Artificial Feeding of All Consecutive Life Stages of Ixodes ricinus
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Feeding
2.2. Artificial Tick Feeding System (ATFS)
2.3. Artificial Membranes
2.4. Animal Hair Extract
2.5. Blood Meal and the Artificial Tick Feeding Procedure
2.6. Data Collection of In Vitro and In Vivo Feedings
2.7. Video
2.8. Statistics
3. Results
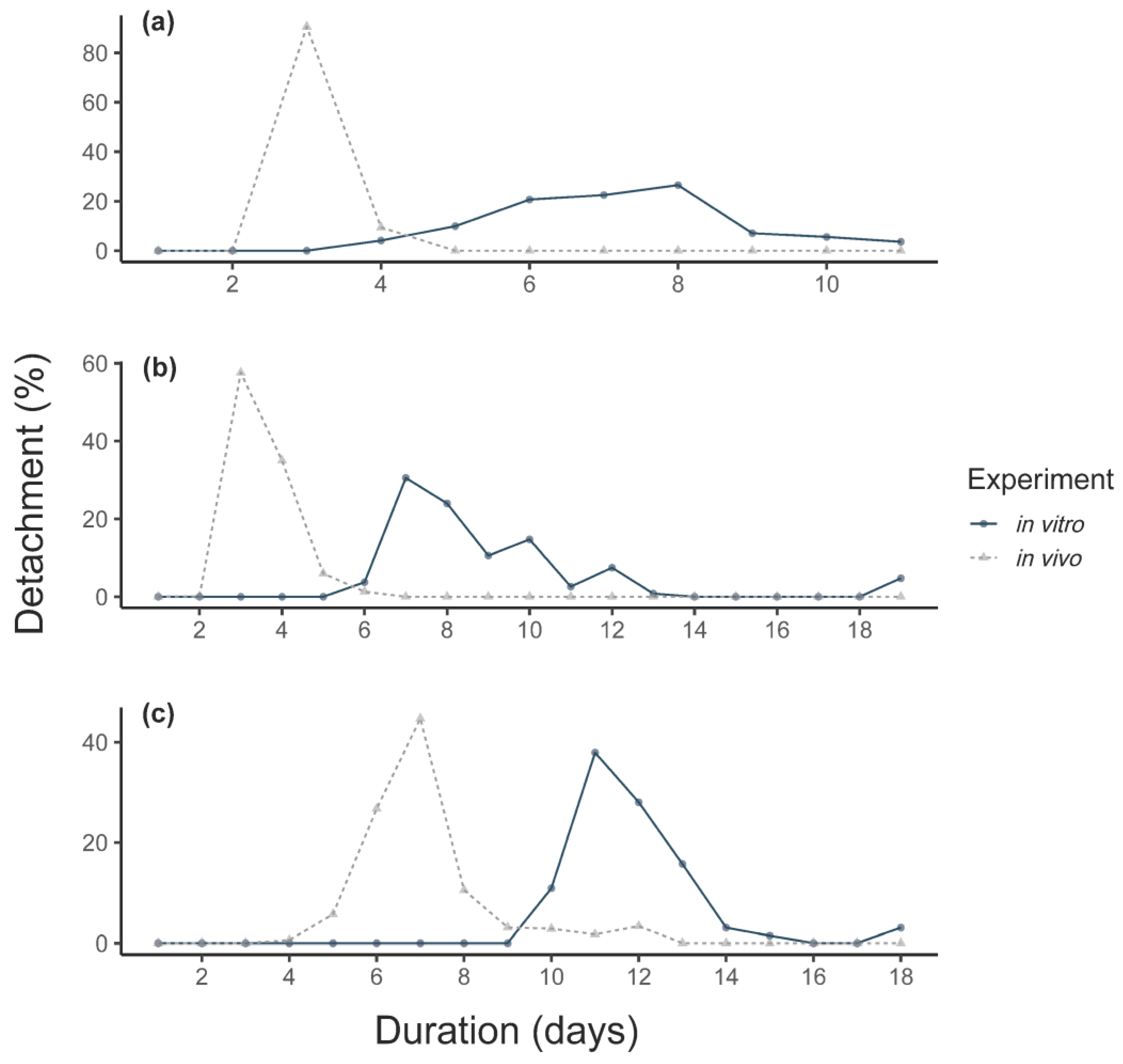
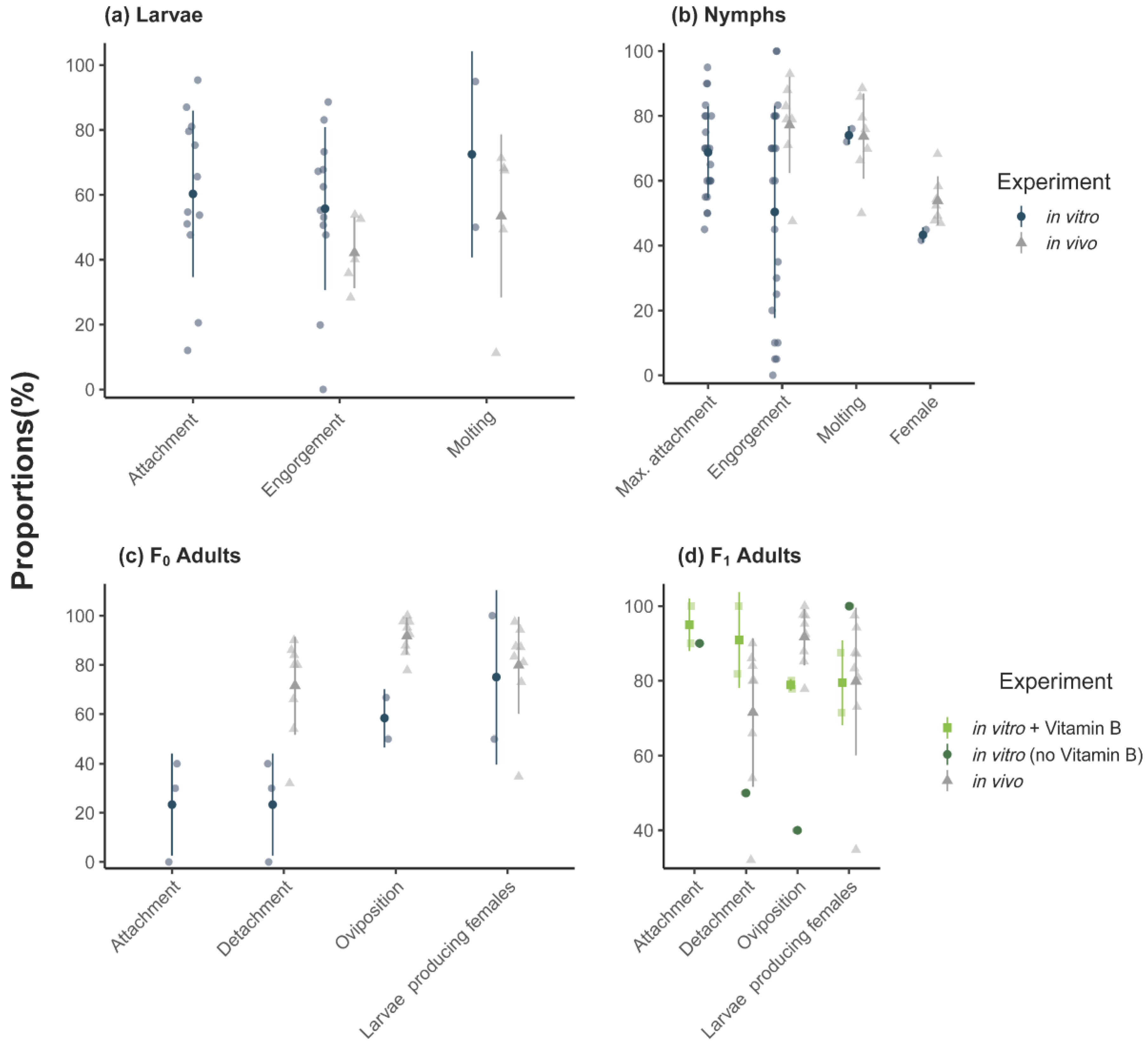
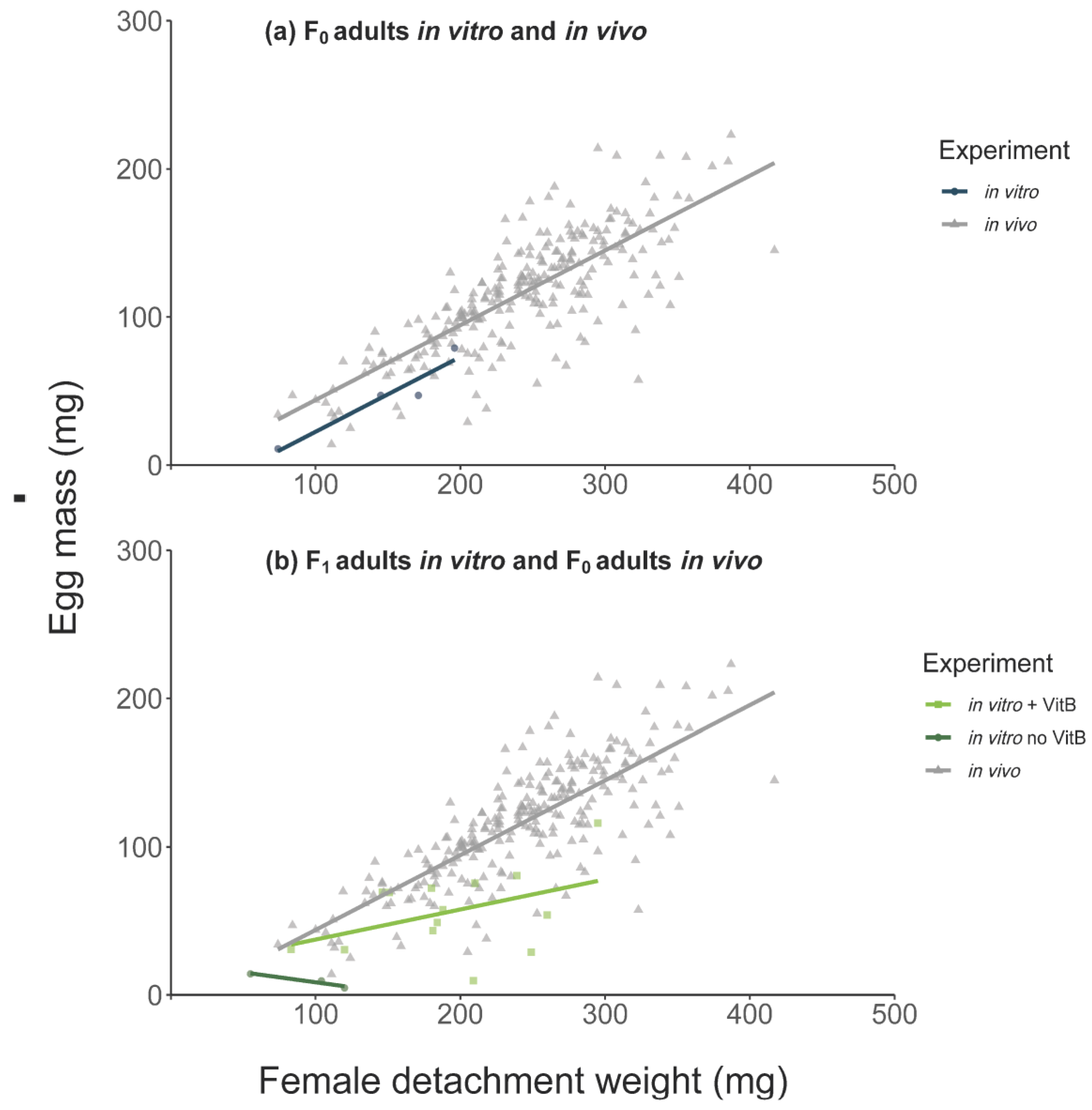
3.1. Feeding of F0 Adult Ticks
3.2. Feeding of Larvae
3.3. Feeding of Nymphs
3.4. Feeding of F1-Adults In Vitro
4. Discussion
4.1. Feeding Duration
4.2. Tick Weights
4.3. Completion of Life Stages
4.4. Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bedford, G. Nuttalliella namaqua, a new genus and species of tick. Parasitology 1931, 23, 230–232. [Google Scholar] [CrossRef]
- Mans, B.J.; De Klerk, D.; Pienaar, R.; Latif, A.A. Nuttalliella namaqua: A living fossil and closest relative to the ancestral tick lineage: Implications for the evolution of blood-feeding in ticks. PLoS ONE 2011, 6, e23675. [Google Scholar] [CrossRef]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Pena, A.; Horak, I.G.; Shao, R.; Barker, S.C. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: A list of valid species names. Zootaxa 2010, 2528, 1–28. [Google Scholar] [CrossRef]
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef]
- Mac, S.; da Silva, S.R.; Sander, B. The economic burden of Lyme disease and the cost-effectiveness of Lyme disease interventions: A scoping review. PLoS ONE 2019, 14, e0210280. [Google Scholar] [CrossRef]
- Petney, T.N.; Pfaeffle, M.P.; Skuballa, J.D. An annotated checklist of the ticks (Acari: Ixodida) of Germany. Syst. Appl. Acarol. 2012, 17, 115–171. [Google Scholar] [CrossRef]
- Brugger, K.; Boehnke, D.; Petney, T.; Dobler, G.; Pfeffer, M.; Silaghi, C.; Schaub, G.A.; Pinior, B.; Dautel, H.; Kahl, O.; et al. A Density Map of the Tick-Borne Encephalitis and Lyme Borreliosis Vector Ixodes ricinus (Acari: Ixodidae) for Germany. J. Med Entomol. 2016, 53, 1292–1302. [Google Scholar] [CrossRef]
- Milne, A. The ecology of the sheep tick, Ixodes ricinus L. Host relationships of the tick: Part 1. Review of previous work in Britain. Parasitology 1949, 39, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Jongejan, F. Ticks feeding on humans: A review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp. Appl. Acarol. 1999, 23, 685–715. [Google Scholar] [CrossRef] [PubMed]
- Hokama, Y.; Lane, R.S.; Howarth, J.A. Maintenance of adult and nymphal Ornithodoros coriaceus (Acari: Argasidae) by artificial feeding through a parafilm membrane. J. Med Entomol. 1987, 24, 319–323. [Google Scholar] [CrossRef]
- Schwan, E.V.; Hutton, D.; Shields, K.J.; Townson, S. Artificial feeding and successful reproduction in Ornithodoros moubata moubata (Murray, 1877) (Acarina: Argasidae). Exp. Appl. Acarol. 1991, 13, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, A.M.; Tyson, K.R. In vitro Feeding Methods for Hematophagous Arthropods and Their Application in Drug Discovery. In Ectoparasites; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 187–204. [Google Scholar] [CrossRef]
- Romano, D.; Stefanini, C.; Canale, A.; Benelli, G. Artificial blood feeders for mosquito and ticks-Where from, where to? Acta Trop. 2018, 183, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, L.C.; Ward, R.A.; Gould, D.J. Studies on the Feeding Response of Mosquitos to Nutritive Solutions in a New Membrane Feeder. Mosq. News 1964, 24, 407–419. [Google Scholar]
- Campbell, E.M.; Burdin, M.; Hoppler, S.; Bowman, A.S. Role of an aquaporin in the sheep tick Ixodes ricinus: Assessment as a potential control target. Int. J. Parasitol. 2010, 40, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.M.; Liu, L.; Jutras, B.L.; Yadav, A.K.; Narasimhan, S.; Gopalakrishnan, V.; Ansari, J.M.; Jefferson, K.K.; Cava, F.; Jacobs-Wagner, C.; et al. Pathogen-mediated manipulation of arthropod microbiota to promote infection. Proc. Natl. Acad. Sci. USA 2017, 114, E781–E790. [Google Scholar] [CrossRef]
- Trentelman, J.J.; Kleuskens, J.A.; van de Crommert, J.; Schetters, T.P. A new method for in vitro feeding of Rhipicephalus australis (formerly Rhipicephalus microplus) larvae: A valuable tool for tick vaccine development. Parasites Vectors 2017, 10, 153. [Google Scholar] [CrossRef]
- Contreras, M.; Alberdi, P.; Fernandez De Mera, I.G.; Krull, C.; Nijhof, A.; Villar, M.; De La Fuente, J. Vaccinomics Approach to the Identification of Candidate Protective Antigens for the Control of Tick Vector Infestations and Anaplasma phagocytophilum Infection. Front. Cell. Infect. Microbiol. 2017, 7, 360. [Google Scholar] [CrossRef]
- Knorr, S.; Anguita, J.; Cortazar, J.T.; Hajdusek, O.; Kopacek, P.; Trentelman, J.J.; Kershaw, O.; Hovius, J.W.; Nijhof, A.M. Preliminary Evaluation of Tick Protein Extracts and Recombinant Ferritin 2 as Anti-tick Vaccines Targeting Ixodes ricinus in Cattle. Front. Physiol. 2018, 9, 1696. [Google Scholar] [CrossRef]
- Antunes, S.; Merino, O.; Mosqueda, J.; Moreno-Cid, J.A.; Bell-Sakyi, L.; Fragkoudis, R.; Weisheit, S.; de la Lastra, J.M.P.; Alberdi, P.; Domingos, A.; et al. Tick capillary feeding for the study of proteins involved in tick-pathogen interactions as potential antigens for the control of tick infestation and pathogen infection. Parasites Vectors 2014, 7. [Google Scholar] [CrossRef]
- Totze, R. Beiträge zur Sinnesphysiologie der Zecken. Z. Für Vgl. Physiol. 1933, 19, 110–161. [Google Scholar]
- Voigt, W.P.; Young, A.S.; Mwaura, S.N.; Nyaga, S.G.; Njihia, G.M.; Mwakima, F.N.; Morzaria, S.P. In vitro feeding of instars of the ixodid tick Amblyomma variegatum on skin membranes and its application to the transmission of Theileria mutans and Cowdria ruminatium. Parasitology 1993, 107 Pt 3, 257–263. [Google Scholar] [CrossRef]
- DeVries, Z.C.; Mick, R.; Schal, C. Feel the heat: Activation, orientation and feeding responses of bed bugs to targets at different temperatures. J. Exp. Biol. 2016, 219, 3773–3780. [Google Scholar] [CrossRef]
- Lees, A.D. The Sensory Physiology of the Sheep Tick, Ixodes ricinus L. J. Exp. Biol. 1948, 25, 145–207. [Google Scholar]
- Matuschka, F.-R.; Richter, D.; Fischer, P.; Spielman, A. Time of repletion of subadult Ixodes ricinus ticks feeding on diverse hosts. Parasitol. Res. 1990, 76, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Waladde, S.; Rice, M. The sensory basis of tick feeding behaviour. In Physiology of Ticks; Obenchain, F.D., Galun, R., Eds.; Elsevier: Oxford, UK, 1982; Volume 1, pp. 71–118. [Google Scholar]
- Kuhnert, F.; Diehl, P.A.; Guerin, P.M. The life-cycle of the bont tick Amblyomma hebraeum in vitro. Int. J. Parasitol. 1995, 25, 887–896. [Google Scholar] [CrossRef]
- Bohme, B.; Krull, C.; Clausen, P.H.; Nijhof, A.M. Evaluation of a semi-automated in vitro feeding system for Dermacentor reticulatus and Ixodes ricinus adults. Parasitol. Res. 2018, 117, 565–570. [Google Scholar] [CrossRef]
- Duron, O.; Morel, O.; Noel, V.; Buysse, M.; Binetruy, F.; Lancelot, R.; Loire, E.; Menard, C.; Bouchez, O.; Vavre, F.; et al. Tick-Bacteria Mutualism Depends on B Vitamin Synthesis Pathways. Curr. Biol. 2018, 28, 1896–1902 e1895. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Jasinskas, A.; Barbour, A.G. Antibiotic treatment of the tick vector Amblyomma americanum reduced reproductive fitness. PLoS ONE 2007, 2, e405. [Google Scholar] [CrossRef]
- Levin, M.L.; Schumacher, L.B. Manual for maintenance of multi-host ixodid ticks in the laboratory. Exp Appl Acarol 2016, 70, 343–367. [Google Scholar] [CrossRef]
- Krober, T.; Guerin, P.M. An in vitro feeding assay to test acaricides for control of hard ticks. Pest Manag. Sci. Former. Pestic. Sci. 2007, 63, 17–22. [Google Scholar] [CrossRef]
- Krober, T.; Guerin, P.M. In vitro feeding assays for hard ticks. Trends Parasitol. 2007, 23, 445–449. [Google Scholar] [CrossRef]
- de Carvalho Ferreira, H.C.; Tudela Zuquete, S.; Wijnveld, M.; Weesendorp, E.; Jongejan, F.; Stegeman, A.; Loeffen, W.L. No evidence of African swine fever virus replication in hard ticks. Ticks Tick-Borne Dis. 2014, 5, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Krull, C.; Bohme, B.; Clausen, P.H.; Nijhof, A.M. Optimization of an artificial tick feeding assay for Dermacentor reticulatus. Parasites Vectors 2017, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Winkham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Fourie, J.J.; Evans, A.; Labuschagne, M.; Crafford, D.; Madder, M.; Pollmeier, M.; Schunack, B. Transmission of Anaplasma phagocytophilum (Foggie, 1949) by Ixodes ricinus (Linnaeus, 1758) ticks feeding on dogs and artificial membranes. Parasites Vectors 2019, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.J.; Xu, G.; Rich, S.M. A silicone membrane for in vitro feeding of Ixodes scapularis (Ixodida: Ixodidae). J. Med Entomol. 2014, 51, 878–879. [Google Scholar] [CrossRef]
- Oliver, J.D.; Lynn, G.E.; Burkhardt, N.Y.; Price, L.D.; Nelson, C.M.; Kurtti, T.J.; Munderloh, U.G. Infection of Immature Ixodes scapularis (Acari: Ixodidae) by Membrane Feeding. J. Med Entomol. 2016, 53, 409–415. [Google Scholar] [CrossRef]
- Körner, S.; Makert, G.R.; Mertens-Scholz, K.; Henning, K.; Pfeffer, M.; Starke, A.; Nijhof, A.M.; Ulbert, S. Uptake and fecal excretion of Coxiella burnetii by Ixodes ricinus and Dermacentor marginatus ticks. Parasites Vectors 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Bonnet, S.; Jouglin, M.; Malandrin, L.; Becker, C.; Agoulon, A.; L’Hostis, M.; Chauvin, A. Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitology 2007, 134, 197–207. [Google Scholar] [CrossRef]
- Gray, J. The development and seasonal activity of the tick Ixodes ricinus: A vector of Lyme borreliosis. Rev. Med Vet. Entomol. 1991, 79, 323–333. [Google Scholar]
- Kocan, K.M.; de la Fuente, J.; Coburn, L.A. Insights into the development of Ixodes scapularis: A resource for research on a medically important tick species. Parasites Vectors 2015, 8, 1–6. [Google Scholar] [CrossRef]
- Musyoki, J.M.; Osir, E.O.; Kiara, H.K.; Kokwaro, E.D. Comparative studies on the infectivity of Theileria parva in ticks fed in vitro and those fed on cattle. Exp. Appl. Acarol. 2004, 32, 51–67. [Google Scholar] [CrossRef]
- González, J.; Valcárcel, F.; Aguilar, A.; Olmeda, A. In vitro feeding of Hyalomma lusitanicum ticks on artificial membranes. Exp. Appl. Acarol. 2017, 72, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Waladde, S.M.; Ochieng, S.A.; Gichuhi, P.M. Artificial-membrane feeding of the ixodid tick, Rhipicephalus appendiculatus, to repletion. Exp. Appl. Acarol. 1991, 11, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Habedank, B.; Hiepe, T. In-vitro feeding of ticks, Dermacentor nuttalli; Olenev 1928 (Acari: Ixodidae) on a silicon membrane. Dermatol. Mon. 1993, 179, 292. [Google Scholar]
- Grenacher, S.; Krober, T.; Guerin, P.M.; Vlimant, M. Behavioural and chemoreceptor cell responses of the tick, Ixodes ricinus, to its own faeces and faecal constituents. Exp. Appl. Acarol. 2001, 25, 641–660. [Google Scholar] [CrossRef]
- Kuhnert, F. Feeding of Hard Ticks In Vitro: New Perspectives for Rearing and for the Identification of Systemic Acaricides. ALTEX 1996, 13, 76–87. [Google Scholar] [PubMed]
- van Duijvendijk, G.; Gort, G.; Sprong, H.; Takken, W. Behavioural responses of Ixodes ricinus nymphs to carbon dioxide and rodent odour. Med Vet. Entomol. 2017, 31, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Lau, M.-J.; Hoffmann, A.A. Does membrane feeding compromise the quality of Aedes aegypti mosquitoes? PLoS ONE 2019, 14, e0224268. [Google Scholar] [CrossRef]
- Matuschka, F.-R.; Spielman, A. Loss of Lyme disease spirochetes from Ixodes ricinus ticks feeding on European blackbirds. Exp. Parasitol. 1992, 74, 151–158. [Google Scholar] [CrossRef]
- Talleklint, L.; Jaenson, T.G.T. Infestation of mammals by Ixodes ricinus ticks (Acari: Ixodidae) in south-central Sweden. Exp. Appl. Acarol. 1997, 21, 755–771. [Google Scholar] [CrossRef]
- Hughes, V.L.; Randolph, S.E. Testosterone depresses innate and acquired resistance to ticks in natural rodent hosts: A force for aggregated distributions of parasites. J. Parasitol. 2001, 49–54. [Google Scholar] [CrossRef]
- Balashov, Y.S. Bloodsucking ticks (Ixodoidea)-vectors of disease in man and animals. Misc. Publ. Entomol. Soc. Am. 1972, 8, 163–376. [Google Scholar]
- Kahl, O.; Hoff, R.; Knulle, W. Gross morphological changes in the salivary glands of Ixodes ricinus (Acari, Ixodidae) between bloodmeals in relation to active uptake of atmospheric water vapour. Exp. Appl. Acarol. 1990, 9, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Rowley, W.A. Relationship between weights of the engorged nymphal stage and resultant sexes in Ixodes scapularis and Dermacentor variabilis (Acari: Ixodidae) ticks. J. Med Entomol. 2000, 37, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Kenny, J. An ivermectin sustained release bolus in cattle: Its effects on the tick Ixodes ricinus. Med Vet. Entomol. 1990, 4, 147–150. [Google Scholar] [CrossRef]
- Jaenson, T.G.; Talleklint, L.; Lundqvist, L.; Olsen, B.; Chirico, J.; Mejlon, H. Geographical distribution, host associations, and vector roles of ticks (Acari: Ixodidae, Argasidae) in Sweden. J. Med Entomol. 1994, 31, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Trentelman, J.J.A.; Teunissen, H.; Kleuskens, J.; van de Crommert, J.; de la Fuente, J.; Hovius, J.W.R.; Schetters, T.P.M. A combination of antibodies against Bm86 and Subolesin inhibits engorgement of Rhipicephalus australis (formerly Rhipicephalus microplus) larvae in vitro. Parasites Vectors 2019, 12, 362. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Parizi, L.F.; Torquato, R.J.S.; Vaz Junior, I.S.; Tanaka, A.S. Novel pseudo-aspartic peptidase from the midgut of the tick Rhipicephalus microplus. Sci. Rep. 2019, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Kemp, D.H.; Agbede, R.I.; Johnston, L.A.; Gough, J.M. Immunization of cattle against Boophilus microplus using extracts derived from adult female ticks: Feeding and survival of the parasite on vaccinated cattle. Int. J. Parasitol. 1986, 16, 115–120. [Google Scholar] [CrossRef]
- Lew-Tabor, A.E.; Bruyeres, A.G.; Zhang, B.; Valle, M.R. Rhipicephalus (Boophilus) microplus tick in vitro feeding methods for functional (dsRNA) and vaccine candidate (antibody) screening. Ticks Tick-Borne Dis. 2014, 5, 500–510. [Google Scholar] [CrossRef]
- Zhang, C.-M.; Li, N.-X.; Zhang, T.-T.; Qiu, Z.-X.; Li, Y.; Li, L.-W.; Liu, J.-Z. Endosymbiont CLS-HI plays a role in reproduction and development of Haemaphysalis longicornis. Exp. Appl. Acarol. 2017, 73, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Guizzo, M.G.; Parizi, L.F.; Nunes, R.D.; Schama, R.; Albano, R.M.; Tirloni, L.; Oldiges, D.P.; Vieira, R.P.; Oliveira, W.H.C.; Leite, M.D.S.; et al. A Coxiella mutualist symbiont is essential to the development of Rhipicephalus microplus. Sci. Rep. 2017, 7, 17554. [Google Scholar] [CrossRef]
- Smith, T.A.; Driscoll, T.; Gillespie, J.J.; Raghavan, R. A Coxiella-like endosymbiont is a potential vitamin source for the Lone Star tick. Genome Biol. Evol. 2015, 7, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Gottlieb, Y. Convergence of nutritional symbioses in obligate blood feeders. Trends Parasitol. 2020, 36, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Koga, R.; Kikuchi, Y.; Meng, X.Y.; Fukatsu, T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl. Acad. Sci. USA 2010, 107, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, E.; Epis, S.; Castelli, M.; Boccazzi, I.V.; Romeo, C.; Desirò, A.; Bazzocchi, C.; Bandi, C.; Sassera, D. Tissue tropism and metabolic pathways of Midichloria mitochondrii suggest tissue-specific functions in the symbiosis with Ixodes ricinus. Ticks Tick-Borne Dis. 2019, 10, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Nikoh, N.; Hosokawa, T.; Moriyama, M.; Oshima, K.; Hattori, M.; Fukatsu, T. Evolutionary origin of insect–Wolbachia nutritional mutualism. Proc. Natl. Acad. Sci. USA 2014, 111, 10257–10262. [Google Scholar] [CrossRef]
- Lake, P.; Friend, W.G. The use of artificial diets to determine some of the effects of Nocardia rhodnii on the development of Rhodnius prolixus. J. Insect Physiol. 1968, 14, 543–562. [Google Scholar] [CrossRef]
| Adult Feeding Experiment | Statistical Analyses (Test, p-Value, df = Degrees of Freedom) | ||||||
|---|---|---|---|---|---|---|---|
| In Vitro | In Vivo n = 450 | ||||||
| F0 n = 30 | F1 | In Vitro F0 to In Vivo | In Vitro F1 (Vitamin B) to In Vivo | In Vitro F0 to In Vitro F1 (Vitamin B) | |||
| No Vitamin B, n = 10 | Vitamin B, n = 21 | ||||||
| Maximum attachment (%) | 20 (CI: 9–37) | 90 (CI: 59–98) | 95 (CI: 77–99) | --- | --- | --- | Z-test, p < 0.0001, df = 1, χ2 = 35,119 |
| Detachment (%) | 20 (CI: 9–37) | 50 (CI: 23–76) | 90 (CI: 71–97) | 71 (CI: 66–75) | Z-test, p < 0.0001, df = 1, χ2 = 33.312 | Z-test, p = 0.0513, df = 1, χ2 = 3.8 | Z-test, p < 0.0001, df = 1, χ2 = 24.55 |
| Mean duration until detachment (days) | 11.5 ± 0.8 (CV: 7.3) | 11 ± 0.7 (CV: 6.4) | 12.3 ± 2.5 (CV: 20.1) | 6.9 ± 1.5 (CV: 21.3) | MWU, p < 0.0001, W = 1860 | MWU, p < 0.0001, W = 5893.5 | MWU, p = 0.921, W = 59 |
| Mean detachment weight (mg) | 136 ± 44.9 (CI: 89–183.3) | 112 ± 37.5 (CI: 72.5–151) | 180 ± 64.1 (CI: 149–211) | 231 ± 72.3 (CI: 222.9–238.9) | t-test, p = 0.003, df = 5.5 | t-test p = 0.003, df = 20.832 | t-test, p = 0.088, df = 12.109 |
| Oviposition of all detached ticks (%) | 67 (CI: 29–90) | 40 (CI: 11–77) | 79 (CI: 56–91) | 91 (CI: 87–94) | Z-test, p = 0.0452, df = 1, χ2 = 4.011 | Z-test, p = 0.088, df = 1, χ2 = 2.912 | Z-test, p = 0.539, df = 1, χ2 = 0.377 |
| Mean duration of oviposition (days) | 22.8 ± 11.4 (CV: 49.9) | 19 ± 4.2 (CV: 22.3) | 12.8 ± 5.4 (CV: 36.7) | 35.8 ± 16.2 (CV: 45.1) | MWU, p = 0.128, W = 322.5 | MWU, p < 0.0001, W = 407 | MWU, p = 0.071, W = 51.5 |
| Mean egg mass (mg) | 46 ± 27.8 (CI: 1.7–90.2) | 7.13 ± 3.37 (CI: 0–37.4) | 56 ± 27.2 (CI: 40.5–71.9) | 116 ± 39.5 (CI: 111–121) | t-test, p = 0.0137, df = −3.195 | t-test, p < 0.0001, df = 16.215 | t-test, p = 0.544, df = 4.781 |
| Mean egg conversion factor | 28.5 ± 10.5 (CI: 11.8–45.2) | 6.5 ± 3.5 (CI: 0–38.3) | 30.4 ± 12.3 (CI: 23.3–37.4) | 47.5 ± 10.2 (CI: 46.2–48.7) | t-test, p = 0.0351, df = 3.0917 | t-test p = 0.0002, df = 14.022 | t-test, p = 0.775, df = 5.621 |
| Larvae producing females per egg batches (%) | 75 (CI: 30–95) | 100 (CI: 34–100) | 80 (CI: 54–93) | 81 (CI: 76–85) | Z-test, p = 0.745, df = 1, χ2 = 0.1056 | Z-test, p = 0.894, df = 1, χ2 = 0.018 | Z-test, p = 0.828, df = 1, χ2 = 0.048 |
| Mean larvae hatch duration (days) | 59.7 ± 1.5 (CV: 2.6) | 69.5 ± 0.7 (CV: 1) | 64.4 ± 12.8 (CV: 19.9) | 67.2 ± 6.4 (CV: 9.5) | MWU, p = 0.071, W = 115 | MWU, p = 0.011, W = 661 | MWU, p = 0.995, W = 16.5 |
| Mean larvae hatching step (1–5) | 3 (IQR: 0) | 3 (IQR: 2) | 5 (IQR: 2) | 5 (IQR: 2) | MWU, p = 0.072, W = 165 | MWU, p = 0.625, W = 960.5 | MWU, p = 0.282, W = 7.5 |
| Larvae prod. females per all fed females (%) | 10 (CI: 3–26) | 20 (CI: 5–51) | 57 (CI: 36–76) | 52 (CI: 47–57) | Z-test, p < 0.0001, df = 1, χ2 = 20.268 | Z-test, p = 0.673, df = 1, χ2 = 0.177 | Z-test, p = 0.0003, df = 1, χ2 = 13.224 |
| Larvae Feeding Experiment | Statistical Analyses (Test, p-Value, df = Degrees of Freedom) In Vitro to In Vivo | ||
|---|---|---|---|
| In Vitro n = 1003 | In Vivo n = 11,737 | ||
| Attachment on day 3 (%) | 60 (CI: 57–63) | --- | --- |
| Engorgement (%) | 55 (CI: 52–58) | 41 (CI: 40–42) | Z-test, p < 0.0001, df = 1, χ2 = 76.44 |
| Mean duration until first engorged tick (days) * | 4.8 ± 0.6 (CV: 12.5) | 3 (CV: 0) | MWU, p = 0.001, W = 55 |
| Mean duration of feeding experiment (days) * | 9.6 ± 1.3 (CV: 13.5) | 3.8 ± 0.4 (CV: 11.8) | MWU, p = 0.0018, W = 55 |
| Mean engorgement (mg) ** | 0.43 ± 0.02 (CV: 4.8) | 0.53 ± 0.03 (CV: 6.4) | MWU, p = 0.0003, W = 1 |
| Molting per engorged tick (%) | 83 (CI: 76–84) | 59 (CI: 57–60) | Z-test, p < 0.0001, df = 1, χ2 = 97 |
| Proportion of deployed larvae reaching the next life stage (%) | 44 (CI: 41–48) | 24 (CI: 23–25) | Z-test, p < 0.0001, df = 1, χ2 = 199.15 |
| Nymph Feeding Experiment | Statistical Analyses (Test, p-Value, df = Degrees of Freedom) In Vitro to In Vivo | ||
|---|---|---|---|
| In Vitro n = 426 | In Vivo n = 800 | ||
| Maximum attachment (%) | 68 (CI: 63–73) | --- | --- |
| Engorgement (%) | 49 (CI: 44–54) | 74 (CI: 70–86) | Z-test, p < 0.0001, df = 1, χ2 = 71.67 |
| Mean duration until first engorged tick (days) * | 6.9 ± 1.4 (CV: 20.7) | 2.9 ± 0.4 (CV: 2.5) | MWU, p < 0.0001, W = 119 |
| Mean duration of feeding experiment (days) * | 11.4 ± 2.9 (CV: 25) | 5.3 ± 0.8 (CV: 14.3) | MWU, p < 0.0001, W = 171 |
| Mean engorgement weight (mg) | 2.82 ± 0.84 (CV: 29.7) | 3.32 ± 0.96 (CV: 28.9) | MWU, p < 0.0001, W = 41,300 |
| Molting rate per engorged tick (%) | 75 (CI: 68–80) | 73 (CI: 69–76) | Z-test, p = 0.6117, df = 1, χ2 = 0.26 |
| Rate of females per molted adults (%) | 43 (CI: 35–51) ♀ = 67; ♂ = 90 | 54 (CI: 49–59) ♀ = 232; ♂ = 197 | Z-test, p = 0.0145, df = 1, χ2 = 5.98 |
| Mean weight of female& male (mg) | ♀ 1.32 ± 0.3 (CI: 1.25–1.39) ♂ 0.81 ± 0.16 (CI: 0.77–0.84) | ♀ 1.68 ± 0.25 (CI: 1.65–1.72) ♂ 0.98 ± 0.18 (CI: 0.95–1) | ♀: t-test, p < 0.0001, df = 97.74 ♂: t-test, p < 0.0001, df = 198.54 |
| Proportion of deployed nymphs reaching the next life stage (%) | 37 (CI: 32–42) | 54 (CI: 50–57) | Z-test, p < 0.0001, df = 1, χ2 = 31.33 |
| Group | Estimates (mg) | 95% CI | n | p | |
|---|---|---|---|---|---|
| Detachment weight | F1 + vitamin B + water bath | reference | |||
| F0, no vitamin B + incubator | −43.52 | (−95.64, 8.61) | 31 | 0.102 | |
| F1, no vitamin B + incubator | −124.68 | (−238.88, −0.48) | 31 | 0.032 | |
| F1, no vitamin B + water bath | −56.48 | (−112.43, −0.54) | 31 | 0.048 | |
| in vivo | +50.51 | (4.55, 96.48) | 338 | 0.031 | |
| Egg mass | F0, no vitamin B + incubator | −10.22 | (−39.65, 19.2) | 21 | 0.496 |
| F1, no vitamin B + incubator | −42 | (−95.73, 11.72) | 21 | 0.121 | |
| F1, no vitamin B + water bath | −49.09 | (−88.33, −9.86) | 21 | 0.014 | |
| in vivo | +59.5 | (30.04, 88.96) | 266 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Militzer, N.; Bartel, A.; Clausen, P.-H.; Hoffmann-Köhler, P.; Nijhof, A.M. Artificial Feeding of All Consecutive Life Stages of Ixodes ricinus. Vaccines 2021, 9, 385. https://doi.org/10.3390/vaccines9040385
Militzer N, Bartel A, Clausen P-H, Hoffmann-Köhler P, Nijhof AM. Artificial Feeding of All Consecutive Life Stages of Ixodes ricinus. Vaccines. 2021; 9(4):385. https://doi.org/10.3390/vaccines9040385
Chicago/Turabian StyleMilitzer, Nina, Alexander Bartel, Peter-Henning Clausen, Peggy Hoffmann-Köhler, and Ard M. Nijhof. 2021. "Artificial Feeding of All Consecutive Life Stages of Ixodes ricinus" Vaccines 9, no. 4: 385. https://doi.org/10.3390/vaccines9040385
APA StyleMilitzer, N., Bartel, A., Clausen, P.-H., Hoffmann-Köhler, P., & Nijhof, A. M. (2021). Artificial Feeding of All Consecutive Life Stages of Ixodes ricinus. Vaccines, 9(4), 385. https://doi.org/10.3390/vaccines9040385






