Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation
Abstract
1. Clinical Importance of Interferon-Based Biopharmaceuticals and Market Overview
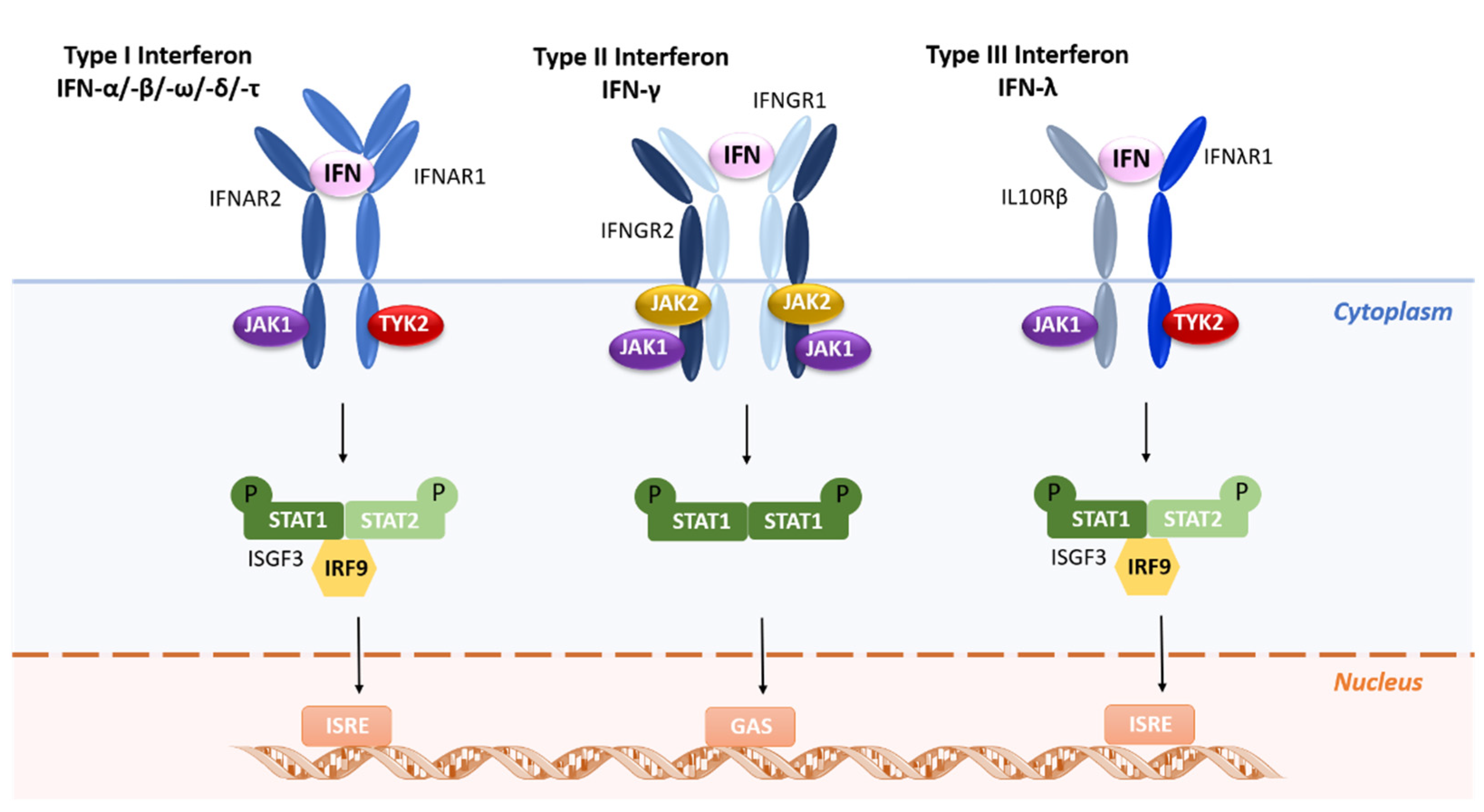
2. Interferons Classification and Mechanisms of Action
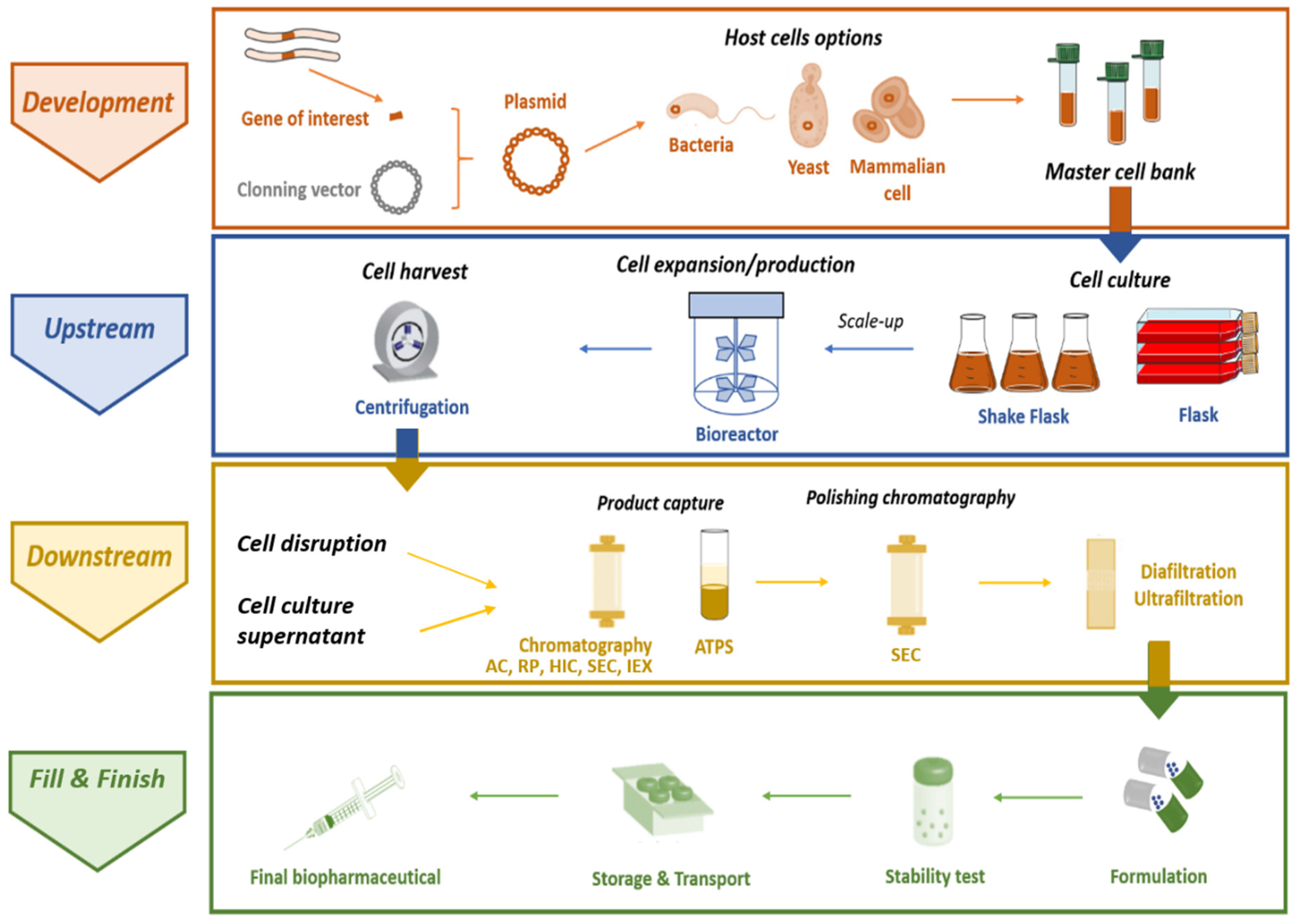
3. Therapeutic Cloned Interferons
3.1. Upstream Stage
3.1.1. Expression Using Escherichia coli
| IFN Type | Strain/Vector | Promotor | Culture Media | Antibiotic | Inducer | Production Scale | Expression | Level of Expression |
|---|---|---|---|---|---|---|---|---|
| IFNα-2, IFNα-8 [59] | BL21(DE3)-RIL pGEM-T | T7 lac | LB + 1% glucose | Ampicillin | IPTG (1 mM) | Shake-flask | Intracellular (IB) | 70.0 mg/L IFNα-2 and 75 mg/L IFNα-8 (refolded IB) |
| Hybrid IFNs [59] | BL21(DE3)-RIL pET-16b | 70.0 mg/L IFNα-828 (refolded IB) | ||||||
| IFNα [46] | BL21-SI pAE | proU | LB without NaCl | Ampicillin | NaCl (0.3 M) | Shake-flask | Intracellular (Soluble) | 75.0 mg/L (native) 210 mg/L (6xHis-tagged) |
| IFNα-2b (GST-fusion) [56] | Origami B pGEX4T1 | tac | LB | Ampicillin | IPTG (0.1, 0.5, 1 mM) | Shake-flask | Intracellular (Soluble) | 100 mg/L (purified) |
| IFNα-2b [60] | JM109(DE3) pET-9 | T7 | Glucose; yeast extract; K2HPO4; KH2PO4; (NH4)2SO4; MgSO4 | Kanamycin | IPTG (1 mM) | Shake-flask; 5L Fermenter | Intracellular (IB) | 13.8 mg IFNα-2b per gram wet cells |
| IFNβ [58] | BL21-SI pTPM13 | T7 | Glucose; K2HPO4; KH2PO4; (NH4)2SO4; MgSO4; thiamine | Ampicillin | NaCl (0.3 M) | Shake-flask | Intracellular (IB) | 61.0 mg/L |
| IFNβ-1b [47] | BL21 (D3) | T7 | TB | Ampicillin | IPTG (0.2 mM) | Bioreactor (2L) | Periplasmatic | 255 mg/L |
| IFNε [45] | DH5α pBV220 | T7 | LB | Ampicillin | 42oC | Shake-Flask | Intracellular (IB) | 8.00 mg/L (purified) |
| IFNγ [54] | BL21-SI (pBAL0; pBAL1; pBAL3) | N/A | Glucose; KH2PO4; (NH4)2SO4; MgSO4; thiamine | Ampicillin | NaCl (0.3 M) | Shake-Flask | Periplasmatic | 45.0 mg/L (post-induction temperature = 20.0 °C) |
| IFNγ [53] | BL21 (DE3) pET14b | T7 | LB M9YE TB | Ampicillin | IPTG (1 mM) | Shake-flask | Intracellular (IB) | 140 mg/g DCW (TB) 130 mg/g DCW (LB) 115 mg/g DCW (M9YE) |
| Bioreactor (1L) | 182 mg/g DCW (TB) 170 mg/g DCW (LB) 160 mg/g DCW (M9YE) | |||||||
| IFNγ [52] | BL21 (DE3) pET3a | lac | M9 modified medium contained (glucose, K2HPO4, KH2PO4, C6H8O7, (NH4)2SO4, MgSO4) | NR | IPTG (2.25 mg/g/L per DCW) | Bioreactor (1L) | NR | 51.0 × 103 mg/L |
| IFN-con [57] | SHuffle Champion™ pET SUMO | T7lac | TB | Kanamycin | IPTG (0.1, 1 mM) | Shake-Flask | Intracellular (Soluble) | 50.0 mg/L (Purified) |
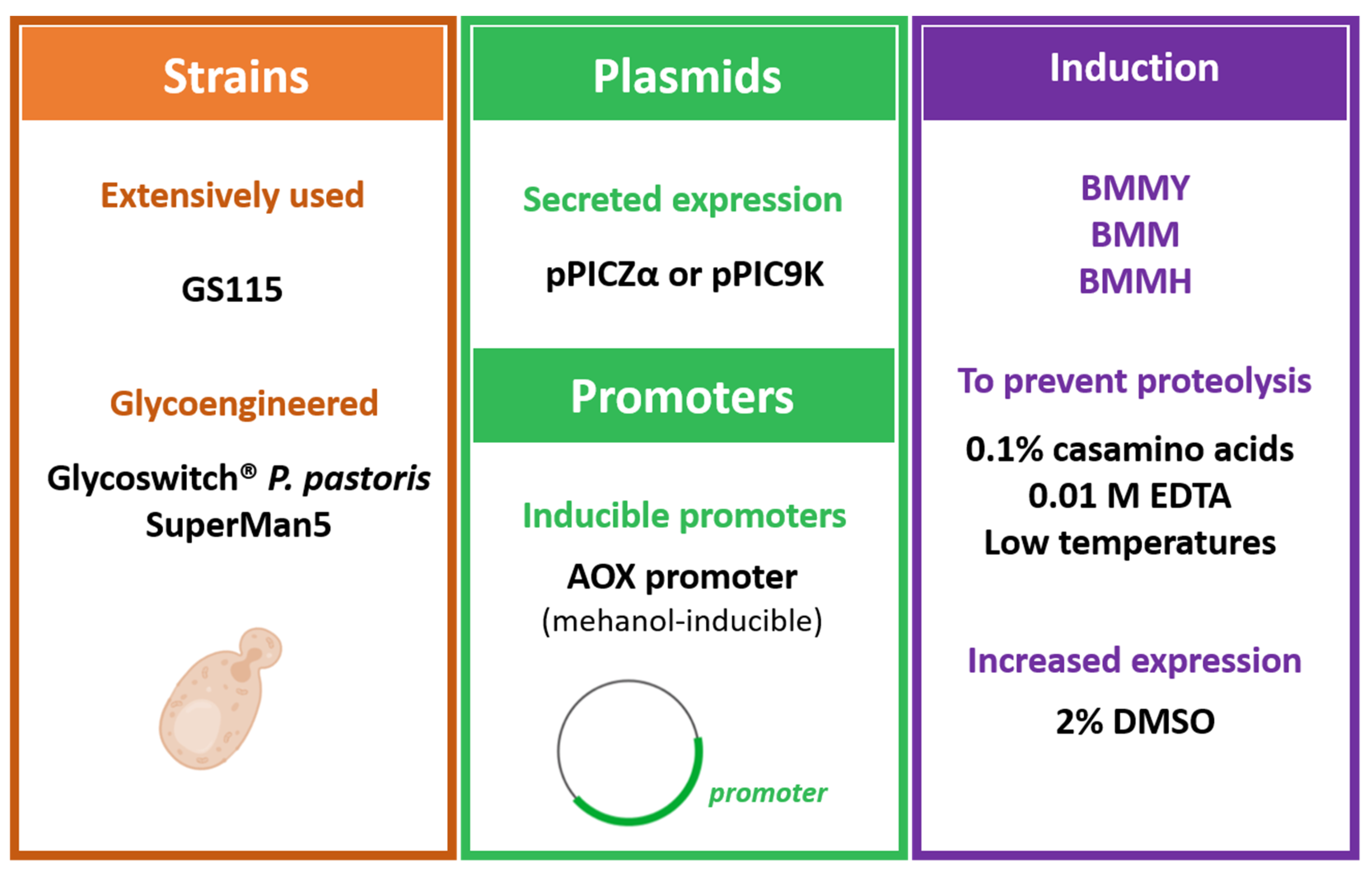
3.1.2. Expression Using Pichia pastoris
| IFN Type | Strain/Plasmid | Promoter | Media | Antibiotic | Inducer | Scale | Type of Expression | Level of Expression |
|---|---|---|---|---|---|---|---|---|
| IFNα-2b [74] | GS115 pPICZα | AOX | BMGY/BMMY | Zeocin | Methanol | Shake-flask | Secreted (αprepro) | 450 mg/L |
| IFNα-2b [72] | KM71H pPICZα-hIFNα-2b | AOX1 | BMGY/BMMY | Zeocin | Methanol | Bioreactor | Secreted (αprepro) | 600 mg/L |
| IFNα-2b [75] | GS115 pPIC9HSS pPIC9IFN pPIC9αIFN | AOX | BMG/BMM | Ampicilin | Methanol | Shake-flask | Secreted (mutated αprepro) | 200 mg/L (pPIC9αIFN) |
| IFNα-2b [76] | GS115 pPIC9KN | AOX | BMGY/BMMY | Geneticin | Methanol | Bioreactor | Secreted (αprepro) | 300 mg/L |
| IFNγ [77] | GS115 pPICZαA | AOX | BMGY/BMMH | Zeocin Geneticin | Methanol | Shake-flask | Secreted (αprepro) | 2.50 mg/L |
| IFNα-2b [78] | Glycoswitch® P. pastoris SuperMan5 | AOX1 | BMGY/BMMY | NR | Methanol | Shake flask | Secreted (N/A) | 436 mg/L |
| N-glycosylated IFNβ-1 [79] | GS115 pPIC9IFN | AOX1 | BMGY/BMMY | NR | Methanol | Shake-flask | Secreted (αprepro) | 6.00–12.0 mg/L |
| IFNλ [69] | GS115 pAO815 | AOX | BMG/BMM | Ampicilin | Methanol | Shake-flask | Secreted (αprepro) | 65.0 mg/L |
| rHSA/IFNα-2b [80] | N/A pPIC9 | AOX | N/A | NR | Methanol | Biostat C 15L fermenter | Secreted (HSA signal peptide) | 250 mg/L |
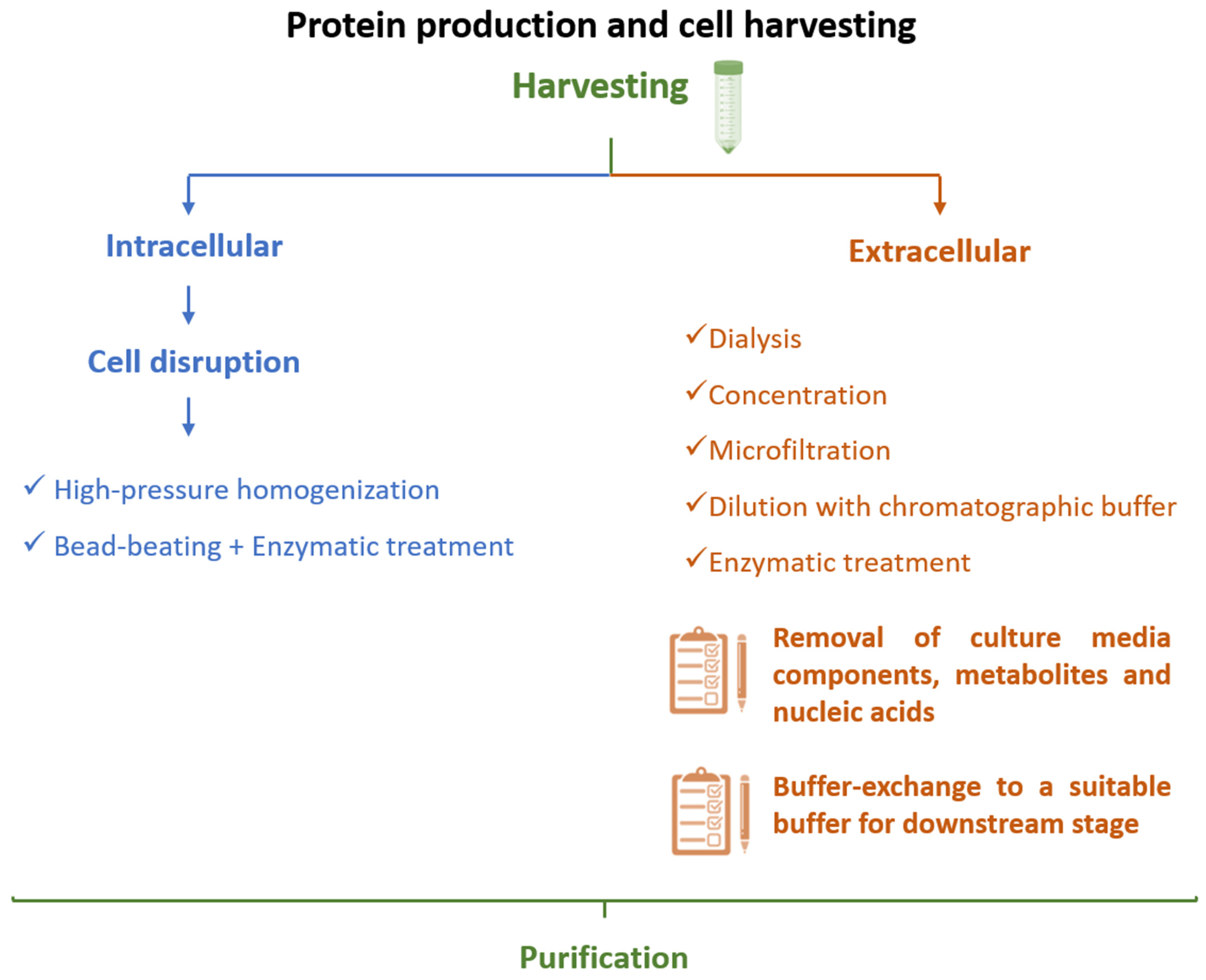
3.2. Downstream Processing of Interferons
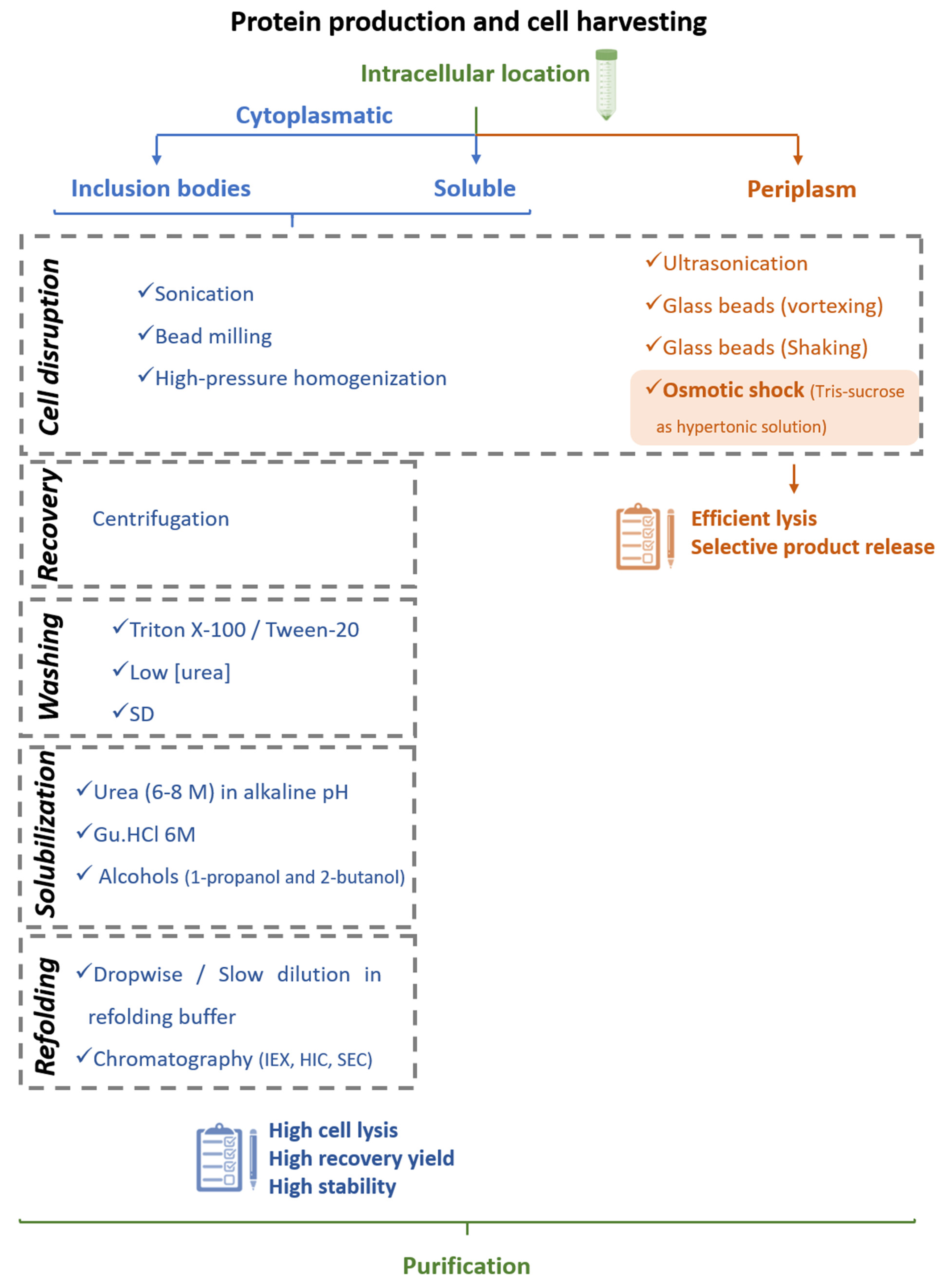
3.2.1. Cell Lysis and Interferon Recovery
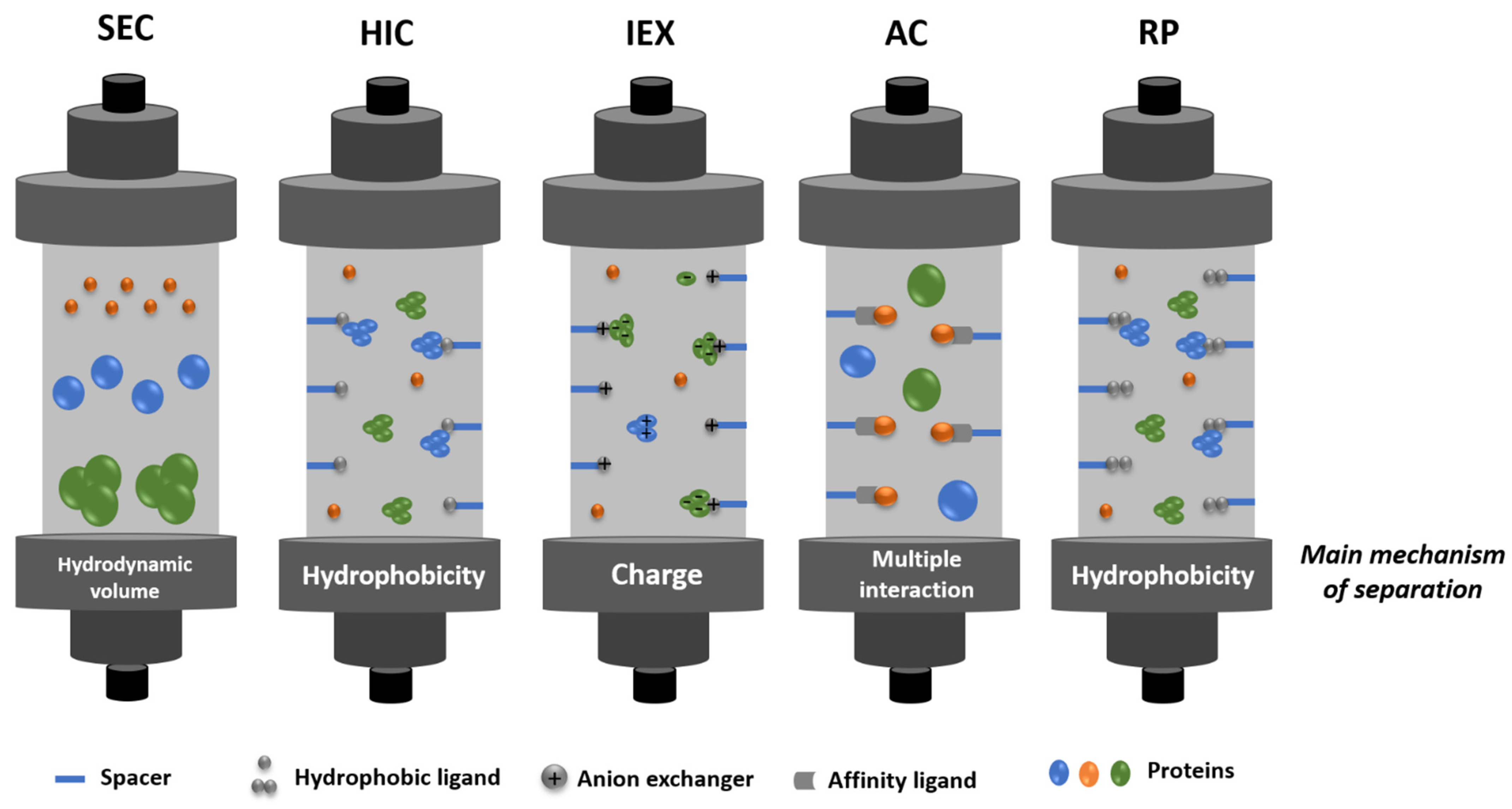
3.2.2. Chromatography-Based Purification
| Chromatography | Column | IFN | Host | IFN Concentration | Recovery Yield (%) | Purity (%) | Specific Activity (IU/mg) |
|---|---|---|---|---|---|---|---|
| IMAC [77] | His-Trap FF affinity column with Ni2+ | 8xHis IFNγ 8x His IFNγ-PDI 8x His IFNγopt | P. pastoris GS115 | 0.009 mg/L | 36.00 | 56.25 | Not reported |
| 0.030 mg/L | 54.54 | 63.83 | |||||
| 0.120 mg/L | 52.17 | 80.00 | |||||
| IMAC [59] | His Bind Quick 900 | 10xHis IFNα-2 | E. coli BL21 (DE3)-RIL | 21.0 mg/L | 16.00 | 18.00 | 1.8 × 108 |
| 10xHis IFNα-8 | 55.0 mg/L | 44.00 | 44.00 | 3.4 × 108 | |||
| 10xHis IFNα-828 | 30.0 mg/L | 26.00 | 24.00 | 7.5 × 108 | |||
| IMAC + SEC [56] | GSTrap Fast Flow + Sephacryl S-100 | GST-IFNα-2 | E. coli BL21 E. coli Origami B | 100 mg/L | NR | NR | 2.0 × 108 |
| IMAC [79] | Hi-Trap FF affinity column with Cu2+ | IFNβ-1 | P. pastoris GS115 | 10.0 mg/L | NR | 80.00 | 2–3 × 107 |
| IMAC + SEC [100] | His-Trap FF affinity column with Ni2+ | IFNα-2 Thymosin α1 | E. coli BL21 (DE3) | 950 mg/L | 69.00 | 98.00 | Biologically active (Not comparable) |
| IMAC + AEX [51] | His-Trap FF affinity column + HiTrap Q HP | MBP-IFNα-2b | E. coli BL21 (DE3) | 14.4 mg/L | 10.50 | 99.80 | Biologically active (Not comparable) |
| IAC [101] | IFNα-2a antibody conjugated to Sepharose 4B | GFE-IFNα-2a | E. coli BL21 (DE3) | 1,05x103 mg/L | 0.520 | >95.00 | 2.5 × 108 |
| AC [91] | Blue-Sepharose Fast Flow | IFNβ | E. coli BL21 (K12) | NR | NR | 93.50 | Biologically active (Not comparable) |
| AEX [49] | Q Sepharose Fast Flow | IFNα-2b | E. coli DH5α | 3.00x103 mg/L | 58.00 | 99.00 | 3 × 109 |
| AEX [76] | Q Sepharose Fast Flow | IFNα-2b | P. pastoris GS115 | 900 mg/L | 93.00 | 90.00 | >2 × 108 |
| CEX [92] | SP-Sepharose Fast Flow | IFNγ | E. coli | 100 mg/L | 54.00 | 95.00 | 7.5 × 105 |
| CEX [102] | SP Sepharose XL | IFNγ | P. pastoris X-33 | 135.2 mg/L | 56.00 | 90.00 | 1–1.4 × 107 |
| CEX + SEC [72] | Sepharose SP + Sephacryl S100 | IFNα-2b | P. pastoris | 183 mg/L | 30.00 | 100.0 | 1.5 × 108 |
| CEX + SEC [69] | SP Sepharose Fast Flow + Superdex 75 | IFNλ-1 | P. pastoris GS115 | NR | NR | >98.00 | NR |
| AEX + SEC [74] | Q Sepharose Fast Flow + Superdex 75 | IFNα-2b | P. pastoris | 298 mg/L | 64.00 | >95.00 | 1.9 × 109 |
| RP [45] | C18 | IFNε | E. coli DH5α | 800 mg/L | NR | NR | 6 × 105 |
| IMAC + RP [57] | His-Trap FF affinity column with Ni2++ C8 | SUMO-IFNcon | E. coli SHuffle™ | 50.0 mg/L | NR | 98.00 | 960 × 106 |
| AEX + CEX [90] | Q Sepharose Fast Flow + SP-Sepharose Fast Flow | NGR-IFNα-2a | E. coli BL21 (DE3) | 18.0 mg/L | NR | >98.00 | 6.2 × 108 |
| AC + HIC + AEX + SEC [80] | Blue Sepharose Fast Flow + Phenyl Sepharose HP + Q Sepharose Fast Flow + Sephadex G25 | HSA-IFNα-2b | P. pastoris | 64.0 mg/L | 25.40 | 97.00 | 6.3 × 105 |
| CEX + AC + SEC [103] | SP Sepharose Fast Flow + Blue Sepharose 6 Fast Flow + Sepharyl S-100 | IFNλ-1 | CHO cells | NR | NR | 90.00 | 1 × 106 |
| SEC [104] | Sephacryl S-200 | IFNα-2a | E. coli BL21 (DE3) | NR | 82.00 | 92.00 | 1.2 × 108 |
| SEC [95] | Superdex 75 | IFNγ | E. coli DH5α | NR | 67.10 | NR | 1.2 × 107 |
3.2.3. Alternative Purification Strategies
3.3. Therapeutics and IFN Delivery
3.3.1. Approved Formulations and Excipients
3.3.2. Chemical Conjugation and Genetically Engineered Fusions
3.3.3. Drug Delivery Systems
| IFN | Drug Delivery System | Composition | Loading (%) | Encapsulation Efficiency (%) | Release | Experimental Conditions |
| IFNγ [135] | Microspheres | Poly(lactic-co-glycolic acid) (PLGA) | 3.2 (w/w) | 100 | ~1.6% ~30–38% in 7 days | |
| IFNα [136] | Nanoparticles | PLGA/Pegylated PLGA | 78–91 | 90% in 16 days | ||
| IFNα-2b [137] | Microspheres | Poly(ethylene glycal/butylenes terephthalate)-PLGA | 86.01 | 16.7% initial burst 83.1% in 23 days | In vivo | |
| IFNγ [138] | Elastomer | Star-poly(Ɛ-caprolactone-co-D,L-lactide) elastomer | NR | NR | 83% in 21 days | BV-2 microglial cells |
| IFNα [139] | Microspheres | PLGA/poloxamer PLGA/poloxamer blend | NR | NR | 2–24% initial burst | Melanoma (A 2058 cells) |
| IFNα-2b [140] | Hybrid | PLGA Nanoparticles-CS/GP | NR | NR | 40% initial burst | In vivo |
| IFNα-2b [141] | Hydrogel | Hydroxypropyl cellulose | NR | 50 | 50% in 5 h 81% in 24 h 90% in 120 h | Gastric Cancer (MKN-45 cells) Melanoma (A375 cells) |
| IFNβ [142] | Hydrogels | P(MAA-g-EG) | 77 | NR | 40% at pH 1.2 70% at pH 6.8 | In vivo |
| IFNα [143] | 60 | NR | Colorectal adenocarcinoma (Caco-2 cells) Colon carcinoma (HT29-MTX cells) | |||
| IFNα [144] | Bioconjugate | Aldehyde-modified hyaluronic acid | Kidney (VERO cells) | |||
| IFNα-2b [145] | Microspheres | Chitosan-carboxymethyl | 11 | 90 | 7.4% in 1 h 89% in 24 h | Lung adenocarcinoma (A549 cells) |
| IFNα-2b [146] | Nanoparticles | Chitosan | NR | 100 | 0.5 h 20.5% in pH 1.2 89.6% in pH 6.8 | Kidney (MDBK cells) |
| IFNβ [147] | Nanoparticles | Chitosan/sulfobutylether-β-cyclodextrin | NR | 88 | 87% | |
| IFNα [148] | Nanoparticles | HSA-IFN-α/poly(sodium-4-styrene) sulphonate/chitosan | 76.13 | 49.1 | In vivo | |
| IFNα [149] | Particles | Calcium phosphate | 0.2–3.1 | 80–96 | 50% in 1 h 80% in 6 h | Cervical cancer (HeLa cells) |
| IFNα [150] | Liposomes | PEGylated lipids | NR | 81 | 30% in 8 h | Vaginal tissue |
| IFNβ [151] | Microparticles | Trimethyl-chitosan (TMC), poly(ethylene glycol)dimethacrylate (PEGDMA) and methacrylic acid (MAA) | 53.25 | In vivo | ||
| IFNα-2b [152] | multivesicular liposome | DOPC, cholesterol, DPPG, triolein | 30 | In vivo | ||
| Clinical Trials | ||||||
| IFN | Drug Delivery System | Composition | Indication | |||
| IFNα-2b [153] | HeberPAG® | Sodium phosphates, Dextran-40, kalium phosphate, sodium chloride, kalium chloride, mannitol, saccharose, and human albumin | Mycosis fungoides | |||
| IFNγ [154] | CIGB-128-A | Trehalose, succinic acid and human serum albumin | Potential application in several malignancies | |||
| INF-α2b [155] | Microspheres | Gelatin, a cationic arginine-rich protein stabilizer, protamine sulphate | Ovarian cancer (SKOV3 cells) | |||
| INF-α2b [156] | Locteron | Poly(ether-ester) microspheres | Hepatitis C therapy | |||
4. Outlook and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nathan, C.; Cars, O. Antibiotic resistance--problems, progress, and prospects. N. Engl. J. Med. 2014, 371, 1761–1763. [Google Scholar] [CrossRef]
- Guiochon, G.; Beaver, L.A. Separation science is the key to successful biopharmaceuticals. J. Chromatogr. A 2011, 1218, 8836–8858. [Google Scholar] [CrossRef]
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018, 36, 1136–1145. [Google Scholar] [CrossRef]
- Jozala, A.F.; Geraldes, D.C.; Tundisi, L.L.; Feitosa, V.A.; Breyer, C.A.; Cardoso, S.L.; Mazzola, P.G.; Oliveira-Nascimento, L.; Rangel-Yagui, C.O.; Magalhaes, P.O.; et al. Biopharmaceuticals from microorganisms: From production to purification. Braz. J. Microbiol. 2016, 47 (Suppl. 1), 51–63. [Google Scholar] [CrossRef]
- Castro, L.S.; Pereira, P.; Passarinha, L.A.; Freire, M.G.; Pedro, A.Q. Enhanced performance of polymer-polymer aqueous two-phase systems using ionic liquids as adjuvants towards the purification of recombinant proteins. Sep. Purif. Technol. 2020, 248, 117051. [Google Scholar] [CrossRef]
- Buckel, P. Recombinant Protein Drugs (Milestones in Drug Therapy). Molecules 2001, 6, 1063. [Google Scholar] [CrossRef]
- Lagassé, H.; Alexaki, A.; Simhadri, V.; Katagiri, N.; Jankowski, W.; Sauna, Z.; Kimchi-Sarfaty, C. Recent advances in (therapeutic protein) drug development. F1000Research 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.D. Biosimilars: Current status and future directions. Expert Opin. Biol. Ther. 2010, 10, 1011–1018. [Google Scholar] [CrossRef]
- Timmermann, C. How to produce ‘marketable and profitable results for the company‘: From viral interference to Roferon, A. Hist Philos. Life Sci. 2019, 41, 30. [Google Scholar] [CrossRef]
- Lindenmann, J.; Schleuning, W.-D. Interferon: The Dawn of Recombinant Protein Drugs, 1st ed.; Lindenmann, J., Schleuning, W.D., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 7, pp. 121–134. [Google Scholar]
- Research and Markets. Interferons Global Market Report 2020-30: COVID-19 Implications and Growth. Available online: https://www.researchandmarkets.com/ (accessed on 5 January 2021).
- ClinicalTrials.gov Database (Search by “Interferon“). Available online: https://clinicaltrials.gov (accessed on 10 January 2021).
- Ryff, J.-C.; Pestka, S. Interferons and Interleukins. In Pharmaceutical Biotechnology, 5th ed.; Crommelin, D., Sindelar, R., Meibohm, B., Eds.; Springer: Cham, Switzerland, 2019; pp. 619–643. [Google Scholar]
- Silva, A.C.; Lobo, J.M.S. Cytokines and Growth Factors. In Current Applications of Pharmaceutical Biotechnology; Silva, A.C., Moreira, J.N., Lobo, J.M.S., Almeida, H., Eds.; Springer: Cham, Switzerland, 2019; pp. 87–113. [Google Scholar]
- Isaacs, A.; Lindenmann, J.; Andrewes, C.H. Virus interference. I. The interferon. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1957, 147, 258–267. [Google Scholar] [CrossRef]
- Pestka, S.; Langer, J.A.; Zoon, K.C.; Samuel, C.E. Interferons and their actions. Annu. Rev. Biochem. 1987, 56, 727–777. [Google Scholar] [CrossRef]
- Borden, E.C.; Sen, G.C.; Uze, G.; Silverman, R.H.; Ransohoff, R.M.; Foster, G.R.; Stark, G.R. Interferons at age 50: Past, current and future impact on biomedicine. Nat. Rev. Drug Discov. 2007, 6, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Interferon nomenclature. Nature 1980, 286, 110. [CrossRef]
- Hauptmann, R.; Swetly, P. A novel class of human type I interferons. Nucleic Acids Res. 1985, 13, 4739–4749. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Leaman, D.W.; Bixby, J.A.; Roberts, R.M. A type I ovine interferon with limited similarity to IFN-alpha, IFN-omega and IFN-tau: Gene structure, biological properties and unusual species specificity. Biochim. Biophys. Acta 1996, 1294, 55–62. [Google Scholar] [CrossRef]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Takaoka, A. A weak signal for strong responses: Interferon-alpha/beta revisited. Nat. Rev. Mol. Cell. Biol. 2001, 2, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef]
- Decker, T.; Müller, M.; Stockinger, S. The Yin and Yang of type I interferon activity in bacterial infection. Nat. Rev. Immunol. 2005, 5, 675–687. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2013, 14, 36–49. [Google Scholar] [CrossRef]
- Takaoka, A. Cross Talk Between Interferon-gamma and -alpha /beta Signaling Components in Caveolar Membrane Domains. Science 2000, 288, 2357–2360. [Google Scholar] [CrossRef]
- Uzé, G.; Monneron, D. IL-28 and IL-29: Newcomers to the interferon family. Biochimie 2007, 89, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Gibbert, K.; Schlaak, J.; Yang, D.; Dittmer, U. IFN-α subtypes: Distinct biological activities in anti-viral therapy. Br. J. Pharm. 2013, 168, 1048–1058. [Google Scholar] [CrossRef]
- Jonasch, E.; Haluska, F.G. Interferon in oncological practice: Review of interferon biology, clinical applications, and toxicities. Oncologist 2001, 6, 34–55. [Google Scholar] [CrossRef] [PubMed]
- Bekisz, J.; Baron, S.; Balinsky, C.; Morrow, A.; Zoon, K.C. Antiproliferative Properties of Type I and Type II Interferon. Pharmaceuticals 2010, 3, 994–1015. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.E. Reoviruses and the Interferon System. In Reoviruses II: Cytopathogenicity and Pathogenesis, 1st ed.; Tyler, K.L., Oldstone, M.B.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 125–145. [Google Scholar]
- Lavoie, T.B.; Kalie, E.; Crisafulli-Cabatu, S.; Abramovich, R.; DiGioia, G.; Moolchan, K.; Pestka, S.; Schreiber, G. Binding and activity of all human alpha interferon subtypes. Cytokine 2011, 56, 282–289. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Walter, L.J.; Hruza, A.; Reichert, P.; Trotta, P.P.; Nagabhushan, T.L.; Walter, M.R. Zinc mediated dimer of human interferon-α2b revealed by X-ray crystallography. Structure 1996, 4, 1453–1463. [Google Scholar] [CrossRef]
- Klaus, W.; Gsell, B.; Labhardt, A.M.; Wipf, B.; Senn, H. The three-dimensional high resolution structure of human interferon α-2a determined by heteronuclear NMR spectroscopy in solution. J. Mol. Biol. 1997, 274, 661–675. [Google Scholar] [CrossRef]
- Ealick, S.; Cook, W.; Vijay-Kumar, S.; Carson, M.; Nagabhushan, T.; Trotta, P.; Bugg, C. Three-dimensional structure of recombinant human interferon-gamma. Science 1991, 252, 698–702. [Google Scholar] [CrossRef]
- Platis, D.; Foster, G.R. Interferon Proteins: Structure, Production and Purification. In The Interferons: Characterization and Application; Meager, A., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2006; pp. 73–83. [Google Scholar]
- Gad, H.H.; Dellgren, C.; Hamming, O.J.; Vends, S.; Paludan, S.R.; Hartmann, R. Interferon-λ Is Functionally an Interferon but Structurally Related to the Interleukin-10 Family. J. Biol. Chem. 2009, 284, 20869–20875. [Google Scholar] [CrossRef]
- Sanchez-Garcia, L.; Martín, L.; Mangues, R.; Ferrer-Miralles, N.; Vázquez, E.; Villaverde, A. Recombinant pharmaceuticals from microbial cells: A 2015 update. Microb Cell Fact 2016, 15. [Google Scholar] [CrossRef]
- Rosano, G.n.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, B.; Gerszberg, A.; Hnatuszko-Konka, K. A Brief Reminder of Systems of Production and Chromatography-Based Recovery of Recombinant Protein Biopharmaceuticals. Biomed. Res. Int. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Overton, T.W. Recombinant protein production in bacterial hosts. Drug Discov. Today 2014, 19, 590–601. [Google Scholar] [CrossRef]
- Pedro, A.Q.; Queiroz, J.A.; Passarinha, L.A. Smoothing membrane protein structure determination by initial upstream stage improvements. Appl. Microbiol. Biotechnol. 2019, 103, 5483–5500. [Google Scholar] [CrossRef]
- Solá, R.J.; Griebenow, K. Glycosylation of Therapeutic Proteins. BioDrugs 2010, 24, 9–21. [Google Scholar] [CrossRef]
- Rosano, G.L.; Morales, E.S.; Ceccarelli, E.A. New tools for recombinant protein production in Escherichia coli: A 5-year update. Protein Sci 2019, 28, 1412–1422. [Google Scholar] [CrossRef]
- Peng, F.W.; Duan, Z.J.; Zheng, L.S.; Xie, Z.P.; Gao, H.C.; Zhang, H.; Li, W.P.; Hou, Y.D. Purification of recombinant human interferon-epsilon and oligonucleotide microarray analysis of interferon-epsilon-regulated genes. Protein Expr. Purif 2007, 53, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Neves, F.O.; Ho, P.L.; Raw, I.; Pereira, C.A.; Moreira, C.; Nascimento, A.L. Overexpression of a synthetic gene encoding human alpha interferon in Escherichia coli. Protein Expr. Purif 2004, 35, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Morowvat, M.H.; Babaeipour, V.; Rajabi Memari, H.; Vahidi, H. Optimization of Fermentation Conditions for Recombinant Human Interferon Beta Production by Escherichia coli Using the Response Surface Methodology. Jundishapur J. Microbiol. 2015, 8, e16236. [Google Scholar] [CrossRef]
- El-Baky, N.A.; Redwan, E.M. Therapeutic Alpha-Interferons Protein: Structure, Production, and Biosimilar. Prep. Biochem. Biotechnol. 2014, 45, 109–127. [Google Scholar] [CrossRef]
- Srivastava, P.; Bhattacharaya, P.; Pandey, G.; Mukherjee, K.J. Overexpression and purification of recombinant human interferon alpha2b in Escherichia coli. Protein Expr. Purif. 2005, 41, 313–322. [Google Scholar] [CrossRef]
- Valente, C.A.; Prazeres, D.M.F.; Cabral, J.M.S.; Monteiro, G.A. Translational Features of Human Alpha 2b Interferon Production in Escherichia coli. Appl. Environ. Microbiol. 2004, 70, 5033–5036. [Google Scholar] [CrossRef]
- Vu, T.T.T.; Jeong, B.; Krupa, M.; Kwon, U.; Song, J.-A.; Do, B.H.; Nguyen, M.T.; Seo, T.; Nguyen, A.N.; Joo, C.H.; et al. Soluble Prokaryotic Expression and Purification of Human Interferon Alpha-2b Using a Maltose-Binding Protein Tag. J. Mol. Microbiol. Biotechnol. 2016, 26, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Babaeipour, V.; Shojaosadati, S.A.; Maghsoudi, N. Maximizing Production of Human Interferon-γ in HCDC of Recombinant, E. coli. Iran. J. Pharm. Res. 2013, 12, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Vaiphei, S.T.; Pandey, G.; Mukherjee, K.J. Kinetic studies of recombinant human interferon-gamma expression in continuous cultures of E. coli. J. Ind. Microbiol. Biotechnol. 2009, 36, 1453–1458. [Google Scholar] [CrossRef]
- Balderas Hernandez, V.E.; Paz Maldonado, L.M.; Medina Rivero, E.; Barba de la Rosa, A.P.; Jimenez-Bremont, J.F.; Ordonez Acevedo, L.G.; De Leon Rodriguez, A. Periplasmic expression and recovery of human interferon gamma in Escherichia coli. Protein Expr. Purif. 2008, 59, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Studier, F.W.; Moffatt, B.A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 1986, 189, 113–130. [Google Scholar] [CrossRef]
- Rabhi-Essafi, I.; Sadok, A.; Khalaf, N.; Fathallah, D.M. A strategy for high-level expression of soluble and functional human interferon α as a GST-fusion protein in E.coli. Protein Eng. Des. Sel. 2007, 20, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Peciak, K.; Tommasi, R.; Choi, J.W.; Brocchini, S.; Laurine, E. Expression of soluble and active interferon consensus in SUMO fusion expression system in E. coli. Protein Expr. Purif. 2014, 99, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, L.M.; Hernandez, V.E.; Rivero, E.M.; Barba de la Rosa, A.P.; Flores, J.L.; Acevedo, L.G.; De Leon Rodriguez, A. Optimization of culture conditions for a synthetic gene expression in Escherichia coli using response surface methodology: The case of human interferon beta. Biomol. Eng. 2007, 24, 217–222. [Google Scholar] [CrossRef]
- Platis, D.; Foster, G.R. High yield expression, refolding, and characterization of recombinant interferon alpha2/alpha8 hybrids in Escherichia coli. Protein Expr. Purif. 2003, 31, 222–230. [Google Scholar] [CrossRef]
- Valente, C.A.; Monteiro, G.A.; Cabral, J.M.; Fevereiro, M.; Prazeres, D.M. Optimization of the primary recovery of human interferon alpha2b from Escherichia coli inclusion bodies. Protein Expr. Purif. 2006, 45, 226–234. [Google Scholar] [CrossRef]
- Graumann, K.; Premstaller, A. Manufacturing of recombinant therapeutic proteins in microbial systems. Biotechnol. J. 2006, 1, 164–186. [Google Scholar] [CrossRef]
- Sørensen, H.P.; Mortensen, K.K. Advanced genetic strategies for recombinant protein expression in Escherichia coli. J. Biotechnol. 2005, 115, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Slouka, C.; Kopp, J.; Hutwimmer, S.; Strahammer, M.; Strohmer, D.; Eitenberger, E.; Schwaighofer, A.; Herwig, C. Custom made inclusion bodies: Impact of classical process parameters and physiological parameters on inclusion body quality attributes. Microb Cell Fact 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Ritz, D. Conversion of a Peroxiredoxin into a Disulfide Reductase by a Triplet Repeat Expansion. Science 2001, 294, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S. Solubility of disulfide-bonded proteins in the cytoplasm ofEscherichia coliand its “oxidizing” mutant. World J. Gastroenterol. 2005, 11. [Google Scholar] [CrossRef] [PubMed]
- Naderi-Manesh, H.; Mohammadian-Mosaabadi, J.; Maghsoudi, N.; Khalilzadeh, R.; Shojaosadati, S.A.; Ebrahimi, M. Effect of oxidative stress on the production of recombinant human interferon-γ in Escherichia coli. Biotechnol. Appl. Biochem. 2005, 41. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Kumar, N.; Monteiro, G.A.; Veeranki, V.D.; Prazeres, D.M.F. Re-engineering of an Escherichia coli K-12 strain for the efficient production of recombinant human Interferon Gamma. Enzym. Microb Technol. 2018, 117, 23–31. [Google Scholar] [CrossRef]
- Goncalves, A.M. Pichia pastoris: A Recombinant Microfactory for Antibodies and Human Membrane Proteins. World J. Microbiol. Biotechnol. 2013, 23, 587–601. [Google Scholar] [CrossRef]
- Xie, Y.F.; Chen, H.; Huang, B.R. Expression, purification and characterization of human IFN-λ1 in Pichia pastoris. J. Biotechnol. 2007, 129, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Pedro, A.Q.; Oppolzer, D.; Bonifácio, M.J.; Maia, C.J.; Queiroz, J.A.; Passarinha, L.A. Evaluation of MutS and Mut+ Pichia pastoris Strains for Membrane-Bound Catechol-O-Methyltransferase Biosynthesis. Appl. Biochem. Biotechnol. 2015, 175, 3840–3855. [Google Scholar] [CrossRef]
- Cregg, J.M.; Cereghino, J.L.; Shi, J.; Higgins, D.R. Recombinant Protein Expression in Pichia pastoris. Mol. Biotechnol. 2000, 16, 23–52. [Google Scholar] [CrossRef]
- Ayed, A.; Rabhi, I.; Dellagi, K.; Kallel, H. High level production and purification of human interferon α2b in high cell density culture of Pichia pastoris. Enzym. Microb Technol. 2008, 42, 173–180. [Google Scholar] [CrossRef]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, D.; Chan, W.; Cheng, L. Efficient expression and purification of human interferon alpha2b in the methylotrophic yeast, Pichia pastoris. Protein Expr. Purif. 2007, 54, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Ghosalkar, A.; Sahai, V.; Srivastava, A. Secretory expression of interferon-alpha 2b in recombinant Pichia pastoris using three different secretion signals. Protein Expr. Purif. 2008, 60, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, S.; Soorapaneni, S.; Prasad, K.S.; Raiker, V.A.; Padmanabhan, S. Strategies to maximize expression of rightly processed human interferon alpha2b in Pichia pastoris. Protein Expr. Purif. 2010, 71, 139–146. [Google Scholar] [CrossRef]
- Prabhu, A.A.; Veeranki, V.D.; Dsilva, S.J. Improving the production of human interferon gamma (hIFN-γ) in Pichia pastoris cell factory: An approach of cell level. Process Biochem. 2016, 51, 709–718. [Google Scholar] [CrossRef]
- Katla, S.; Karmakar, B.; Tadi, S.R.R.; Mohan, N.; Anand, B.; Pal, U.; Sivaprakasam, S. High level extracellular production of recombinant human interferon alpha 2b in glycoengineered Pichia pastoris: Culture medium optimization, high cell density cultivation and biological characterization. J. Appl. Microbiol. 2019, 126, 1438–1453. [Google Scholar] [CrossRef]
- Skoko, N.; Argamante, B.; Grujicic, N.K.; Tisminetzky, S.G.; Glisin, V.; Ljubijankic, G. Expression and characterization of human interferon-beta1 in the methylotrophic yeast Pichia pastoris. Biotechnol. Appl. Biochem. 2003, 38, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Chen, Z.; Yang, Z.Y.; Wang, T.Y.; Zhou, L.; Wu, J.B.; Zhou, L.F. Preparation and characterization of a potent, long-lasting recombinant human serum albumin-interferon-alpha2b fusion protein expressed in Pichia pastoris. Eur. J. Pharm. Biopharm. 2007, 67, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Agashe, V.R.; Hartl, F.U. Roles of molecular chaperones in cytoplasmic protein folding. Semin. Cell Dev. Biol. 2000, 11, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Solà, A.; Jouhten, P.; Maaheimo, H.; Sánchez-Ferrando, F.; Szyperski, T.; Ferrer, P. Metabolic flux profiling of Pichia pastoris grown on glycerol/methanol mixtures in chemostat cultures at low and high dilution rates. Microbiology 2007, 153, 281–290. [Google Scholar] [CrossRef][Green Version]
- Rumjantsev, A.M.; Bondareva, O.V.; Padkina, M.V.; Sambuk, E.V. Effect of Nitrogen Source and Inorganic Phosphate Concentration on Methanol Utilization andPEXGenes Expression in Pichia pastoris. Sci. World J. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Saraswat, M.; Musante, L.; Ravidá, A.; Shortt, B.; Byrne, B.; Holthofer, H. Preparative Purification of Recombinant Proteins: Current Status and Future Trends. Biomed. Res. Int. 2013, 2013, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A. Downstream Processing for Biopharmaceuticals Recovery. In Pharmaceuticals from Microbes, 1st ed.; Divya, A., Chetan, S., Sundeep, J., Eric, L., Eds.; Springer: Cham, Switzerland, 2019; pp. 163–190. [Google Scholar]
- Ramanan, R.N.; Ling, T.C.; Ariff, A.B. The performance of a glass bead shaking technique for the disruption of Escherichia coli cells. Biotechnol. Bioprocess Eng. 2008, 13, 613–623. [Google Scholar] [CrossRef]
- Ramanan, R.N.; Tan, J.S.; Mohamed, M.S.; Ling, T.C.; Tey, B.T.; Ariff, A.B. Optimization of osmotic shock process variables for enhancement of the release of periplasmic interferon-α2b from Escherichia coli using response surface method. Process. Biochem. 2010, 45, 196–202. [Google Scholar] [CrossRef]
- Protein production and purification. Nat. Methods 2008, 5, 135–146. [CrossRef]
- Walther, C.; Mayer, S.; Sekot, G.; Antos, D.; Hahn, R.; Jungbauer, A.; Dürauer, A. Mechanism and model for solubilization of inclusion bodies. Chem. Eng. Sci. 2013, 101, 631–641. [Google Scholar] [CrossRef]
- Meng, J.; Yan, Z.; Wu, J.; Li, L.; Xue, X.; Li, M.; Li, W.; Hao, Q.; Wan, Y.; Qin, X.; et al. High-yield expression, purification and characterization of tumor-targeted IFN-α2a. Cytotherapy 2007, 9, 60–68. [Google Scholar] [CrossRef]
- Ashnagar, F.; Khodabandeh, M.; Arpanaei, A.; Sadigh, Z.A.; Rahimi, F.; Shariati, P. Optimizing Primary Recovery and Refolding of Human Interferon-b from Escherichia coli Inclusion Bodies. Iran. J. Biotechnol. 2014, 12, 26–34. [Google Scholar] [CrossRef][Green Version]
- Jin, T.; Guan, Y.-X.; Fei, Z.-Z.; Yao, S.-J.; Cho, M.-G. A Combined Refolding Technique for Recombinant Human Interferon-γ Inclusion Bodies by Ion-exchange Chromatography with a Urea Gradient. World J. Microbiol. Biotechnol. 2005, 21, 797–802. [Google Scholar] [CrossRef]
- Nekoufar, S.; Fazeli, A.; Fazeli, M.R. Solubilization of Human Interferon β-1b Inclusion Body Proteins by Organic Solvents. Adv. Pharm. Bull. 2020, 10, 233–238. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Miyazaki, M. Refolding Techniques for Recovering Biologically Active Recombinant Proteins from Inclusion Bodies. Biomolecules 2014, 4, 235–251. [Google Scholar] [CrossRef]
- Guan, Y.-X.; Pan, H.-X.; Gao, Y.-G.; Yao, S.-J.; Cho, M.-G. Refolding and purification of recombinant human interferon-γ expressed as inclusion bodies in Escherichia coli using size exclusion chromatography. Biotechnol. Bioprocess Eng. 2005, 10, 122–127. [Google Scholar] [CrossRef]
- Geng, X.; Bai, Q.; Zhang, Y.; Li, X.; Wu, D. Refolding and purification of interferon-gamma in industry by hydrophobic interaction chromatography. J. Biotechnol. 2004, 113, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Y.; Li, J.; Ma, G.; Su, Z. On-column refolding of consensus interferon at high concentration with guanidine-hydrochloride and polyethylene glycol gradients. J. Chromatogr A 2006, 1115, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Dashbolaghi, A.; Khatami, S.; Sardari, S.; Cohan, R.A.; Norouzian, D. Improved Refolding Efficacy of Recombinant Human Interferon α-2b via pH Modulation. Trop. J. Pharm. Res. 2015, 14. [Google Scholar] [CrossRef]
- Hanke, A.T.; Ottens, M. Purifying biopharmaceuticals: Knowledge-based chromatographic process development. Trend Biotechnol. 2014, 32, 210–220. [Google Scholar] [CrossRef]
- Aslam, M.S.; Gull, I.; Mahmood, M.S.; Iqbal, M.M.; Abbas, Z.; Tipu, I.; Ahmed, A.; Athar, M.A. High yield expression, characterization, and biological activity of IFNα2-Tα1 fusion protein. Prep. Biochem. Biotechnol. 2019, 50, 281–291. [Google Scholar] [CrossRef]
- Yan, Z.; Lu, L.; Shi, J.; Bao, C.; Han, W.; Wu, Y.; Zhang, Y. Expression, Refolding, and Characterization of GFE Peptide-Fused Human Interferon-α2a in Escherichia coli. Appl. Biochem. Biotechnol. 2006, 133, 149–162. [Google Scholar] [CrossRef]
- Wang, D.A.N.; Ren, H.U.I.; Xu, J.-W.; Sun, P.-D.; Fang, X.-D. Expression, purification and characterization of human interferon-γ in Pichia pastoris. Mol. Med. Rep. 2014, 9, 715–719. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, W.-M.; Zhang, W.-J.; Ma, F.-L.; Li, J.-S.; Zhang, Q.; Zheng, L.-S. IFN-λ1 in CHO cells: Its expression and biological activity. Cell. Mol. Biol. Lett. 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Shi, L.; Xu, L.X. Refolding of recombinant human interferon α-2a from Escherichia coli by urea gradient size exclusion chromatography. Appl Biochem. Microbiol. 2012, 49, 11–17. [Google Scholar] [CrossRef]
- Lin, Y.K.; Ooi, C.W.; Ramanan, R.N.; Ariff, A.; Ling, T.C. Recovery of Human Interferon Alpha-2b from Recombinant Escherichia coli by Aqueous Two-Phase System. Sep. Sci. Technol. 2012, 47, 1023–1030. [Google Scholar] [CrossRef]
- Lin, Y.K.; Ooi, C.W.; Tan, J.S.; Show, P.L.; Ariff, A.; Ling, T.C. Recovery of human interferon alpha-2b from recombinant Escherichia coli using alcohol/salt-based aqueous two-phase systems. Sep. Purif. Technol. 2013, 120, 362–366. [Google Scholar] [CrossRef]
- Pandey, R.; Prabhu, A.A.; Dasu, V.V. Purification of recombinant human interferon gamma from fermentation broth using reverse micellar extraction: A process optimization study. Sep. Sci. Technol. 2017, 53, 487–495. [Google Scholar] [CrossRef]
- Cao, Y.; Bai, G.; Chen, J.; Tian, W.; Wang, S.; Yang, W. Preparation and characterization of magnetic microspheres for the purification of interferon α-2b. J. Chromatogr. B 2006, 833, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.S.P.; Freire, M.G.; Pedro, A.Q. Progress in the Development of Aqueous Two-Phase Systems Comprising Ionic Liquids for the Downstream Processing of Protein-Based Biopharmaceuticals. Am. Pharm. Rev. 2019, 2019, 1–6. [Google Scholar]
- Yau, Y.K.; Ooi, C.W.; Ng, E.-P.; Lan, J.C.-W.; Ling, T.C.; Show, P.L. Current applications of different type of aqueous two-phase systems. Bioresour. Bioprocess 2015, 2. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef]
- AlQahtani, A.D.; O’Connor, D.; Domling, A.; Goda, S.K. Strategies for the production of long-acting therapeutics and efficient drug delivery for cancer treatment. Biomed. Pharm. 2019, 113. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Khurana, V.; Patel, S.; Mitra, A.K. Long-term delivery of protein therapeutics. Expert Opin. Drug Deliv. 2014, 12, 415–440. [Google Scholar] [CrossRef]
- Jiskoot, W.; Randolph, T.W.; Volkin, D.B.; Russell Middaugh, C.; Schöneich, C.; Winter, G.; Friess, W.; Crommelin, D.J.A.; Carpenter, J.F. Protein Instability and Immunogenicity: Roadblocks to Clinical Application of Injectable Protein Delivery Systems for Sustained Release. J. Pharm. Sci. 2012, 101, 946–954. [Google Scholar] [CrossRef]
- George, P.M.; Badiger, R.; Alazawi, W.; Foster, G.R.; Mitchell, J.A. Pharmacology and therapeutic potential of interferons. Pharmacol. Ther. 2012, 135, 44–53. [Google Scholar] [CrossRef]
- Wang, W. Advanced protein formulations. Protein Sci. 2015, 24, 1031–1039. [Google Scholar] [CrossRef]
- Frokjaer, S.; Otzen, D.E. Protein drug stability: A formulation challenge. Nat. Rev. Drug Discov. 2005, 4, 298–306. [Google Scholar] [CrossRef]
- Kerwin, B.A. Polysorbates 20 and 80 Used in the Formulation of Protein Biotherapeutics: Structure and Degradation Pathways. J. Pharm. Sci. 2008, 97, 2924–2935. [Google Scholar] [CrossRef]
- Qian, J.; Tang, Q.; Cronin, B.; Markovich, R.; Rustum, A. Development of a high performance size exclusion chromatography method to determine the stability of Human Serum Albumin in a lyophilized formulation of Interferon alfa-2b. J. Chromatogr. A 2008, 1194, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.M.; Fiscella, M.; Lamousé-Smith, A.; Zeuzem, S.; McHutchison, J.G. Albinterferon α-2b: A genetic fusion protein for the treatment of chronic hepatitis C. Nat. Biotechnol. 2007, 25, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Lipiäinen, T.; Peltoniemi, M.; Sarkhel, S.; Yrjönen, T.; Vuorela, H.; Urtti, A.; Juppo, A. Formulation and Stability of Cytokine Therapeutics. J. Pharm. Sci. 2015, 104, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.T.; Dalhus, B.; Viuff, D.; Ravn, B.T.; Gunnarsen, K.S.; Plumridge, A.; Bunting, K.; Antunes, F.; Williamson, R.; Athwal, S.; et al. Extending Serum Half-life of Albumin by Engineering Neonatal Fc Receptor (FcRn) Binding. J. Biol. Chem. 2014, 289, 13492–13502. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Patel, R.; Patel, M. Poloxamers: A pharmaceutical excipients with therapeutic behaviors. Int. J. PharmTech Res. 2009, 1, 299–303. [Google Scholar]
- Bodratti, A.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Available online: http://www.fda.gov (accessed on 15 January 2021).
- European Medicines Agency. Available online: http://www.ema.europa.eu (accessed on 15 January 2021).
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Foster, G.R. Pegylated interferons: Chemical and clinical differences. Aliment Pharmacol. Ther. 2004, 20, 825–830. [Google Scholar] [CrossRef]
- RxList. Available online: http://www.rxlist.com (accessed on 15 January 2021).
- Bajracharya, R.; Song, J.G.; Back, S.Y.; Han, H.-K. Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery. Comput. Struct. Biotechnol. J. 2019, 17, 1290–1308. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-J.; Xu, S.; Wang, H.-M.; Ling, Y.; Dong, J.; Xia, R.-D.; Sun, X.-H. Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmsciTech 2019, 20. [Google Scholar] [CrossRef]
- Bocci, V. Evaluation of Routes of Administration of Interferon in Cancer: A Review and a Proposal. Cancer Drug Deliv. 1984, 1, 337–351. [Google Scholar] [CrossRef]
- Dutta, R. Drug Carriers in Pharmaceutical Design: Promises and Progress. Curr. Pharm. Des. 2007, 13, 761–769. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Bannerjee, S.; Bhati, L.; Pandey, S.; Pandey, P.; Sriwastawa, B. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2. [Google Scholar] [CrossRef]
- Yang, J.; Cleland, J.L. Factors Affecting the in Vitro Release of Recombinant Human Interferon-γ (rhIFN-γ) from PLGA Microspheres. J. Pharm. Sci. 1997, 86, 908–914. [Google Scholar] [CrossRef]
- Feczkó, T.; Fodor-Kardos, A.; Sivakumaran, M.; Haque Shubhra, Q.T. In vitro IFN-α release from IFN-α- and pegylated IFN-α-loaded poly(lactic-co-glycolic acid) and pegylated poly(lactic-co-glycolic acid) nanoparticles. Nanomedicine 2016, 11, 2029–2034. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Liu, Y.; Zhang, H.; Li, X.; Luo, F.; Mei, X. Development of interferon alpha-2b microspheres with constant release. Int. J. Pharm. 2011, 410, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Younes, H.M.; El-Kadi, A.O.S.; Neufeld, R.J.; Amsden, B.G. Sustained interferon-γ delivery from a photocrosslinked biodegradable elastomer. J. Control. Release 2005, 102, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Tobío, M.A.; González, L.; Fabra, A.; Alonso, M.A.J. Biodegradable micro- and nanoparticles as long-term delivery vehicles for interferon-alpha. Eur. J. Pharm. Sci. 2003, 18, 221–229. [Google Scholar] [CrossRef]
- Dai, J.; Long, W.; Liang, Z.; Wen, L.; Yang, F.; Chen, G. A novel vehicle for local protein delivery to the inner ear: Injectable and biodegradable thermosensitive hydrogel loaded with PLGA nanoparticles. Drug Dev. Ind. Pharm. 2017, 44, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, D.; Qian, H.; Chu, Y.; Yang, Y.; Shao, J.; Xu, Q.; Liu, B. Superior Antitumor Efficacy of IFN-α2b-Incorporated Photo-Cross-Linked Hydrogels Combined with T Cell Transfer and Low-Dose Irradiation Against Gastric Cancer. Int. J Nanomed. 2020, 3669–3680. [Google Scholar] [CrossRef]
- Kamei, N.; Morishita, M.; Chiba, H.; Kavimandan, N.J.; Peppas, N.A.; Takayama, K. Complexation hydrogels for intestinal delivery of interferon β and calcitonin. J. Control. Release 2009, 134, 98–102. [Google Scholar] [CrossRef]
- Caldorera-Moore, M.; Vela Ramirez, J.E.; Peppas, N.A. Transport and delivery of interferon-α through epithelial tight junctions via pH-responsive poly(methacrylic acid-grafted-ethylene glycol) nanoparticles. J. Drug Target. 2019, 27, 582–589. [Google Scholar] [CrossRef]
- Montagner, I.M.; Merlo, A.; Carpanese, D.; Dalla Pietà, A.; Mero, A.; Grigoletto, A.; Loregian, A.; Renier, D.; Campisi, M.; Zanovello, P.; et al. A site-selective hyaluronan-interferonα2a conjugate for the treatment of ovarian cancer. J. Control. Release 2016, 236, 79–89. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, J.; Bao, P.; Ding, Y.; Shen, Y.; Webster, T.J.; Xu, Y. Construction and in vivo/in vitro evaluation of a nanoporous ion-responsive targeted drug delivery system for recombinant human interferon α-2b delivery. Int. J. Nanomed. 2019, 14, 5339–5353. [Google Scholar] [CrossRef] [PubMed]
- Cánepa, C.; Imperiale, J.C.; Berini, C.A.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Development of a Drug Delivery System Based on Chitosan Nanoparticles for Oral Administration of Interferon-α. Biomacromolecules 2017, 18, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- González, L.F.; Acuña, E.; Arellano, G.; Morales, P.; Sotomayor, P.; Oyarzun-Ampuero, F.; Naves, R. Intranasal delivery of interferon-β-loaded nanoparticles induces control of neuroinflammation in a preclinical model of multiple sclerosis: A promising simple, effective, non-invasive, and low-cost therapy. J. Control. Release 2020. [Google Scholar] [CrossRef] [PubMed]
- Kristó, K.; Szekeres, M.; Makai, Z.; Márki, Á.; Kelemen, A.; Bali, L.; Pallai, Z.; Dékány, I.; Csóka, I. Preparation and investigation of core-shell nanoparticles containing human interferon-α. Int. J. Pharm. 2020, 573. [Google Scholar] [CrossRef]
- Morçöl, T.; Weidner, J.M.; Mehta, A.; Bell, S.J.D.; Block, T. Calcium Phosphate Particles as Pulmonary Delivery System for Interferon-α in Mice. AAPS PharmsciTech 2017, 19, 395–412. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Basnet, P.; Acharya, G.; Škalko-Basnet, N. PEGylated liposomes for topical vaginal therapy improve delivery of interferon alpha. Eur. J. Pharm. Biopharm. 2017, 113, 132–139. [Google Scholar] [CrossRef]
- Kondiah, P.P.D.; Tomar, L.K.; Tyagi, C.; Choonara, Y.E.; Modi, G.; du Toit, L.C.; Kumar, P.; Pillay, V. A novel pH-sensitive interferon-β (INF-β) oral delivery system for application in multiple sclerosis. Int. J. Pharm. 2013, 456, 459–472. [Google Scholar] [CrossRef]
- Qiu, J.; Wei, X.-H.; Geng, F.; Liu, R.; Zhang, J.-W.; Xu, Y.-H. Multivesicular liposome formulations for the sustained delivery of interferon alpha-2b1. Acta Pharmacol. Sin. 2005, 26, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- García-Vega, Y.; García-García, I.; Collazo-Caballero, S.E.; Santely-Pravia, E.E.; Cruz-Ramírez, A.; Tuero-Iglesias, Á.D.; Alfonso-Alvarado, C.; Cabrera-Placeres, M.; Castro-Basart, N.; Duncan-Roberts, Y.; et al. Pharmacokinetic and pharmacodynamic characterization of a new formulation containing synergistic proportions of interferons alpha-2b and gamma (HeberPAG®) in patients with mycosis fungoides: An open-label trial. BMC Pharmacol. Toxicol. 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- García-García, I.; Hernández-González, I.; Díaz-Machado, A.; González-Delgado, C.A.; Pérez-Rodríguez, S.; García-Vega, Y.; Campos-Mojena, R.; Tuero-Iglesias, Á.D.; Valenzuela-Silva, C.M.; Cruz-Ramírez, A.; et al. Pharmacokinetic and pharmacodynamic characterization of a novel formulation containing co-formulated interferons alpha-2b and gamma in healthy male volunteers. BMC Pharmacol. Toxicol. 2016, 17. [Google Scholar] [CrossRef]
- Gulia, M.; Rai, S.; Jain, U.K.; Katare, O.P.; Katyal, A.; Madan, J. Sustained-release protamine sulphate-impregnated microspheres may reduce the frequent administration of recombinant interferon α-2b in ovarian cancer. Anti-Cancer Drugs 2014, 25, 63–71. [Google Scholar] [CrossRef]
- De Leede, L.G.J.; Humphries, J.E.; Bechet, A.C.; Van Hoogdalem, E.J.; Verrijk, R.; Spencer, D.G. Novel Controlled-Release Lemna-Derived IFN-α2b (Locteron): Pharmacokinetics, Pharmacodynamics, and Tolerability in a Phase I Clinical Trial. J. Interferon Cytokine Res. 2008, 28, 113–122. [Google Scholar] [CrossRef] [PubMed]


| Interferon (IFN) Type/Subtype | Clinical Indication | Commercial Name | Active Pharmaceutical Ingredient | Approval Date | |
|---|---|---|---|---|---|
| IFNα (I) | IFNα-2a | Hairy cell leukemia; AIDS-related Kaposi’s sarcoma; Chronic myelogenous leukemia; Cutaneous T-cell lymphoma; Chronic hepatitis B and C; Follicular lymphoma; Malignant melanoma | Roferon A® Hoffmann–La Roche (Basel, Switzerland) | IFNα-2a (E. coli) | 1986 (EU) 1986 (USA) |
| Chronic hepatitis B; Chronic myelogenous leukemia; Melanoma | Pegasys® Hoffmann–La Roche (Basel, Switzerland) | PEGylated IFNα-2a (E. coli) | 2002 (USA and EU) | ||
| IFNα-2b | Multiple myeloma; Chronic myelogenous leukemia; Chronic hepatitis B and C; Carcinoid tumor; Hairy cell leukemia; Follicular lymphoma; Malignant melanoma; Condylomata acuminate; Kaposi’s sarcoma | Intron A®, Alfatronol® (Merck Sharp & Dohme Corp., Kenilworth, NJ, USA) | IFNα-2b (E. coli) | 1986 (USA) 1986 (EU) | |
| Chronic hepatitis B and C | Viraferon® (Schering-Plough Corporation, Brussels, Belgium) | IFNα-2b (E. coli) | 2000 (EU) | ||
| Chronic hepatitis C | Rebetron® (Schering-Plough Corporation, Brussels, Belgium) | ribavirin/IFNα-2b (E. coli) | 1999 (USA) | ||
| Chronic hepatitis C | ViraferonPeg® (Merck Sharp & Dohme Corp., Kenilworth, NJ, USA) | PEGylated IFNα-2b (E. coli) | 2000 (EU) | ||
| Chronic hepatitis C | PegIntron® (Schering-Plough Corporation, Brussels, Belgium) | PEGylated IFNα-2b (E. coli) | 2001 (USA) 2000 (EU) | ||
| Chronic hepatitis C | Albinterferon®/Albuferon® (Novartis—Basel, Switzerland; Human Genome Sciences, Rockville, MD, USA) | Fusion protein of albumin and IFNα-2b (E. coli) | 2010 (USA) | ||
| Melanoma | Sylatron™ (Merck & Co., Inc, Kenilworth, NJ, USA) | PEGylated IFNα-2b (E. coli) | 2011 (USA) | ||
| IFNα-2c | Chronic viral hepatitis; HIV infection | Berofor® (Boehringer Ingelheim, Lda, Ingelheim am Rhein, Germany) | IFNα-2c (E. coli) | 1989 (USA) | |
| IFNα (I) | IFNα-n3 | Condyloma acuminate | Alferon N® AIM ImmunoTech (Philadelphia, PA, USA) | IFNα-n3 (human leukocytes) | 1987 (USA) |
| IFNα-n1 (lymphoblastoid) | Chronic hepatitis B and C; Hairy cell leukemia; HPV infection | Wellferon®Glaxo Wellcome (London, United Kingdom) | IFNα-n1 (human lymphoblastoid cells) | 1997 (USA) | |
| IFNα-con-1 | Chronic hepatitis C | Infergen® (Three Rivers Pharmaceuticals, Warrendale, USA) | IFNα (E. coli) IFNα + Ribavirin (E. coli) | 2001(USA) | |
| IFNβ (I) | INFβ-1a | Multiple sclerosis | Avonex® (Biogen Idec, Maidenhead, United Kingdom) | IFNβ-1a (CHO cells) | 1996 (USA) 1997 (EU) |
| Rebif® (EMD Serono, London, United Kingdom) | Glycosylated IFNβ-1a (CHO cells) | 2002 (USA) 1998 (EU) | |||
| Plegridy® (Biogen Idec, Maidenhead, United Kingdom) | PEGylated IFNβ-1a (CHO) | 2014 (EU and US) | |||
| INFβ-1b | Multiple sclerosis | Betaseron® (Chiron—Emeryville, USA; Berlex Laboratories, Richmond, VA, USA) | IFNβ-1b (differs from human protein in that Cysteine-17 is replaced by Serine) (E. coli) | 1993 (USA) | |
| Betaferon® (Bayer Pharma, Leverkusen, Germany) | 1995 (EU) | ||||
| Extavia® (Novartis Europharm, Camberley, United Kingdom; Novartis Pharmaceuticals, East Hanover, NJ, USA) | IFNβ-1b (E. coli) | 2008 (US) 2009 (EU) | |||
| IFNγ (II) | INFγ-1b | Chronic granulomatous disease; Osteopetrosis | Actimmune® (Vidara Therapeutics, Dublin, Ireland) | IFNγ-1b (E. coli) | 1990 (US) |
| Imukin® (Boehringer Ingelheim, Lda, Ingelheim am Rhein, Germany) | 1996 (US) | ||||
| IFN Type | Class | Discovery Year | Receptor Binding |
|---|---|---|---|
| I | α | 1957 | High binding affinity to IFNAR2, which then recruits low-affinity IFNAR1 to form the signaling competent ternary complex |
| β | 1957 | ||
| ω | 1985 | ||
| τ | 1996 | ||
| II | γ | Early 1970s | Affinity for IFNGR (IFNGR1 and IFNGR2) |
| III | λ1 | 2003 | High binding affinity to IFNLR1, which then recruits low-affinity IL-10Rβ to form signaling competent ternary complex |
| λ2 | |||
| λ3 | |||
| λ4 |
| Excipient | Proposed Role | IFN Formulations | Highlights | |
|---|---|---|---|---|
| Buffers | Sodium phosphate pH 7 | Adjust pH to maximize the conformational stability of IFNs | IntronA®, PegIntron®, ViraferonPeg®, Alferon N® | Contrary to IFNα-2a, biological activity of IFNα-2b is high at pH 7; Acetate is not suitable for dry products due to the volatility of acetate and changes in pH during lyophilization |
| Acetate buffer pH 5 | Roferon® | |||
| Acetate buffer pH 6 | Pegasys® | |||
| Surfactants | Polysorbate 20 | Inhibit protein aggregation and adsorption to surfaces | ViraferonPeg®, Imukin® | Widely used independent of the type of IFN |
| Polysorbate 80 | Roferon®, ViraferonPeg®, Pegasys® | |||
| Poloxamer 188 | Rebif® | |||
| Chelating agents | Edetate disodium | Mitigate risk of oxidation and immunogenicity from aggregates | IntronA® | |
| Salts | NaCl | Tonicity modifier | IntronA®, Roferon®, Pegasys® | Liquid formulations |
| Sugars and polyols | Sucrose | Lyoprotectant and tonicity modifier | ViraferonPeg® | Powder formulation |
| Mannitol | Lyoprotectant | Actimmune®, Immukin® | ||
| Preservatives | Benzyl alcohol | Oxidation inhibition | Pegasys® | |
| Proteins | Human serum albumin | Prevents aggregation | IntronA®, Betaseron® | Higher albumin concentrations if IFNβ products due to their higher tendency to aggregate than IFNα-based products |
| Amino acids | Arginine | Increase protein solubility and stability and preserves biological activity | Avonex® | Often used as an alternative to albumin |
| Glycine | Prevents aggregation | Betaseron® | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, L.S.; Lobo, G.S.; Pereira, P.; Freire, M.G.; Neves, M.C.; Pedro, A.Q. Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation. Vaccines 2021, 9, 328. https://doi.org/10.3390/vaccines9040328
Castro LS, Lobo GS, Pereira P, Freire MG, Neves MC, Pedro AQ. Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation. Vaccines. 2021; 9(4):328. https://doi.org/10.3390/vaccines9040328
Chicago/Turabian StyleCastro, Leonor S., Guilherme S. Lobo, Patrícia Pereira, Mara G. Freire, Márcia C. Neves, and Augusto Q. Pedro. 2021. "Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation" Vaccines 9, no. 4: 328. https://doi.org/10.3390/vaccines9040328
APA StyleCastro, L. S., Lobo, G. S., Pereira, P., Freire, M. G., Neves, M. C., & Pedro, A. Q. (2021). Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation. Vaccines, 9(4), 328. https://doi.org/10.3390/vaccines9040328









