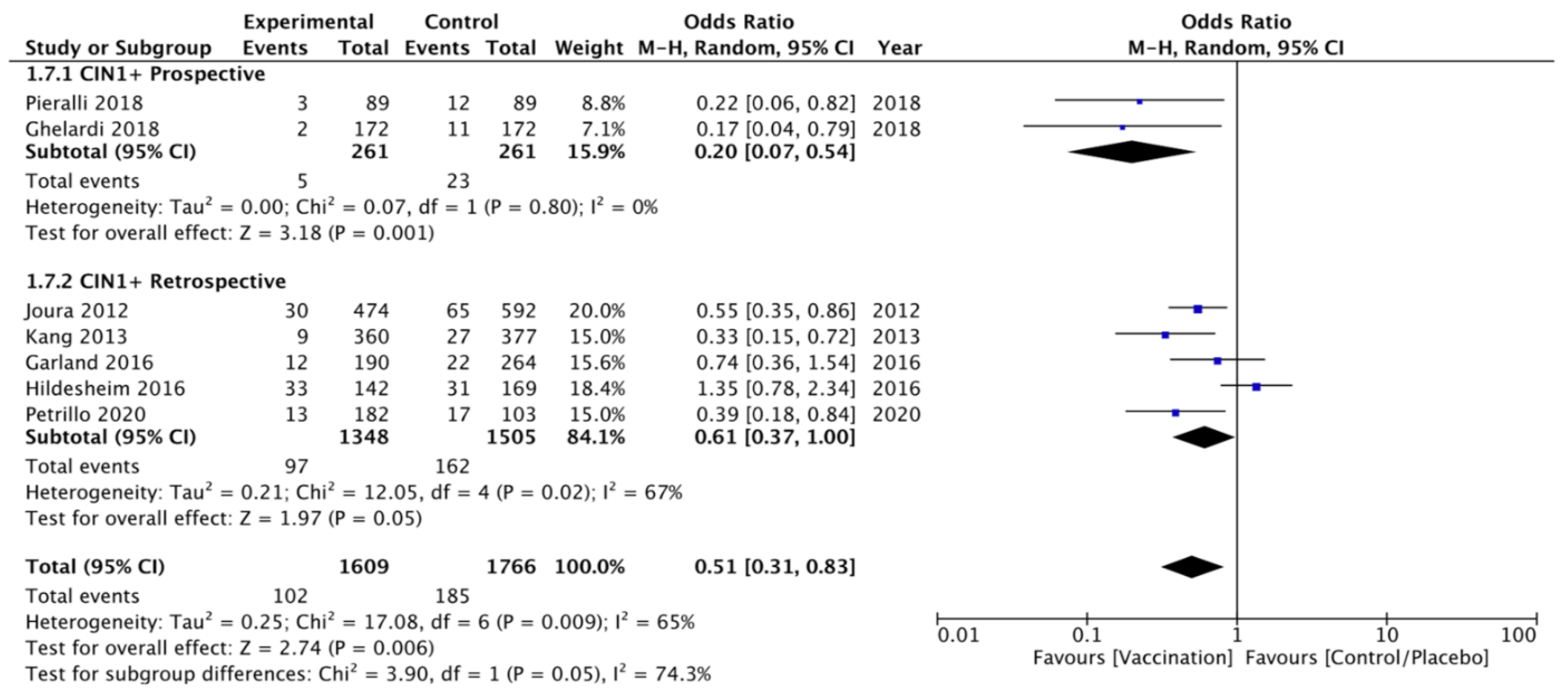
CIN 1+ Recurrence
3.3.2. Secondary Outcomes
HPV Persistence
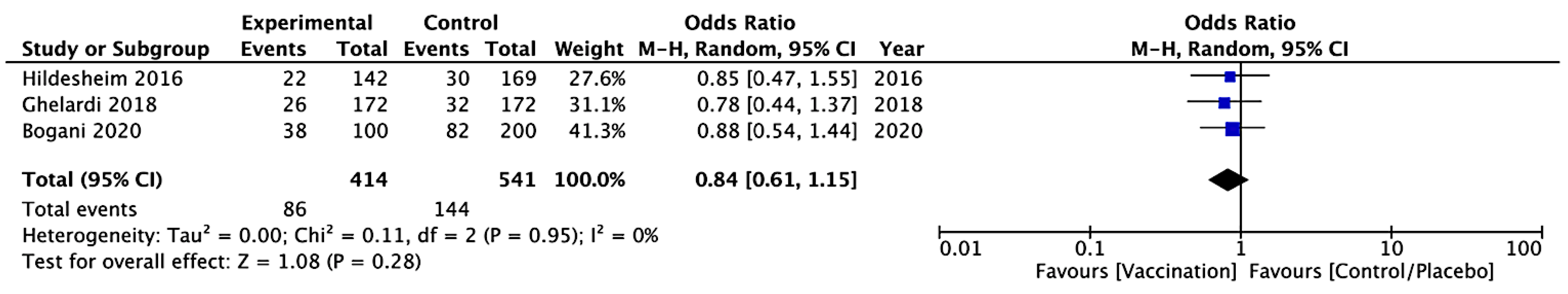
CIN 3 Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy (Pubmed/Medline Database)
- Cervical cancer: “uterine cervical neoplasms” [MeSH Terms] OR (“uterine”[All Fields] AND “cervical”[All Fields] AND “neoplasms”[All Fields]) OR “uterine cervical neoplasms”[All Fields] OR (“cervical”[All Fields] AND “cancer”[All Fields]) OR “cervical cancer”[All Fields] (111,287 results)
- Cervical disease: “uterine cervical diseases”[MeSH Terms] OR (“uterine”[All Fields] AND “cervical”[All Fields] AND “diseases”[All Fields]) OR “uterine cervical diseases”[All Fields] OR (“cervical”[All Fields] AND “disease”[All Fields]) OR “cervical disease”[All Fields] (118,317 results)
- Cervical intraepithelial neoplasia: “cervical intraepithelial neoplasia”[MeSH Terms] OR (“cervical”[All Fields] AND “intraepithelial”[All Fields] AND “neoplasia”[All Fields]) OR “cervical intraepithelial neoplasia”[All Fields] (14,638 results)
- Human papillomavirus: “alphapapillomavirus”[MeSH Terms] OR “alphapapillomavirus”[All Fields] OR (“human”[All Fields] AND “papillomavirus”[All Fields]) OR “human papillomavirus”[All Fields] (44,121 results)
- Vaccine: “vaccine”[Supplementary Concept] OR “vaccine”[All Fields] OR “vaccination”[MeSH Terms] OR “vaccination”[All Fields] OR “vaccinable”[All Fields] OR “vaccinal”[All Fields] OR “vaccinate”[All Fields] OR “vaccinated”[All Fields] OR “vaccinates”[All Fields] OR “vaccinating”[All Fields] OR “vaccinations”[All Fields] OR “vaccination’s”[All Fields] OR “vaccinator”[All Fields] OR “vaccinators”[All Fields] OR “vaccine’s”[All Fields] OR “vaccined”[All Fields] OR “vaccines”[MeSH Terms] OR “vaccines”[All Fields] OR “vaccine”[All Fields] OR “vaccins”[All Fields] (409,438 results)
- Vaccination: “vaccin”[Supplementary Concept] OR “vaccin”[All Fields] OR “vaccination”[MeSH Terms] OR “vaccination”[All Fields] OR “vaccinable”[All Fields] OR “vaccinal”[All Fields] OR “vaccinate”[All Fields] OR “vaccinated”[All Fields] OR “vaccinates”[All Fields] OR “vaccinating”[All Fields] OR “vaccinations”[All Fields] OR “vaccination’s”[All Fields] OR “vaccinator”[All Fields] OR “vaccinators”[All Fields] OR “vaccine’s”[All Fields] OR “vaccined”[All Fields] OR “vaccines”[MeSH Terms] OR “vaccines”[All Fields] OR “vaccine”[All Fields] OR “vaccins”[All Fields] (409,438 results)
- 1 OR 2 OR 3 (144,114 results)
- 5 OR 6 (409,438 results)
- 5 AND 7 AND 8 (6503 results)
References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Tjalma, W. HPV negative cervical cancers and primary HPV screening. Facts Views Vis. ObGyn 2018, 10, 107–113. [Google Scholar]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Prabhu, V.S.; Snedecor, S.J.; Corman, S.; Kuter, B.J.; Nwankwo, C.; Chirovsky, D.; Myers, E. Economic Value of Lost Productivity Attributable to Human Papillomavirus Cancer Mortality in the United States. Front. Public Health 2020, 8, 624092. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018, 5, CD009069. [Google Scholar] [CrossRef]
- Koh, W.-J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Clark, R.; Cohn, D.; et al. Cervical Cancer, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 64–84. [Google Scholar] [CrossRef] [PubMed]
- Dostalek, L.; Åvall-Lundqvist, E.; Creutzberg, C.L.; Kurdiani, D.; Ponce, J.; Dostalkova, I.; Cibula, D. ESGO Survey on Current Practice in the Management of Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R. HPV vaccination: The most pragmatic cervical cancer primary prevention strategy. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. 1), S33–S35. [Google Scholar] [CrossRef] [PubMed]
- Pils, S.; Joura, E.A. From the monovalent to the nine-valent HPV vaccine. Clin. Microbiol. Infect. 2015, 21, 827–833. [Google Scholar] [CrossRef]
- Radley, D.; Saah, A.; Stanley, M. Persistent infection with human papillomavirus 16 or 18 is strongly linked with high-grade cervical disease. Hum. Vaccines Immunother. 2016, 12, 768–772. [Google Scholar] [CrossRef]
- Huh, W.K.; Joura, E.A.; Giuliano, A.R.; Iversen, O.-E.; de Andrade, R.P.; Ault, K.A.; Bartholomew, D.; Cestero, R.M.; Fedrizzi, E.N.; Hirschberg, A.L.; et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: A randomised, double-blind trial. Lancet 2017, 390, 2143–2159. [Google Scholar] [CrossRef]
- Khan, M.J.; Smith-McCune, K.K. Treatment of cervical precancers: Back to basics. Obstet. Gynecol. 2014, 123, 1339–1343. [Google Scholar] [CrossRef]
- Kocken, M.; Helmerhorst, T.J.M.; Berkhof, J.; Louwers, J.A.; Nobbenhuis, M.A.E.; Bais, A.G.; Hogewoning, C.J.; Zaal, A.; Verheijen, R.H.M.; Snijders, P.J.F.; et al. Risk of recurrent high-grade cervical intraepithelial neoplasia after successful treatment: A long-term multi-cohort study. Lancet Oncol. 2011, 12, 441–450. [Google Scholar] [CrossRef]
- Barra, F.; Della Corte, L.; Noberasco, G.; Foreste, V.; Riemma, G.; Di Filippo, C.; Bifulco, G.; Orsi, A.; Icardi, G.; Ferrero, S. Advances in therapeutic vaccines for treating human papillomavirus-related cervical intraepithelial neoplasia. J. Obstet. Gynaecol. Res. 2020, 46, 989–1006. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Zarchi, M.; Allahqoli, L.; Nehmati, A.; Kashi, A.M.; Taghipour-Zahir, S.; Alkatout, I. Can the prophylactic quadrivalent HPV vaccine be used as a therapeutic agent in women with CIN? A randomized trial. BMC Public Health 2020, 20, 274. [Google Scholar] [CrossRef]
- Wang, R.; Pan, W.; Jin, L.; Huang, W.; Li, Y.; Wu, D.; Gao, C.; Ma, D.; Liao, S. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer Lett. 2020, 471, 88–102. [Google Scholar] [CrossRef]
- Bartels, H.C.; Postle, J.; Rogers, A.C.; Brennan, D. Prophylactic human papillomavirus vaccination to prevent recurrence of cervical intraepithelial neoplasia: A meta-analysis. Int. J. Gynecol. Cancer 2020, 30, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Lichter, K.; Krause, D.; Xu, J.; Tsai, S.H.L.; Hage, C.; Weston, E.; Eke, A.; Levinson, K. Adjuvant Human Papillomavirus Vaccine to Reduce Recurrent Cervical Dysplasia in Unvaccinated Women: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2020, 135, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
- Jentschke, M.; Kampers, J.; Becker, J.; Sibbertsen, P.; Hillemanns, P. Prophylactic HPV vaccination after conization: A systematic review and meta-analysis. Vaccine 2020, 38, 6402–6409. [Google Scholar] [CrossRef] [PubMed]
- Mariz, F.C.; Bender, N.; Anantharaman, D.; Basu, P.; Bhatla, N.; Pillai, M.R.; Prabhu, P.R.; Sankaranarayanan, R.; Eriksson, T.; Pawlita, M.; et al. Peak neutralizing and cross-neutralizing antibody levels to human papillomavirus types 6/16/18/31/33/45/52/58 induced by bivalent and quadrivalent HPV vaccines. NPJ Vaccines 2020, 5, 14. [Google Scholar] [CrossRef]
- Athanasiou, A.; Bowden, S.; Paraskevaidi, M.; Fotopoulou, C.; Martin-Hirsch, P.; Paraskevaidis, E.; Kyrgiou, M. HPV vaccination and cancer prevention. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Ault, K.A. Human papillomavirus vaccines and the potential for cross-protection between related HPV types. Gynecol. Oncol. 2007, 107, S31–S33. [Google Scholar] [CrossRef]
- De Vincenzo, R.; Ricci, C.; Conte, C.; Scambia, G. HPV vaccine cross-protection: Highlights on additional clinical benefit. Gynecol. Oncol. 2013, 130, 642–651. [Google Scholar] [CrossRef]
- Söderlund-Strand, A.; Kjellberg, L.; Dillner, J. Human papillomavirus type-specific persistence and recurrence after treatment for cervical dysplasia. J. Med. Virol. 2014, 86, 634–641. [Google Scholar] [CrossRef]
- Byun, J.M.; Jeong, D.H.; Kim, Y.N.; Jung, E.J.; Lee, K.B.; Sung, M.S.; Kim, K.T. Persistent HPV-16 infection leads to recurrence of high-grade cervical intraepithelial neoplasia. Medicine 2018, 97, e13606. [Google Scholar] [CrossRef] [PubMed]
- Saftlas, A.F.; Spracklen, C.N.; Ryckman, K.K.; Stockdale, C.K.; Penrose, K.; Ault, K.; Rubenstein, L.M.; Pinto, L.A. Influence of a loop electrosurgical excision procedure (LEEP) on levels of cytokines in cervical secretions. J. Reprod. Immunol. 2015, 109, 74–83. [Google Scholar] [CrossRef]
- Scott, M.E.; Shvetsov, Y.B.; Thompson, P.J.; Hernandez, B.Y.; Zhu, X.; Wilkens, L.R.; Killeen, J.; Vo, D.D.; Moscicki, A.; Goodman, M. Cervical cytokines and clearance of incident human papillomavirus infection: Hawaii HPV cohort study: Mucosal cytokines and cervical HPV clearance. Int. J. Cancer 2013, 133, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Frazer, I.H. Interaction of human papillomaviruses with the host immune system: A well evolved relationship. Virology 2009, 384, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0; updated March 2011; The Cochrane Collaboration: London, UK; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Di Donato, V.; Kontopantelis, E.; Aletti, G.; Casorelli, A.; Piacenti, I.; Bogani, G.; Lecce, F.; Benedetti Panici, P. Trends in Mortality after Primary Cytoreductive Surgery for Ovarian Cancer: A Systematic Review and Metaregression of Randomized Clinical Trials and Observational Studies. Ann. Surg. Oncol. 2017, 24, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, V.; Casorelli, A.; Bardhi, E.; Vena, F.; Marchetti, C.; Muzii, L.; Benedetti Panici, P. Bartholin gland cancer. Crit Rev. Oncol. Hematol. 2017, 117, 1–11. [Google Scholar] [CrossRef]
- Di Donato, V.; Bardhi, E.; Tramontano, L.; Capomacchia, F.M.; Palaia, I.; Perniola, G.; Plotti, F.; Angioli, R.; Giancotti, A.; Muzii, L.; et al. Management of morbidity associated with pancreatic resection during cytoreductive surgery for epithelial ovarian cancer: A systematic review. Eur. J. Surg. Oncol. 2020, 46, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, V.; Caruso, G.; Bogani, G.; Giannini, A.; D’Oria, O.; Perniola, G.; Palaia, I.; Plotti, F.; Angioli, R.; Muzii, L.; et al. Preoperative frailty assessment in patients undergoing gynecologic oncology surgery: A systematic review. Gynecol. Oncol. 2021. [Google Scholar] [CrossRef]
- Kontopantelis, E.; Springate, D.A.; Reeves, D. A Re-Analysis of the Cochrane Library Data: The Dangers of Unobserved Heterogeneity in Meta-Analyses. PLoS ONE 2013, 8, e69930. [Google Scholar] [CrossRef]
- Joura, E.A.; Garland, S.M.; Paavonen, J.; Ferris, D.G.; Perez, G.; Ault, K.A.; Huh, W.K.; Sings, H.L.; James, M.W.; Haupt, R.M. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: Retrospective pooled analysis of trial data. BMJ 2012, 344, e1401. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.D.; Choi, H.S.; Kim, S.M. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2–3)? Gynecol. Oncol. 2013, 130, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.M.; Paavonen, J.; Jaisamrarn, U.; Naud, P.; Salmerón, J.; Chow, S.; Apter, D.; Castellsagué, X.; Teixeira, J.C.; Skinner, S.R.; et al. Prior human papillomavirus-16/18 AS04-adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: Post-hoc analysis from a randomized controlled trial. Int. J. Cancer 2016, 139, 2812–2826. [Google Scholar] [CrossRef]
- Hildesheim, A.; Gonzalez, P.; Kreimer, A.R.; Wacholder, S.; Schussler, J.; Rodriguez, A.C.; Porras, C.; Schiffman, M.; Sidawy, M.; Schiller, J.T.; et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment. Am. J. Obstet. Gynecol. 2016, 215, 212.e1–212.e15. [Google Scholar] [CrossRef] [PubMed]
- Ghelardi, A.; Parazzini, F.; Martella, F.; Pieralli, A.; Bay, P.; Tonetti, A.; Svelato, A.; Bertacca, G.; Lombardi, S.; Joura, E.A. SPERANZA project: HPV vaccination after treatment for CIN2+. Gynecol. Oncol. 2018, 151, 229–234. [Google Scholar] [CrossRef]
- Pieralli, A.; Bianchi, C.; Auzzi, N.; Fallani, M.G.; Bussani, C.; Fambrini, M.; Cariti, G.; Scarselli, G.; Petraglia, F.; Ghelardi, A.; et al. Indication of prophylactic vaccines as a tool for secondary prevention in HPV-linked disease. Arch. Gynecol. Obstet. 2018, 298, 1205–1210. [Google Scholar] [CrossRef]
- Ortega-Quiñonero, P.; Remezal-Solano, M.; Carazo-Díaz, M.C.; Prieto-Merino, D.; Urbano-Reyes, M.I.; de Guadiana-Romualdo, L.G.; Martínez-Cendán, J.P. Impact of the human papillomavirus vaccination on patients who underwent conization for high-grade cervical intraepithelial neoplasia. Eur. J. Gynaecol. Oncol. 2019, 40, 402–407. [Google Scholar]
- Sand, F.L.; Kjaer, S.K.; Frederiksen, K.; Dehlendorff, C. Risk of cervical intraepithelial neoplasia grade 2 or worse after conization in relation to HPV vaccination status. Int. J. Cancer 2020, 147, 641–647. [Google Scholar] [CrossRef]
- Petrillo, M.; Dessole, M.; Tinacci, E.; Saderi, L.; Muresu, N.; Capobianco, G.; Cossu, A.; Dessole, S.; Sotgiu, G.; Piana, A. Efficacy of HPV Vaccination in Women Receiving LEEP for Cervical Dysplasia: A Single Institution’s Experience. Vaccines 2020, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- del Pino, M.; Martí, C.; Torras, I.; Henere, C.; Munmany, M.; Marimon, L.; Saco, A.; Torné, A.; Ordi, J. HPV Vaccination as Adjuvant to Conization in Women with Cervical Intraepithelial Neoplasia: A Study under Real-Life Conditions. Vaccines 2020, 8, 245. [Google Scholar] [CrossRef]
- Bogani, G.; Raspagliesi, F.; Sopracordevole, F.; Ciavattini, A.; Ghelardi, A.; Simoncini, T.; Petrillo, M.; Plotti, F.; Lopez, S.; Casarin, J.; et al. Assessing the Long-Term Role of Vaccination against HPV after Loop Electrosurgical Excision Procedure (LEEP): A Propensity-Score Matched Comparison. Vaccines 2020, 8, 717. [Google Scholar] [CrossRef] [PubMed]
- Coskuner, E.R.; Ozkan, T.A.; Karakose, A.; Dillioglugil, O.; Cevik, I. Impact of the quadrivalent HPV vaccine on disease recurrence in men exposed to HPV Infection: A randomized study. J. Sex. Med. 2014, 11, 2785–2791. [Google Scholar] [CrossRef]
- Ghelardi, A.; Marrai, R.; Bogani, G.; Sopracordevole, F.; Bay, P.; Tonetti, A.; Lombardi, S.; Bertacca, G. Surgical Treatment of Vulvar HSIL: Adjuvant HPV Vaccine Reduces Recurrent Disease. Vaccines 2021, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Grzes, B.; Heimrath, J.; Ciszek, M. Minimally invasive surgery with the complementing immunotherapy in the treatment of intraepithelial neoplasia of cervix in women of child-bearing age. Onkol. Pol. 2011, 14, 125–130. [Google Scholar]

| Study, Year | Study Design | N. of Patients Age (Years) | Primary Endpoint | HPV Vaccine Type and Time of Vaccination | Surgical Treatment |
|---|---|---|---|---|---|
| Joura et al., 2012 [37] | Post-hoc-pooled analysis of 2 RCT (FUTURE I and II) Follow-up 2.5 years (median) | 1066 15–26 | CIN 2+ (HPV-type independent) | Quadrivalent at day 1, month 2, and month 6 after surgery | LEEP (84.7%), cervical conization (12.5%), cryotherapy (0.7%), and other n.a. (2.1%) |
| CIN 2+ (HPV 16, 18) | |||||
| CIN 1+ (HPV-type independent) | |||||
| CIN 1+ (HPV 16, 18) | |||||
| CIN 3 (HPV-type independent) | |||||
| Kang et al., 2013 [38] | Retrospective case-control Follow-up 3.5 years (median) | 737 20–45 | CIN 2+ (HPV-type independent) | Quadrivalent at week 1, month 2, and month 6 after surgery | LEEP |
| CIN 2+ (HPV 16, 18) | |||||
| CIN 1+ (HPV-type independent) | |||||
| CIN 1+ (HPV 16, 18) | |||||
| Garland et al., 2016 [39] | Post-hoc analysis of a RCT (PATRICIA) Follow-up 4 years | 454 15–25 | CIN 2+ (HPV-type independent) | Bivalent at months 0, 1, and 6 after surgery | LEEP |
| CIN 2+ (HPV 16, 18) | |||||
| CIN 1+ (HPV-type independent) | |||||
| CIN 1+ (HPV 16, 18) | |||||
| Hildesheim et al., 2016 [40] | Subgroup analysis of a RCT Follow-up 27.3 mo (median) | 311 18–25 | CIN 2+ (HPV-type independent) | Bivalent, 3 doses over 6 months after surgery | LEEP |
| CIN 2+ (HPV 16, 18) | |||||
| CIN 1+ (HPV-type independent) | |||||
| CIN 1+ (HPV 16, 18) | |||||
| Ghelardi et al., 2018 [41] | Prospective case-control (SPERANZA project) Follow-up 4 years | 344 18–45 | CIN 2+ (HPV-type independent) | Quadrivalent at day 30, month 2, and month 6 after surgery | LEEP |
| CIN 1+ (HPV-type independent) | |||||
| Pieralli et al., 2018 [42] | RCT Follow-up 3 years | 178 <45 | CIN 2+ (HPV-type independent) | Quadrivalent at months 0, 2 and 6 after surgery | Conization (83%), other n.a. (17%) |
| CIN 1+ (HPV-type independent) | |||||
| Ortega-Quinonero et al., 2019 [43] | Retrospective Follow-up 2 years | 242 18–65 | CIN 2+ (HPV-type independent) | Bi-/Quadrivalent, first dose 0-1 months before or 0-1 months after surgery, other 2 doses over 6 months | LEEP |
| CIN 2+ (HPV 16, 18) | |||||
| Sand et al., 2020 [44] | Prospective cohort | 17128 17–51 | CIN 2+ (HPV-type independent) | Bi-/Quadrivalent, first dose 0-3 months before or 0-12 months after surgery | Conization |
| Petrillo et al., 2020 [45] | Retrospective Follow-up 2 years | 285 32–47 | CIN 2+ (HPV-type independent) | Bi-/Quadrivalent, first dose 0-1 months after surgery | LEEP |
| CIN 1+ (HPV-type independent) | |||||
| Del Pino et al., 2020 [46] | Prospective Follow up 22.4 mo median | 265 26–64 | CIN 2+ (HPV-type independent) | Bivalent at 0, 1 and 6 months after surgery Quadrivalent at 0, 2 and 6 months after surgery | Conization |
| Bogani et al., 2020 [47] | Retrospective, multicenter Follow-up 5 years | 300 18–89 | CIN 2+ (HPV-type independent) | Bi-/Quadrivalent | LEEP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Donato, V.; Caruso, G.; Petrillo, M.; Kontopantelis, E.; Palaia, I.; Perniola, G.; Plotti, F.; Angioli, R.; Muzii, L.; Benedetti Panici, P.; et al. Adjuvant HPV Vaccination to Prevent Recurrent Cervical Dysplasia after Surgical Treatment: A Meta-Analysis. Vaccines 2021, 9, 410. https://doi.org/10.3390/vaccines9050410
Di Donato V, Caruso G, Petrillo M, Kontopantelis E, Palaia I, Perniola G, Plotti F, Angioli R, Muzii L, Benedetti Panici P, et al. Adjuvant HPV Vaccination to Prevent Recurrent Cervical Dysplasia after Surgical Treatment: A Meta-Analysis. Vaccines. 2021; 9(5):410. https://doi.org/10.3390/vaccines9050410
Chicago/Turabian StyleDi Donato, Violante, Giuseppe Caruso, Marco Petrillo, Evangelos Kontopantelis, Innocenza Palaia, Giorgia Perniola, Francesco Plotti, Roberto Angioli, Ludovico Muzii, Pierluigi Benedetti Panici, and et al. 2021. "Adjuvant HPV Vaccination to Prevent Recurrent Cervical Dysplasia after Surgical Treatment: A Meta-Analysis" Vaccines 9, no. 5: 410. https://doi.org/10.3390/vaccines9050410
APA StyleDi Donato, V., Caruso, G., Petrillo, M., Kontopantelis, E., Palaia, I., Perniola, G., Plotti, F., Angioli, R., Muzii, L., Benedetti Panici, P., & Bogani, G. (2021). Adjuvant HPV Vaccination to Prevent Recurrent Cervical Dysplasia after Surgical Treatment: A Meta-Analysis. Vaccines, 9(5), 410. https://doi.org/10.3390/vaccines9050410






