Abstract
Zika virus (ZIKV) infection and its associated congenital and other neurological disorders, particularly microcephaly and other fetal developmental abnormalities, constitute a World Health Organization (WHO) Zika Virus Research Agenda within the WHO’s R&D Blueprint for Action to Prevent Epidemics, and continue to be a Public Health Emergency of International Concern (PHEIC) today. ZIKV pathogenicity is initiated by viral infection and propagation across multiple placental and fetal tissue barriers, and is critically strengthened by subverting host immunity. ZIKV immune evasion involves viral non-structural proteins, genomic and non-coding RNA and microRNA (miRNA) to modulate interferon (IFN) signaling and production, interfering with intracellular signal pathways and autophagy, and promoting cellular environment changes together with secretion of cellular components to escape innate and adaptive immunity and further infect privileged immune organs/tissues such as the placenta and eyes. This review includes a description of recent advances in the understanding of the mechanisms underlying ZIKV immune modulation and evasion that strongly condition viral pathogenesis, which would certainly contribute to the development of anti-ZIKV strategies, drugs, and vaccines.
1. Introduction
Between 2011 and 2018, World Health Organization (WHO) identified, alerted and tracked more than 1400 epidemic events in 172 countries due to emerging viruses, such as influenza, Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), Ebola, Yellow Fever, Zika, and others [1]. These epidemic diseases are a prelude of the new global era the world is facing, and hallmark of high-impact, fast-spreading outbreaks that are increasingly difficult to manage, as is occurring nowadays with the new worldwide infection by the emerged SARS Coronavirus 2 (SARS-CoV-2) responsible for the ongoing coronavirus disease 2019 (COVID-19) pandemic [2]. Remarkably, in the last five years, WHO has declared three global health emergencies associated with emerging viruses: SARS-CoV-2 in 2020 [2], Ebola virus (EBOV) in 2014 [3,4,5,6] and Zika virus (ZIKV) in 2016 [7]. These threats were the first warnings of the socio-economic impact of emerging viruses, as the world is now experiencing with the SARS-CoV-2 pandemic which has become a global crisis [8]. The impact of the ZIKV pandemic put two of the biggest world sports events at risk of cancellation, both in Brazil, the Olympic Games (2016) and the World Soccer Championship (2018) [9,10].
In the context of ZIKV, WHO called Zika an “extraordinary event that needed a coordinated response, constituting a public health emergency of international concern (PHEIC)” [7], due to the description of a large outbreak of rash illness [11,12,13], with short-term and low-grade fever [13], not in all cases, and a cluster of microcephaly in newborns to infected mothers [14,15,16,17,18,19,20,21,22] together with neurological abnormalities and the Guillain-Barré syndrome (GBS) in Brazil [11,23,24,25,26,27,28,29,30]. In fact, ZIKV infection carries the risk of adverse pregnancy outcomes including increased risk of preterm birth, fetal death and stillbirth, and congenital malformations collectively characterized as congenital Zika syndrome (CZS), including the above-mentioned microcephaly, abnormal brain development, limb contractures, eye abnormalities, brain calcifications, and other neurologic manifestations [17,31,32,33,34,35]. ZIKV has been found in the cerebrospinal fluid (CSF) and brain of adults infected by the virus who manifested neurological disorders [24,36,37,38,39]. This flavivirus causes harmful effects in the adult brain, such as GBS [36,38,40,41,42], encephalitis [36,41,42,43], meningoencephalitis [24,44], acute myelitis [36,42,45] and encephalomyelitis [36,39,41,46,47], among sensory polyneuropathy [48] and other neurological complications [49,50]. After Brazil, other ZIKV outbreaks and rapid transmission were subsequently reported throughout the Americas and Africa, as well as other regions of the world [12,23,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65]. Taken together, these data prompted the scientific community to retrospectively seek a potential association between ZIKV and reported cases of microcephaly and also GBS in French Polynesia (2013–2014) [66,67,68], providing a correlation of the risk of microcephaly, congenital malformations and fatal cases of fetuses and neonates from ZIKV infected mothers [69,70,71].
ZIKV is a mosquito-borne, positive-sense single-stranded RNA virus belonging to the family Flaviviridae (genus Flavivirus) [72,73,74,75,76,77,78,79,80,81,82,83]. ZIKV is further classified by homology to the Spondweni virus (SPONV) in the Spondweni viral clade or serogroup [73,84,85], both viruses were first characterized in Africa in 1947 and 1952 [82,84], respectively. ZIKV was isolated from the serum of a pyrexial rhesus monkey caged in the canopy of the Zika Forest in Uganda [82], and discovered during the study of the vector responsible for the cycle of sylvan Yellow fever virus (YFV) in Uganda [85]. The first confirmed human infections by ZIKV occurred in Nigeria (1954) [86], further cases were reported in Uganda (1962–63) [54] and outside Africa in central Java, Indonesia (1977) [87]. ZIKV presents three major phylogenetic related East African, West African and Asian/American lineages [88,89,90,91,92], constituting a single serotype [93]. These ZIKV linages are thought to have emerged from East Africa in the late 1800s or early 1900s [88,94], with the Asian lineage being responsible for all ZIKV outbreaks in the Pacific and the Americas [23,95,96,97,98,99,100].
ZIKV is principally transmitted to humans by infected Aedes aegypti and Aedes albopictus mosquitoes [100,101,102,103]. An increase of Aedes mosquito populations has been observed in tropical developing countries [104]. Thus, infected mosquitoes, highly populated areas, and global commercial and tourist activities together with modern transportation constitute an efficient scenario to spread infected mosquitoes and viruses such as ZIKV around the world [105], as reported with Dengue [104] and as occurred with epidemic ZIKV episodes in 2007 [95], 2013 [96,106] and 2014–2015 [107,108,109], declared as a PHEIC by WHO [7]. The scientific community efforts have rapidly increased knowledge about this virus. However, understanding the complexity of ZIKV infection, transmission and pathogenesis remains an urgent challenge. Hence, it is crucial to study competent vectors and natural reservoirs for ZIKV, its viral genetic diversity and flavivirus coinfection [110,111,112,113], as well as potential cross-immune reactivity (i.e., challenging the immune diagnosis) and immune enhancement of infection (i.e., antibody-dependent enhancement (ADE)) [114,115,116,117,118,119,120], fortunately not yet observed in humans [121], together with environmental factors that may suddenly trigger ZIKV epidemic expansion and a worse viral pathogenesis [12,30,55,122,123,124].
Due to the enormous challenge to develop a ZIKV vaccine [125,126,127,128,129], it is still not possible to immunize against ZIKV infection and associated pathology. Furthermore, the understanding of the immune events underlying ZIKV infection, transmission and pathology is key for meeting this ZIKV challenge. Based on the above, the present article aims to review the mechanisms of ZIKV immune evasion related to viral non-structural (NS) proteins, genomic and non-coding viral RNA, as well as microRNA (miRNA) generated during ZIKV infection to modulate cellular environment, in order to escape immunity and cause ZIKV complex pathology.
2. Mechanisms of ZIKV Immune Evasion
Apart from the CZS that covers the pathogenic events associated with maternal-fetal ZIKV transmission and the above summarized GBS [130], primary ZIKV infection is generally asymptomatic or mild in adults [131,132,133,134,135]. Factors determining asymptomatic or mild ZIKV infection, and severe manifestations, as well as chronic sequelae, are still to be determined. Understanding the importance of being infected by a particular ZIKV lineage, the influence of comorbidities and previous flavivirus infections (i.e., Dengue virus (DENV)), as well as viral load and the molecular mechanisms underlying severe infection, such as host genetic susceptibility to infection, immunosuppression and/or failure of the innate immunity are key to improving the overall knowledge of the complex ZIKV disease, diagnosis, prophylaxis and treatment [136,137,138,139,140,141,142,143,144,145,146,147].
The interplay between ZIKV and immune responses is initiated once the virus invades different cells, tissues and organs from the early infection. In infected individuals and non-human primates, the virus is fast cleared from blood, but persisting in saliva, urine, semen, breast milk and the central nervous system (CNS) for months [12,148,149,150,151]. Several in vitro and ex vivo studies indicate that ZIKV replicates in human endothelial and epithelial cells [152], peripheral blood mononuclear cells (PBMCs) [152], astrocyte and microglial cells [153,154], different placenta cells, such as trophoblasts [155], Hofbauer cells in chorionic villi and amniotic epithelial cells [156], as well as fibroblasts (placental, uterine, pulmonary) [157]. Moreover, ZIKV has a broad cell tropism in vitro, infecting human skin cells (i.e., dermal fibroblasts and epidermal keratinocytes), human myeloid cells (i.e., dendritic cells (DCs) and macrophages), and human progenitor cells of neuronal, placental and testicular origin (reviewed in [158,159]).
It is important to understand how ZIKV interacts with these cells and tissues, as well as with the host immune system to cause severe disease. The following sections describe the mechanisms underlying interferon (IFN) immune response against flavivirus, and the available knowledge concerning the events triggered by the ZIKV genome and proteins to escape or neutralize the antiviral IFN functions to infect and induce pathogenesis in immune privileged organs such as the brain and eye [140,160,161,162].
2.1. Antiviral IFN and Associated Signals
Host immune defense against ZIKV implies: (1) efficient recognition of the virus as the first line of defense (see Figure 1 for a schematic representation of ZIKV components that could be recognized by host defenses); (2) the induction of the IFN cascade intermediates and related signals, as a central mediator that allows antiviral action, inhibiting viral replication, and (3) initiation of a specific adaptive immune response [158,159,163,164,165,166], as occurs with other flaviviruses [159,167]. Nevertheless, this scheme is partially blurred by the action of the components of the virus. ZIKV responds to the assault by the infected cell by (1) mounting multiple camouflage strategies evading free genomic RNA recognition in cytosol, (2) avoiding the activation of multiple interactions of IFN cascades and finally (3) turning off the signaling that serves as a warning signal of infection.
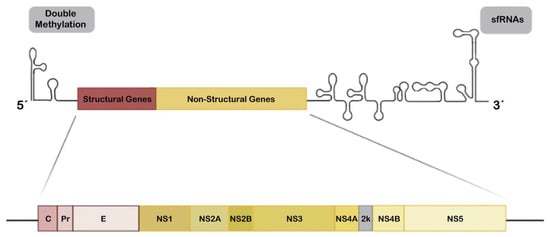
Figure 1.
Schematic overview of the Zika virus (ZIKV) genome. The ZIKV genome is a single-stranded positive-sense RNA (+ssRNA) of approximately 11 Kb and codes a polyprotein which is cleaved in the endoplasmic reticulum (ER) lumen by the host and/or viral proteases to release three structural proteins (C, PrM and E; red boxes) and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5; yellow boxes). The 5′ untranslated region (UTR) is susceptible for specific double methylation (represented in grey box at left) by the viral C-terminal methyltransferase domain of NS5 to behave like messenger RNA (mRNA) and hijacks host factors for translation and for masking the viral RNA to prevent cellular recognition and degradation. UTR loops at 3′ end are subgenomic flavivirus RNAs (sfRNAs) (shown in the grey box on the right), that play a role in the immune response evasion (see Section 2.4 in the main text).
The positive-sense single-stranded RNA (+ssRNA) genome of ZIKV is approximately 11 Kb in size and codes one large polyprotein that is processed by viral and host proteases into three structural (C, prM/M, and E) and seven non-structural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) [73,168,169] (Figure 1), as reported for other flaviviruses [170,171]. In infected cells, all these proteins are produced following genome replication and translation in the cytoplasm, where the structural proteins together with some NS proteins drive virion assembly at endoplasmic reticulum (ER) membranes and subsequent egress or new viral particles [172]. Furthermore, the enzymatic and protective battery of NS proteins are important for viral polypeptide processing, replication and modulation of host responses, which are needed for viral success [172]. Evolution studies of the ZIKV polyproteins suggest that NS maintains a high grade of conservation and presents several sites that mediate interaction with the host immune system [173] or that may regulate viral RNA synthesis [173,174]. Many of these functional sites have evolved to act against similar factors and functions making the host immune system antagonism redundant but adequately efficient, due to the need to competently escape the immune surveillance and to establish infection in the host [173,174]. Variability at these sites may modulate ZIKV infection capacity, optimization of immune evasion and pathogenicity, and this could be associated with the infection severity degree of the different viral phenotypes or lineages [140,142,175,176,177,178].
Once inside the target cell, as occurs with other RNA virus, the delivered +ssRNA genome of ZIKV could be recognized by pattern recognition receptors (PRRs), such as melanoma differentiation-associated gene 5 (MDA5) and retinoic acid-inducible gene 1 (RIG-I). This recognition activates PRRs synergistically and the subsequent signal pathway leads to expression of type I IFNs and other antiviral genes mediated by IFN regulatory factors (IRFs) [166,179,180], in general, accompanied with production of pro-inflammatory cytokines, in a nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) dependent manner [181]. Furthermore, the active MDA5/RIG-I pathway joins several key factors, such as the mitochondrial antiviral signaling protein (MAVS), inhibitor of kappa-B kinase epsilon (IKKε), TANK-binding kinase 1 (TBK1), and IRF3 and IRF7, together playing a pivotal role in modulating the expression of type I IFN genes [180]. Type I IFNs continue the antiviral cascade in an autocrine and paracrine manner, by activation of type-I IFN receptors (IFNARs) and the crucial Janus kinase/signal transducers and activators of transcription (JAK/STAT (STAT1 and STAT2)) pathway in target cells. The activation of this axis evokes the second round of upregulation of antiviral genes which implies IFN-stimulated response elements (ISREs) that trigger the expression of IFN-stimulated genes (ISGs) to restrict ZIKV infection as a key action of the innate immune response [182,183,184]. These antiviral IFN-triggered ISGs exert diverse and different antiviral functions, such as inhibition of viral entry and viral protein synthesis, clearance of both viral protein and genome and inhibition of viral replication and release [183,185], as reported for ISG15 that limits ZIKV infection [186]. In addition, the antiviral responses failed during ZIKV infection in ISG15-/- mice, with lower expression of RIG-I and IFN alpha-inducible protein 6 (IFI6) related ISGs and increased severity of retinal lesions [186]. Moreover, over-expression of IFI6 restricts ZIKV replication and prevents ZIKV-mediated cell death [187], as similarly reported for the IFN-inducible factor 16 (IFI16) [188]. However, ISG15 has also recently been reported to be involved in promoting ZIKV infection by regulating JAK/STAT and ISGylation (the term that stands for protein conjugation with ISG15 [189]) pathways [190].
IFNs are produced after ZIKV entry into target cells by recognition of the viral +ssRNA genome into the endosomal compartments by the membrane associated Toll-like receptors (TLR)-3 or -7 [191,192,193], as further confirmed by specific, small interference RNA (siRNA) knock-down of TLR-3 in human skin cells that strongly favors ZIKV infection [180]. ZIKV infection could also promote host mitochondrial DNA (mitoDNA) release (i.e., mitochondrial failure) [194] that is sensed by cyclic GMP-AMP synthase (cGAS) and signals through the ER associated intermediate stimulator of IFN genes (STING) [195]. Through these signals, as reported for DENV (reviewed in [196]), ZIKV may further activate TBK1 and IKKε which in turn phosphorylate IRF-3/7 [185], and IKKε activates NF-κB [197]. Finally, IRF3 and NF-κB act as transcription factors to promote expression of type-I IFNβ and some antiviral or proinflammatory genes [197].
Furthermore, paracrine IFN signaling in non-infected cells renders these cells refractory to viral infection. All type I IFNs are able to signal through the type-I IFNAR that is composed of two heterodimeric subunits (IFNAR1 and IFNAR2) which are generally widely distributed throughout the body (reviewed in [198,199]). This canonical type I IFN signal induces a plethora of ISREs which drive ISGs expression to establish a cellular antiviral state ([200,201,202,203]) (Figure 2). The protective role of IFN-I against ZIKV has been demonstrated in IFN-I signaling-deficient mice which are highly susceptible to viral infection [204]. Type I and II IFN-pretreated primary skin fibroblasts showed a non-permissive status against ZIKV, observing a strong and dose-dependent inhibition of viral replication [180]. In a different work, IFN-β shows moderated anti-ZIKV activity [205]. However, elevated secretion of IFN-β, detected in human lung epithelial cells during ZIKV infection appears to be responsible for preventing virus-mediated cell death by apoptosis [205]. Concerning the protective role of ISGs against ZIKV infection, it has been reported that placental cells could resist viral infection due to actions of IFN-λ (type III IFN) [206]. Likewise, it seems that small membrane-associated IFN-inducible transmembrane proteins (IFITMs), particularly IFITM1 and 3, could inhibit ZIKV infection early in the viral life cycle [207]. In this work, results point to the possibility that IFITM3 could also prevent cell death mediated by ZIKV infection [207].
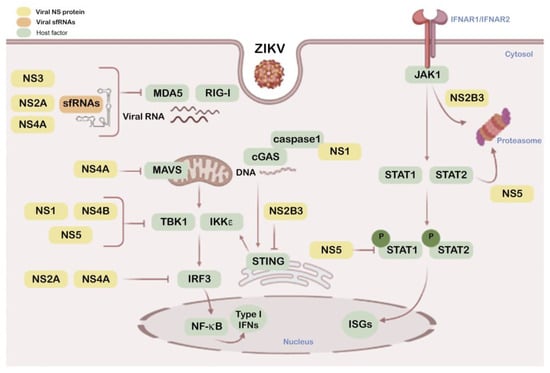
Figure 2.
Schematic overview of ZIKV NS proteins and sfRNAs as modulators of antiviral interferon (IFN) and associated signals. This diagram shows the interactions of cellular factors (green boxes) involved in the IFN pathway with the NS viral proteins (yellow boxes) and sfRNAs (sfRNA1 and 2) (orange box) that play a role in this IFN pathway. Color code inbox is indicated top left.
In this scenario, ZIKV replication appears to be either impaired in IFN-α- or IFN-β-pre-treated primary skin fibroblasts [180], which are key IFNs for autocrine and paracrine antiviral effects [208], or promoted by anti-IFN-α/β receptor (IFNAR2) antibodies (Abs) in human infected DCs [209], or in mice lacking IFNAR1 or IRF3, 5 and 7 which developed more severe neurological disease compared to wild-type [210].
Therefore, ZIKV needs to neutralize and evade these type I IFN antiviral responses to infect and persist. In order to do this, the virus carries several components that are able to antagonize IFN antiviral functions, such as the structural E protein, NS proteins, genomic and non-coding viral RNA which are summarized in the sections below.
2.2. ZIKV Structural E Protein Inhibits IFN Production
In viral particles, the ZIKV E surface glycoprotein is responsible for the binding of the virion to different receptors at target cells, thereby determining viral tropism, infection and disease (reviewed in [168]). Furthermore, the ZIKV E protein elicits a strong humoral immune response in infected individuals that has positioned it as an ideal candidate for the development of vaccines. However, there is a disparity between the highly immunogenic nature of ZIKV E and the poor neutralizing capacity of the polyclonal Ab response induced against it [211].
A plethora of host receptors has been reported to mediate in ZIKV E-dependent attachment and/or entry and infection, such as heparan sulfate glycosaminoglycans (HS-GAGs) [212], the C-type (calcium dependent) lectin, dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin (DC-SIGN)/CD209 [180], and phosphatidylserine (PS) receptors of the TIM (T-cell immunoglobulin and mucin domain) and TAM (Tyro3, Axl, and Mer) families (reviewed in [168]). ZIKV indirectly binds to the Axl (Anexelekto; a Greek word that means uncontrolled) TAM kinase through Gas6 (growth arrest-specific 6) soluble factor, the natural ligand of Axl (i.e., both factors constituting an Axl-Gas6 complex). The functional role of this TAM kinase in ZIKV infection is mediated by the ability of Gas6 to bridge with protein S and the PS on the viral envelope [213,214,215,216]. These ZIKV-PS/Gas6-Axl interactions therefore mediate the attachment of the Zika virion to the host cell-surface [153,154,217].
It is important to note that activated Axl kinase suppresses type I IFN signaling [218]. ZIKV could therefore condition immune escape from the first steps of the viral life cycle, through activating Axl and impairing IFN production, then negatively acting on IFN-defenses [154,213]. Thus, the efficiency of ZIKV to target the different host receptors during the entry mechanism could compromise the efficiency of the IFN protection, which could explain the lack of an effective vaccine against ZIKV E, since this viral antigen may suppress IFN defenses [213,219,220,221,222].
2.3. ZIKV NS Proteins as Modulators of Antiviral IFN and Associated Signals
Besides their central roles in the viral lifecycle [223], NS proteins modulate innate responses altering IFN cascades and associated protective mechanisms (Figure 2). NS1 targets the IFN-associated cGAS-STING pathway by promoting cGAS processing by caspase-1. NS1 stabilizes caspase-1 by recruiting the deubiquitinase USP8 that deubiquitinates caspase-1 at K134 residue, increasing its stability and facilitating its action to cleave cGAS which results in reduced type I IFN signaling and enhanced ZIKV replication [195]. In addition, a natural, evolutionary NS1 mutation (bearing 188V residue) in the Asian ZIKV lineage has been described which facilitates its interaction with TBK1, diminishing TBK1 phosphorylation that results in the inhibition of IFN-β production in human cells, and viral replication enhancement in immunocompetent C57BL/6J mice [224]. Moreover, NS1 together with NS4B target type I IFN production by stabilizing NS2B-NS3 (NS2B3) during viral infection [225].
NS2A down-regulates the promoter activity of IFN-β by acting on several factors that are key for IFN-β activation [226]. Hence, NS2A suppresses RIG-I and MDA5, two pattern recognition receptors (PRRs) of the viral RNA genome for triggering IFN production and antiviral functions [166,179]. It is noteworthy that NS2A inhibits IFN-β induction by all forms of the IRFs (IRF3) [226], which act as IFN promoters in the JAK/STAT pathway [182]. Down-stream from IFN signaling, the JAK/STAT pathway promotes antiviral ISGs which target different steps of the viral cycle [182]. As regards NS2B, overexpression of the ZIKV NS2B3 precursor also interferes with the JAK/STAT pathway, as it is involved the viral helicase domain in interacting with JAK1 and driving it to the proteasome degradative route [225]. On the other hand, and confirming the NS2B3 interplay with the antiviral IFN function, it has been reported that type I IFN signaling promotes NS2B3 autophagic clearance in a STAT1-dependent manner, thus limiting ZIKV replication [225]. Moreover, NS2B3 helps viral replication by inhibiting protective RIG-I-like receptor (RLRs)-mediated cell-apoptosis in a JAK1-independent manner [225]. RLRs recognize cytosolic viral RNA [227], acting as sensors for ZIKV and other RNA viruses [167]. Moreover, NS2B3 is able to target the cGAS/STING pathway by cleaving STING, a degradative action that abrogates ISGs expression in response to cGAMP and neutralizes anti-ZIKV defenses [228].
NS3 protein also blocks RIG-I- and MDA5-mediated IFN antiviral actions, but acts on the 14–3-3 family of adaptor proteins [229] These factors complex with either RIG-I or MDA5 to translocate them to mitochondria where they promote antiviral IFN induction [230,231]. Thus, NS3 binds to 14–3-3 proteins preventing 14–3-3 factors from working together with RIG-I or MDA5 to recognize viral patterns and neutralize ZIKV infection [229]. Regardless of these multiple mechanisms for evasion of viral sensing, NS2B-NS3 also induces nucleoporins (NUPs) degradation and causes a marked inhibition of mature messenger RNAs (mRNAs) and nuclear proteins export to the cytoplasm [232], performing novel functions hijacking the protective role of nuclear components relevant in triggering immune response pathways, thereby inducing a favorable environment for viral replication
The ZIKV-NS4A protein performs an anti-IFN activity, reducing its production by blocking RIG-I and MDA5 signaling, also at the mitochondrial level [226,233]. NS4A competitively binds to the N-terminal caspase recruitment and activation domain (CARD) of the mitochondrial MAVS factor, abrogating MAVS interaction with activated RIG-I or MDA5, resulting in the inhibition of type-I IFN production. It has also been reported that NS4A inhibits the activities of both IFN-β and IRF3 reporters, blocking the type-I IFN response [234,235]. Furthermore, NS4B has also been observed interacting with TBK1 kinase, preventing its oligomerization and phosphorylation that are responsible for its activation and subsequent IFN production [225]. Besides, NS4B in cooperation with NS1 also inhibits type I IFN production by stabilizing NS2B3 during viral infection [225].
ZIKV NS5 is the most highly conserved protein encoded by ZIKV genome not only because of the requirement to maintain two enzymatic activities indispensable for virus replication, methyltransferase and RNA-dependent RNA polymerase, but also in its IFN-I antagonism [173,236,237,238,239,240].
NS5 targets each level of the IFN activation axis in host cells, particularly impairing genomic RNA sensing at its 5′ untranslated region (UTR) capping by RIG-I by repressing RIG-I polyubiquitination by means of the NS5-MTase (methyltransferase) domain but MTase function independent, thereby making RIG-I incompetent to activate IRF3 and therefore IFN-β production [241], or by acting as a barrier of IFN activation (Figure 1). Hence, NS5 abrogates IRF3 and NF-κB signaling [234], as it is able to interact with IRF3 avoiding its activation [224], or binding to IKKε, affecting IKKε protein levels and phosphorylation, which also finally results in IRF3 inactivation [242]. Furthermore, NS5 protein antagonizes IFN production by impairing the activation of TBK1 and, therefore, the IRF3 factor, a TBK1 substrate for phosphorylation [243]. In addition, NS5 competitively binds to the ubiquitin-like domain of TBK1, affecting its interaction with TRAF6 (TNF (tumor necrosis factor) receptor-associated factor 6). This complex is required for TBK1-mediated IRF3 phosphorylation and activation, thus interfering with type I and III IFN transcription [243].
Additionally, in IFN-induced cells, ZIKV NS5 expression results in proteasomal degradation of the IFN-regulated transcriptional activator STAT2 in humans but not in mice, and also affects STAT1 phosphorylation levels, suppressing INF-mediated JAK/STAT signal transduction [244,245], which show that ZIKV-induced disease takes advantage of NS5-promoted IFN deficiency. Moreover, despite the fact that the role that NS5 plays in the nucleus remains enigmatic, the latest works suggest that subcellular localization of NS5 is important for its function in innate immune suppression which provides new insight into ZIKV pathogenesis [239,246,247]. Hence, NS5 allows ZIKV evasion of the IFN-mediated innate immunity.
2.4. Viral Genomic and Subgenomic Rnas Inhibit IFN Signaling
The ZIKV genome contains 5′ and 3′UTRs (Figure 1), about 107 and 428 nucleotides long, respectively [73,248,249]. The RNA genome of ZIKV and other flavivirus are processed by cellular exoribonucleases, such as the host 5′-3′ exoribonuclease (XRN-1) to produce subgenomic flavivirus RNAs (sfRNAs) once inside the cell [250]. These incompletely degraded sfRNAs result from the stalling of the host 5′-3′ XRN1 enzyme or its homologous Pacman in insects on conserved RNA structures in the 3′UTR of the ZIKV RNA [250,251,252,253]. Furthermore, XRN1 is halted in its degradative action by two structured XRN-1-resistant RNA elements (xrRNAs) on ZIKV, xrRNA1 and xrRNA2 [254,255] which protect the remaining 3′UTR leading to the accumulation of sfRNAs during ZIKV infection. The ZIKV genome also presents an additional putative xrRNA3 formed by a structured RNA element known as dumbbell (DB1) [255,256].
As regards IFN modulation, ZIKV sfRNAs seem to suppress RIG-I- and MDA-5-mediated IFN induction, as reported by an experimental approach based on transient expression of the ZIKV-3′UTR together with transfection of the RIG-I or MDA-5 agonist [257]. Moreover, nucleotide deletions in the DB-1 structure of the ZIKV-3′UTR region, in a live-attenuated vaccine candidate, impair viral RNA synthesis and increase the sensitivity to type I IFN inhibition [258]. Despite all these reported data, the mechanism underlying the observed attenuation of type I IFN production by sfRNAs is not well understood [248].
However, it is conceivable that sfRNAs may be involved in ZIKV efficient infection and neuro-pathogenicity in vivo, since these events have been related to impairment of the IFN response in several mouse models of ZIKV infection [204,259,260,261,262] and in other scientific evidence obtained from patients [206,235,263,264]. In this regard but without targeting IFN production, recent studies using wild-type ZIKV point to the importance of sfRNAs for viral infection in both human cells and mosquitoes [265]. ZIKV-sfRNAs do not negatively affect IFN levels, since high IFN and proinflammatory cytokines levels were induced after infection of human cells with wild-type virus [265]. However, IFN levels were strongly diminished in cells infected by a recombinant ZIKV unable to generate sfRNAs [265]. Of note, efficient viral clearance was observed in cells infected with sfRNA-deficient ZIKV. Further studies are needed to clarify this apparent contradictory observation concerning the absence of sfRNA-mediated regulation of IFN immunity. However, these data correlated well with the strong induction of ISGs which occurred in these infected cells, thereby suggesting that the deficiency in sfRNA is associated with an uncontrolled IFN signaling which strongly activates ISGs to further clear ZIKV [265]. Therefore, sfRNAs are required for ZIKV propagation in human cells. Furthermore, results obtained with recombinant ZIKV indicated that the absence of sfRNAs results in inefficient viral infection and transmission in Aedes aegypti mosquitoes [265]. Similarly, a recent study has demonstrated the importance of the sfRNAs for productive infection and virus transmission in mosquitos by suppressing ZIKV infection-mediated apoptosis and assuring the ability of the virus to disseminate and reach saliva in the invertebrate vector [266].
On the other hand, viral genomic RNA (gRNA) double methylation during flavivirus infection appears to be key for avoiding gRNA rapid degradation and recognition by antiviral host factors, and for controlling the efficiency of mRNA translation and [248,267]. 2′-O cap methylation of the 5′UTR end mediates the evasion of flavivirus from the antiviral IFN-induced proteins with tetratricopeptide repeats (IFIT) response [268], thereby inhibiting their binding to viral gRNA [267]. Hence, the West Nile virus (WNV), DENV and Japanese encephalitis virus (JEV) mutants, deficient in 2′-O methylation activity, are unable to escape viral suppression by IFN and IFIT proteins [268,269,270]. In this respect, 2′-O methylation of the 5′-end of the ZIKV gRNA has also been reported [271,272], with the NS5 MTase activity itself being responsible for this modification [271,272,273]. Thus, 2′-O methylated 5′-cap gRNA mimics cellular RNAs, evading its recognition by antiviral host factors and following translation by the host machinery [272]. It is plausible, therefore, that 2′-O methylation of the ZIKV gRNA mediates immune escape, viral infection and pathology.
These observations have promoted the development of ZIKV and other flavivirus antiviral drugs [271,274,275,276] and vaccines based on deficiency in 2′-O methylation, in order to potentiate IFN and IFIT antiviral functions and attenuating flavivirus virulence [270,277,278,279,280].
2.5. MicroRNA, ZIKV Infection, Immunity and Pathogenicity
A microRNA (miRNA or miR (consensus nomenclature reviewed in [281])) is an evolutionarily conserved small non-coding RNA, 20–25 nucleotide-long, that performs a wide regulatory activity on several biological processes such as cellular immune responses against viruses in the case of viral miRNAs [282]. miRNAs modulate gene expression through base pairing of the miRNA seed sequence, mainly located within the 3′UTR to its target mRNA, which leads to either translational repression or mRNA cleavage, blocking target protein synthesis [283,284]. In the context of ZIKV infection and pathology a new field of research has emerged to find potential viral and cellular miRNAs that could regulate immune responses to favor the viral life cycle and thereby contributing to ZIKV complex pathology.
In infection experiments of human neuronal stem cells (hNSCs) with ZIKV MR766 and Paraiba strains, data obtained indicate that genes regulating stem cell survival, NESTIN (neuroectodermal stem cell marker) and PAX6 (paired box 6), cell cycle and neurogenesis are repressed in a miRNA-dependent manner [285]. In this work, ZIKV infection upregulated miR-124–3p and let-7c which downregulated transferrin receptor (TFRC) and HMGA2 (high-mobility group AT-hook 2) mRNAs, respectively, thereby downregulating these target genes, including the established regulator of NSC renewal mRNA in hNSCs and mouse brain tissue [285]. Moreover, ZIKV infection upregulated miR-125a-5p together with miR-125a-3p, both showing repressive activity on MAVS which is central in the RIG-I and type I IFN response pathways of the innate immune system [286]. Additionally, ZIKV infection upregulated miR-7–5p and miR-320c, both miRNAs are able to target “SIN3 transcription regulator homolog A (SIN3A)” mRNA, which codes for a paired amphipathic helix protein that bears a STAT3-interacting repressor, essential for IFN-mediated antiviral response [287]. The authors proposed that this miRNA-mRNA interplay could be key for suppression of IFN signaling in ZIKV-infected hNSCs and suggest that these miRNAs could orchestrate the suppression of gene networks important for immunity, fostering neurodegeneration and microcephaly.
In astrocytes, ZIKV infection has been mainly related to the downregulation of several miRNAs with the unfolded protein response (UPR) pathway as a major downregulated target [288]. Apart from this, a downregulated key gene during the course of ZIKV infection is the DICER1 transcripts, which may account for global downregulation of miRNAs. On the contrary, ZIKV infection upregulates some miRNAs, such as miR-431-5p, together with upregulation of CHOP (C/EBP homologous protein) and GADD34 (growth arrest and DNA damage-inducible 34) genes that are autophagy associated factors [288]. Considering all these data, it seems that many flaviviruses facilitate their own viral replication and persistence by targeting the UPR pathway [288,289,290]. The alteration of this cellular quality control mechanism promotes the accumulation of misfolded proteins and triggers ER stress and autophagy [291]. Thus, ZIKV infection activates the expression of proteins related to the UPR pathway in mouse neural cells, such as IRE1 (inositol-requiring enzyme)-XBP1 (X-box binding protein 1) and ATF6 (activating transcription factor 6) [292], also observing the upregulation of several RNA transcripts of genes related to the autophagy function and pathway, such as atf4, gadd34, chop, and edem-1 genes. A recent study in ZIKV-infected primary mouse neurons states that ZIKV-modulated miRNAs include miR-155, miR-203, miR-29a and miR-124-3p [293]. Furthermore, these ZIKV-modulated miRNAs seem to interact with mRNAs that regulate neurological development and neuroinflammatory responses. Thus, some of these miRNAs have been involved in the control of flavivirus infection, anti-viral immunity and brain injury [294,295,296,297,298,299]. Likewise, it has been reported that ZIKV modulates mRNA levels of enzymes involved in the generation of miRNAs, such as Drosha, Dicer, Ago2, Ago3 and DGCR8, during infection in liver, lung and kidney cells [300]. Additionally, virus-derived siRNAs (vsiRNAs) have been observed in ZIKV infected human neural progenitor cells (hNPCs), as well as in mosquito cells [301]. These vsiRNAs seem to exert an immune antiviral effect, since targeting key RNA interference (RNAi) machinery components significantly enhances ZIKV replication [301]. This antiviral action exerted by vsiRNA has been confirmed by using brain organoids treated with the RNAi enhancer enoxacin [302]. The increase of antiviral RNAis by enoxacin inhibits ZIKV-induced microcephalic phenotypes in brain organoids [301]. Therefore, the regulation of these RNA transcripts and proteins may account for the permissivity of these organs for ZIKV infection and damage.
On the other hand, several studies challenging human and mouse neuronal cells with ZIKV have not reported any change in the expression level of miRNAs expression in cells of the astrocyte lineage [303,304,305].
Apart from these discrepancies and the necessity of an accurate mechanistic characterization of ZIKV induction or repression of miRs, in target cells and tissues, it is conceivable that the regulation by ZIKV of the interplay between miR and mRNA transcripts of key genes for immune functions, cell differentiation and fate would be crucial to understand the events underlying viral immune escape and neuropathogenesis, during active ZIKV infection and transmission Therefore, these ZIKV related miRs are good candidates to develop therapeutics for the management of ZIKV neurological disease.
2.6. ZIKV Infection and Adaptive Immune Responses Understanding Cross-Immune Protection and Vaccine Challenges
The innate immune responses imply a rapid sensing of viruses to eliminate them, and ZIKV deploys different strategies to subvert these mechanisms, as presented above. Adaptive immunity against viruses provides a more finely tuned repertoire of recognition for these nonself-antigens (reviewed in [306,307,308,309]). The adaptive immune response is initiated by activation of naïve T cells through antigen presentation, where CD4+ and CD8+ T cells are important players in the control of viral infection, representing the memory cell repertoire of defense for future expositions to the virus together with B cells and neutralizing Abs (NAbs) (reviewed in [306,308,309,310,311]).
Some studies on the role of T cells in ZIKV infection indicate that CD8+ T cells are important for controlling the viral load (viremia) of ZIKV, as depletion of CD8+ T cells in mice resulted in enhanced virus replication [312], whereas CD4+ T cells presented a Th1 profile that produces the antiviral IFN-γ together with TNF-α, and interleukin 2 (IL-2) [312]. These data have been confirmed by other studies of primary ZIKV infection in animal models, where CD8+ T cells exert a key protective action against ZIKV [313,314]. CD8+ T cell protective responses against ZIKV have been reported to target structural proteins such as E, prM, and C [315], whereas CD8+ T cell responses promoted by DENV infection are predominantly against NS proteins, such as NS3, NS4B and NS5 [316,317,318].
Cross-reactive T cell-mediated protective action against ZIKV infection has gained interest since ZIKV affects regions where DENV used to be endemic [159,319,320], and the scientific community is concerned about potential reinfection events possibly causing acute or more severe ZIKV infection and pathogenesis [114,320,321]. Thus, characterization of this flaviviral T cell cross-protection is crucial for vaccine development and preparedness actions for future outbreaks [322,323,324,325]. In this regard, CD8+ T cell cross-response against ZIKV has been reported with CD8+ T cells generated during a previous DENV infection. In mice, DENV-elicited CD8+ T cells protect against a posterior challenge with ZIKV either in non-pregnant [326,327] or pregnant mice [328]. In addition, both immunization with ZIKV-specific or ZIKV/DENV cross-reactive peptides, followed by ZIKV challenge, elicited CD8+ T cell responses that were protective as depletion of CD8+ T cells resulted in increased ZIKV infection [326]. Similar data have been reported with DENV2. Hence, DENV2-promoted protective CD8+ T cells against ZIKV infection could recognize epitopes in different NS and structural ZIKV proteins, mainly NS3, NS5, prM and E [319]. This cross-immune protection has been corroborated in humans by a study in DENV-endemic countries (Sri Lanka and Nicaragua) and by comparing ZIKV cross-reactive T cells from non-infected individuals vs. individuals that have been previously exposed to DENV [315]. This study discovered an interesting observation relating the distinct specificity for ZIKV proteins and epitopes shown by protective CD8+ T cells generated in ZIKV infected individuals that have previously experienced a DENV infection compared with individuals without any previous reported DENV infection. Thus, CD8+ T cells from DENV-immune individuals showed a response to epitopes in ZIKV NS3 and NS5 proteins, whereas cells from DENV-naïve individuals targeted C, E, and prM structural proteins [315]. The predominance of DENV-elicited CD8+ T cell cross-immune reactions against ZIKV-NS proteins have also been observed in other human studies, mainly recognizing NS3 protein [329,330] with poor cross-reaction reported against structural proteins, such as ZIKV C protein [330]. Therefore, human pre-existing T cell responses against DENV have been found to recognize some peptide sequences in ZIKV proteins, which could be explained by the high degree of homology in sequence and structures (DENV and ZIKV polyprotein homology is about 55–56%) together with functional long-range interactions between the two flaviviruses [319,331]. These facts allow previous DENV infection to promote reactive T cells that cross-react and protect against ZIKV infection, as further reported by other studies in humans [332,333,334].
It has been reported that peptide immunization or ZIKV infection-elicited CD4+ T cell responses are necessary for protection against virus infection and further viral clearance in primary infection. However, the involvement of viral antigen specific CD4+ T cells in the control of ZIKV infection and disease is still controversial [335,336]. ZIKV-triggered CD4+ T cell mediated Ab response, but not CD8+ T cells, seemed to be responsible for viral clearance in an intravaginal infection model [336]. These data point to the relevance of CD4+ T cell immunity in blocking sexual transmission. Activation of CD4+ T cells and associated humoral immunity should therefore be considered as a challenge for vaccine designs that aim to interfere with ZIKV sexual transmission. Vaccine-mediated T cell protective recruitment is also key to achieving protection against ZIKV in the maternal-fetal interface during pregnancy. This is a real challenge to be accomplished by vaccines, since T cells mobilization and access to the maternal-fetal interface is restricted during pregnancy [337]. In this regard, attenuated ZIKV used as vaccine failed to elicit strong T cells responses during pregnancy in mice, when compared with vaccine efficiency at that level in non-pregnant mice. Humoral protection of this tissue in pregnant mice requires higher NAbs titers in order to protect and block vertical transmission of ZIKV [338]. Therefore, ZIKV could enter an immune privileged interface and tissues during pregnancy, therefore a vaccine should offer high potency in eliciting CD4+ T cell activation to cooperate with B cell to assure long-term associated humoral immunity, together with protective CD8+ T cells.
For the above reasons, the efforts to develop anti-ZIKV vaccines are focused on generating protective CD8+ and CD4+ T cells to control ZIKV infection and promote virus clearance. In this respect, an NS3-based vaccine has recently been reported to generate specific CD8+ T cell response protecting against ZIKV infection [339]. These vaccine-elicited polyfunctional CD8+ T cells are required to prevent death in lethally infected adult mice and fetal growth restriction in infected pregnant mice. Moreover, this NS3 vaccine design and strategy appears to be safe, since it failed to induce a NAb response [339], thereby avoiding serious concerns related to potential ADE that could increase the severity of a reinfection event [340] that in the flavivirus family could occur with a secondary exposure with a different serotype of the same virus or with heterologous flavivirus [340]. Although it has been reported that anti-DENV Abs (i.e., generated during DENV infection) could enhance ZIKV infection and disease severity in mice [117,341,342] as well as in human placental explants [343], there is not any evidence that clearly demonstrates ZIKV-ADE in humans [340,344]. This safety concern is always behind several anti-ZIKV vaccine designs and developments that are conceived to elicit NAb responses, and use prM and E (prM-E) proteins in the majority of the anti-ZIKV vaccine candidates developed so far [345]. Furthermore, a DNA vaccine expressing ZIKV NS1 protein protected against viremia and disease in BALB/c mice infected by ZIKV [346]. In this case, it seems that viremia is decreased by the NAbs generated by the NS1 DNA vaccine, with CD4+ and CD8+ T cells also being key for viral control, since this vaccine failed to protect against a lethal ZIKV challenge in Ifnar1−/− mice [346]. Therefore, and considering that ADE effect is not demonstrated in humans for ZIKV infection [340], this NS1-based vaccine design that elicits specific and efficient humoral and cellular immune responses may be a good candidate for anti-ZIKV vaccine in humans to elicit long-term protection. Additionally, the above mentioned NS3-based vaccine that activates protective CD8+ T cells against ZIKV in a highly susceptible mouse model with human-relevant T cell responses, prompted the suggestion that this vaccine might afford protection in vulnerable pregnant individuals [339], representing a promising candidate for a safe anti-ZIKV vaccine in humans and evading ADE-associated concerns [344]. Moreover, an adenovirus type 4 vector-based vaccine expressing ZIKV prM-E achieved protection against lethal ZIKV challenge in mice, without generation of anti-ZIKV NAbs [347], which could be also considered a safe vaccine candidate.
A vaccine to protect against ZIKV infection, transmission and pathogenesis is a challenge that requires deep molecular and cell/tissue knowledge on how the virus infects and persists through several cell types and organs, particularly, in immune privileged tissues where vaccines should induce IFN-mediated innate antiviral immunity, and recruit long-term memory cellular and humoral protective immunity.
2.7. ZIKV Invades and Infects the Fetal Brain and Eye: Evading Immune Responses in Immuno-Privileged Organs
ZIKV is able to persist for months in immune-privileged sites, such as testes that allows it to be sexually transmitted [348], eyes [160] and the CNS [140,162]. The infection of the placenta and fetus by ZIKV is proof of the innate and adaptive immune evasion of this virus in relevant cell types and tissues/organs which are related to the CZS and neuro-damage and disorders [130].
The interplay of ZIKV with these relevant tissues could disturb metabolism and the fetus-mother exchange of different important factors, such as nutrients, growth factors and gas (CO2/O2)-exchange in the placenta during pregnancy, and altering vasculature and nervous system development [349]. ZIKV is able to infect and replicate in different primary cell types isolated from placental chorionic villi and the chorioamniotic membranes (e.g., cytotrophoblast cells, Hofbauer cells, amniotic epithelial cells, and umbilical cord endothelial cells) [156,350]. In this physiological scenario, the blood/CSF provides a barrier in the choroid plexus for ZIKV invasion to the nervous system, which could regulate virus access to the interior brain and spinal cord surfaces. The choroid plexus is a complex structure to develop a model to study its interplay with ZIKV to characterize ZIKV infection and trafficking into the fetal brain. However, it is conceivable that ZIKV may traffic across the choroid epithelium via transcytosis and exocytosis into the CSF [351]. Indeed, ZIKV-mediated injury patterns around the lateral ventricles (where CSF flows from) have been reported in both humans and non-human primates [352]. Likewise, ZIKV persistence in the placental chorionic villi points to a potential establishment of a viral reservoir in this tissue that sustains maternal viremia throughout pregnancy [16]. Furthermore, the blood/brain barrier (BBB) could mediate in the access of the ZIKV to the outer brain and spinal cord regions, whereas the blood/retinal barrier could regulate ZIKV invasion to the developing ocular tissues [130,349,353].
Overall, at the maternal/fetal interface, different cell types express cell-surface factors that allow viral entry and infection, replication and further transmission to the fetus, such as Axl, Tyro3 and TIM1 [156] that belong to the ZIKV PS receptors of the TAM (i.e., Tyro3 and Axl) and TIM families [168]. Animal models show that ZIKV infection targets neural progenitor cells during pregnancy that results in reduced fetal cerebral cortical surface area [354], which could trigger the sequence of malformations associated with CZS. This ZIKV cytotoxic effect on neural progenitor cells has also been described by infecting cells, neurospheres and/or cerebral organoids structures made of neural stem cells [140,290,355]. In non-human primates, ZIKV infection has been associated with reduced NSCs proliferation across the subventricular zone, compromising neurogenic output [356,357]. It has also been reported that the multi-ciliated ependymal epithelium, a ventricular-subventricular stem cell niche during human brain development [358], could be injured by ZIKV thus impairing CSF transport and outflow through the dura, which might contribute to ventriculomegaly, both in non-human primates and humans [70]. Different data point to the possibility that ZIKV could pass through the BBB to access outer brain cells at later stages of fetal development, since ZIKV infection of this tissue is well correlated with the presence of posterior cortical/subcortical calcifications and gliosis, and polymicrogyria (i.e., late migration defects) [359,360]. These cytopathic events could be responsible for or contribute to motor, vision, auditory, arthrogryposis, and dystonic disorders [359,361]. Once ZIKV crosses the BBB to access outer parenchyma, the virus could invade outer NSCs, specialized neurogenic daughter cells (i.e., intermediate progenitors (IPs)), neurons, as well as astrocytes and microglia [304,305,362,363]. This infection process leads to a loss of progenitors and neuroinflammation together with immune activation and cytokine release that abrogate neurogenesis (reviewed in [351]). Additionally, innate antiviral pathways are negatively altered by ZIKV infection in early pregnancy and, particularly, when congenital microcephaly is relevant [156]. There is an important body of knowledge about the harmful effects exerted by this flavivirus on the developing brain. However, the understanding of the mechanisms underlying the consequences of ZIKV infection on mature CNS requires more research. As introduced in the present review, a broad spectrum of neurological disorders has been reported in adult patients infected by ZIKV [36], detecting ZIKV in CSF and brains of adult patients [24,36,37,39,41]. In animal models, ZIKV has also been found in CSF of infected monkeys, persisting for weeks in this cerebral fluid, even after viral clearance of the rest of the organism [151], whereas ZIKV infects neural progenitors, affecting their proliferation in the adult brain of mice [364]. Additionally, in adult infected mice, genomic RNA of ZIKV has been detected in the frontal cortex and hippocampus, and observing synapsis impairment by the virus, thereby indicating that ZIKV could target memory-related brain regions [365]. These negative effects of ZIKV on synapsis and memory in mice appear to be related to the upregulation of the TNF-α signaling, subsequent microglial activation and inflammation together with significant increase of C1q/C3 proteins of the complement system. In this work, data demonstrate that ZIKV replicates in ex vivo tissues isolated from human adult brain, and targets mature neurons [365]. Altogether these data suggest that ZIKV could infect and persist in adult CNS, accounting for the long-term neurodegenerative impact of ZIKV infection in the brain of adult patients.
The observation of conjunctivitis and uveitis events together with other ocular injuries associated with ZIKV infection in children and adults, such as optic nerve hypoplasia, coloboma, foveal reflex loss, vascular defects, and gliosis and optic nerve cupping/neuritis related to inflammation at later fetal stages are indicative that the virus is able to infect and injure the eye and the retina [349,366,367], and that it may cross the nervous system protective blood/retinal barrier.
Taken together these data are key to understanding the biological processes, as well as the cellular and molecular mechanisms that could favor ZIKV maternal/fetal transmission and trafficking into the brain, as reported in ZIKV infected monocytes that present enhancing adhesion and transmigration capacities favoring viral dissemination to neural cells [368], in order to elicit fetal protection and develop antiviral therapeutics or vaccines [128,129,369,370,371,372].
3. Conclusions
ZIKV persistence and pathogenesis involve a complex immune-evasion strategy to facilitate ZIKV trafficking, infection and spread through different cell types and tissues to finally cross protective barriers to the immune-privileged fetus, affecting the development of the fetal brain and eyes.
ZIKV uses its own viral proteins, gRNA and sfRNA elements to evade antiviral immunity, particularly the anti-ZIKV IFN response and associated signals and factors, as mentioned above. Hence, multiple NS proteins of ZIKV negatively modulate the antiviral response at various levels by inhibiting type I IFN production and the expression of downstream ISGs, acting on the IFN-associated cGAS-STING pathway, targeting with TBK1 and therefore impairing IRF3 promoters, or modulating RIG-I- and MDA5-directed type I IFN induction, as well as different steps of the antiviral type I IFN system against RNA viruses such as ZIKV. The NS proteins could cooperate to overcome the INF antiviral functions, limiting immune-protection and the understanding of ZIKV immune-evasion and pathogenesis. Moreover, gRNA-associated modifications that function as an antagonist of the innate type-I IFN response, and sfRNA stability against XRN-1, 5′-3′ exoribonuclease, which help to efficiently suppress RIG-I- and MDA-5-mediated IFN have been identified as an important proviral pathway, abrogating IFN neutralization of ZIKV infection during the early-phase of the viral life cycle. Moreover, ZIKV infection modifies the miRNA landscape of host cells in order to evade innate and adaptive immune responses and promote viral replication and survive. Although the involvement of viral antigen specific CD4+ T cells in the control of ZIKV infection and disease is still controversial, the CD8+ T cell response is associated with the control of the ZIKV infection and pathogenesis. This protective action has been more clearly demonstrated by cross-immune reactions of human DENV-elicited CD8+ T cells [326] which react against ZIKV-NS (i.e., mainly recognizing NS3 protein [329,330]). Anti-ZIKV vaccines are therefore focused on generating protective CD8+ and CD4+ T cells in order to control ZIKV infection and promote virus clearance, thereby avoiding harmful ADE effects with potential reinfection events by ZIKV or other flaviviruses [121,128,373,374,375].
In order to reach the human fetus, ZIKV must cross the placenta barrier which develops within days of conception and is indispensable for pregnancy [376,377]. Furthermore, fetal damage by ZIKV infection can be observed after the first trimester, and persistence infection, late during pregnancy, results in fetal disease or adverse pregnancy outcomes [13,378].
Recently, as a result of the global impact of ZIKV and its teratogen effects, such as microcephaly in newborns to infected mothers along with neurological abnormalities and GBS, and comparison with previous congenital pathogens such as Toxoplasma gondii, other (e.g., syphilis, human immunodeficiency virus (HIV) or parvovirus B19), rubella virus, cytomegalovirus (CMV) and herpes simplex virus (HSV) (i.e., all of which are grouped under the term of TORCH pathogens) [379]. ZIKV has been proposed as a new TORCH pathogen but more complex in the associated pathogenesis [379]. ZIKV-induced congenital microcephaly abnormality alarmed the whole of the planet [19] together with other developmental abnormalities that determine the CZS (i.e., severe microcephaly with partially collapsed skull, thin cerebral cortices with subcortical calcifications, macular scarring and focal pigmentary retinal mottling, congenital contractures and marked early hypertonia) [380,381], and placental insufficiency and fetal loss [71]. ZIKV is able to infect, traffic and spread through several cells and tissues during fetal differentiation, just after overcoming/crossing several protective barriers such as those mentioned above. ZIKV is able to infect eye and brain immune-privileged organs, avoiding innate IFN defenses and provoking neuroinflammation and cytokine release that halt neurogenesis [351], thereby favoring viral spread and tissue damage.
Therefore, understanding how ZIKV interacts with the INF system and influences the outcome of infection in barrier tissues such as the placenta, eyes and brain during pregnancy may help in the development of therapeutic strategies to clear ZIKV from the organism, based on the action mechanism of the IFN system, and keeping immune-privileged organs safe during both fetal development and in adults.
Moreover, determining the molecular interplay between the ZIKV genome and viral proteins with the IFN and T cell responses and antiviral signals is also crucial to improving vaccine strategies based on mutant live-attenuated viral candidates that need to induce adaptive cellular and humoral immune protective responses, also helping type-I IFN antiviral responses [128,129,169,382].
Author Contributions
A.V.-F. wrote and drafted the manuscript, J.E.-H. drafted the manuscript and S.P.-Y. and R.C.-R. prepared the diagrams. All the authors D.M.-A., R.T.-G., J.-D.M., R.M. read, supervised the scientific content, edited and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research and A.V.-F.’s Lab was funded by RTI2018-093747-B-100 (“Ministerio de Ciencia, Innovación y Universidades”, Spain) and the European Regional Development Fund (ERDF), ProID2020010093 (RIS-3 Canarias Strategy-“María del Carmen Betancourt y Molina” Program, “Consejería de Economía, Conocimiento y Empleo, Gobierno de Canarias”), UNLL10-3E-783 (ERDF and “Fundación CajaCanarias”), and by the Spanish AIDS network “Red Temática Cooperativa de Investigación en SIDA” RD12/0017/0034 as part of the Plan Nacional R+D+I and cofunded by Spanish “Instituto de Salud Carlos III (ISCIII)-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER)”. R.C.-R., S.P.-Y. and D.M.-A. are funded by RD12/0017/0034, M-ULL PhD-training contract (“Contratos predoctorales M-ULL para la Formación de Doctores, Convocatoria 2019, Universidad de La Laguna and Ministerio de Ciencia, Innovación y Universidades”, Spain), “Fundación Doctor Manuel Morales” and “TESIS2017010116” (“Programa Predoctoral de Formación del Personal Investigador, Agencia Canaria de Investigación, Innovación y Sociedad de la Información” and European Social Fund-fellowships). J.E.-H. is funded by the Cabildo of Tenerife “Agustin de Betancourt” 2017 Program.
Acknowledgments
All figures were created with BioRender.com.
Conflicts of Interest
The author declares no conflict of interest.
References
- A World at Risk: Annual Report on Global Preparedness for Health Emergencies. Global Preparedness Monitoring Board. 2019. Available online: https://apps.who.int/gpmb/assets/annual_report/GPMB_annualreport_2019.pdf?utm_source=mandiner&utm_medium=link&utm_campaign=mandiner_202004 (accessed on September 2019).
- Coronavirus Disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ (accessed on 29 December 2020).
- Statement on the 1st Meeting of the ihr Emergency Committee on the 2014 Ebola Outbreak in West Africa. 2014. Available online: https://www.who.int/mediacentre/news/statements/2014/ebola-20140808/en/ (accessed on 8 August 2014).
- Koenig, K.L.; Majestic, C.; Burns, M.J. Ebola virus disease: Essential public health principles for clinicians. West. J. Emerg. Med. 2014, 15, 728–731. [Google Scholar] [CrossRef][Green Version]
- McCoy, C.E.; Lotfipour, S.; Chakravarthy, B.; Schultz, C.; Barton, E. Emergency medical services public health implications and interim guidance for the ebola virus in the united states. West. J. Emerg. Med. 2014, 15, 723–727. [Google Scholar] [CrossRef]
- Safari, S.; Baratloo, A.; Rouhipour, A.; Ghelichkhani, P.; Yousefifard, M. Ebola hemorrhagic fever as a public health emergency of international concern: A review article. Emergency (TehranIran) 2015, 3, 3–7. [Google Scholar]
- Who Statement on the First Meeting of the International Health Regulations (2005) (ihr 2005) Emergency Committee on Zika Virus and Observed Increase in Neurological Disorders and Neonatal Malformations. 2016. Available online: https://www.who.int/news/item/01-02-2016-who-statement-on-the-first-meeting-of-the-international-health-regulations-(2005)-(ihr-2005)-emergency-committee-on-zika-virus-and-observed-increase-in-neurological-disorders-and-neonatal-malformations (accessed on 1 February 2016).
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (covid-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Wilson, M.E.; Touch, S.; McCloskey, B.; Mwaba, P.; Bates, M.; Dar, O.; Mattes, F.; Kidd, M.; Ippolito, G.; et al. Rapid spread of zika virus in the americas--implications for public health preparedness for mass gatherings at the 2016 brazil olympic games. Int. J. Infect. Dis. 2016, 44, 11–15. [Google Scholar] [CrossRef]
- Salvador, F.S.; Fujita, D.M. Entry routes for zika virus in brazil after 2014 world cup: New possibilities. Travel Med. Infect. Dis. 2016, 14, 49–51. [Google Scholar] [CrossRef]
- Miranda-Filho Dde, B.; Martelli, C.M.; Ximenes, R.A.; Araújo, T.V.; Rocha, M.A.; Ramos, R.C.; Dhalia, R.; França, R.F.; Marques Júnior, E.T.; Rodrigues, L.C. Initial description of the presumed congenital zika syndrome. Am. J. Public Health 2016, 106, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika virus. N. Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar] [CrossRef]
- Brasil, P.; Pereira, J.P., Jr.; Moreira, M.E.; Ribeiro Nogueira, R.M.; Damasceno, L.; Wakimoto, M.; Rabello, R.S.; Valderramos, S.G.; Halai, U.A.; Salles, T.S.; et al. Zika virus infection in pregnant women in rio de janeiro. N. Engl. J. Med. 2016, 375, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Kleber de Oliveira, W.; Cortez-Escalante, J.; De Oliveira, W.T.; do Carmo, G.M.; Henriques, C.M.; Coelho, G.E.; Araújo de França, G.V. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed zika virus transmission during the first trimester of pregnancy—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 242–247. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika virus and birth defects—Reviewing the evidence for causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef]
- Driggers, R.W.; Ho, C.Y.; Korhonen, E.M.; Kuivanen, S.; Jääskeläinen, A.J.; Smura, T.; Rosenberg, A.; Hill, D.A.; DeBiasi, R.L.; Vezina, G.; et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med. 2016, 374, 2142–2151. [Google Scholar] [CrossRef]
- Martines, R.B.; Bhatnagar, J.; Keating, M.K.; Silva-Flannery, L.; Muehlenbachs, A.; Gary, J.; Goldsmith, C.; Hale, G.; Ritter, J.; Rollin, D.; et al. Notes from the field: Evidence of zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika virus associated with microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.; Horovitz, D.D.; Cavalcanti, D.P.; Pessoa, A.; Doriqui, M.J.; Neri, J.I.; Neto, J.M.; Wanderley, H.Y.; et al. Possible association between zika virus infection and microcephaly—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef]
- Soares de Araújo, J.S.; Regis, C.T.; Gomes, R.G.; Tavares, T.R.; Rocha Dos Santos, C.; Assunção, P.M.; Nóbrega, R.V.; Pinto, D.F.; Bezerra, B.V.; Mattos, S.D. Microcephaly in north-east brazil: A retrospective study on neonates born between 2012 and 2015. Bull. World Health Organ. 2016, 94, 835–840. [Google Scholar] [CrossRef]
- Calvet, G.; Aguiar, R.S.; Melo, A.S.O.; Sampaio, S.A.; de Filippis, I.; Fabri, A.; Araujo, E.S.M.; de Sequeira, P.C.; de Mendonça, M.C.L.; de Oliveira, L.; et al. Detection and sequencing of zika virus from amniotic fluid of fetuses with microcephaly in brazil: A case study. Lancet Infect. Dis. 2016, 16, 653–660. [Google Scholar] [CrossRef]
- Dyer, O. Zika virus spreads across americas as concerns mount over birth defects. BMJ (Clin. Res. Ed.) 2015, 351, h6983. [Google Scholar] [CrossRef]
- WHO. WHO Situation Report. Zika Virus Microcephaly Guillain-Barré Syndrome. 2017. Available online: http://reliefweb.int/report/world/zika-virus-microcephaly-and-guillain-barr-syndrome-situation-report-10-march-2017 (accessed on 10 March 2017).
- Carteaux, G.; Maquart, M.; Bedet, A.; Contou, D.; Brugières, P.; Fourati, S.; Cleret de Langavant, L.; de Broucker, T.; Brun-Buisson, C.; Leparc-Goffart, I.; et al. Zika virus associated with meningoencephalitis. N. Engl. J. Med. 2016, 374, 1595–1596. [Google Scholar] [CrossRef]
- Barbi, L.; Coelho, A.V.C.; Alencar, L.C.A.; Crovella, S. Prevalence of guillain-barré syndrome among zika virus infected cases: A systematic review and meta-analysis. Braz. J. Infect. Dis. 2018, 22, 137–141. [Google Scholar] [CrossRef]
- Costello, A.; Dua, T.; Duran, P.; Gülmezoglu, M.; Oladapo, O.T.; Perea, W.; Pires, J.; Ramon-Pardo, P.; Rollins, N.; Saxena, S. Defining the syndrome associated with congenital zika virus infection. Bull. World Health Organ. 2016, 94, 406–406a. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.; Rodriguez, A.; Almiron, M.; Sanhueza, A.; Ramon, P.; de Oliveira, W.K.; Coelho, G.E.; Badaró, R.; Cortez, J.; Ospina, M.; et al. Zika virus and the guillain-barré syndrome—Case series from seven countries. N. Engl. J. Med. 2016, 375, 1598–1601. [Google Scholar] [CrossRef]
- Hoen, B.; Schaub, B.; Funk, A.L.; Ardillon, V.; Boullard, M.; Cabié, A.; Callier, C.; Carles, G.; Cassadou, S.; Césaire, R.; et al. Pregnancy outcomes after zikv infection in french territories in the americas. N. Engl. J. Med. 2018, 378, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Zika Virus Disease Epidemic: Potential Association with Microcephaly and Guillain-Barré Syndrome (First Update). Eur. Cent. Dis. Prev. ControlStockh. Swed. 2016. Available online: http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf (accessed on 21 January 2016).
- Pierson, T.C.; Diamond, M.S. The emergence of zika virus and its new clinical syndromes. Nature 2018, 560, 573–581. [Google Scholar] [CrossRef]
- Dudley, D.M.; Van Rompay, K.K.; Coffey, L.L.; Ardeshir, A.; Keesler, R.I.; Bliss-Moreau, E.; Grigsby, P.L.; Steinbach, R.J.; Hirsch, A.J.; MacAllister, R.P.; et al. Miscarriage and stillbirth following maternal zika virus infection in nonhuman primates. Nat. Med. 2018, 24, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Van der Eijk, A.A.; van Genderen, P.J.; Verdijk, R.M.; Reusken, C.B.; Mögling, R.; van Kampen, J.J.; Widagdo, W.; Aron, G.I.; GeurtsvanKessel, C.H.; Pas, S.D.; et al. Miscarriage associated with zika virus infection. N. Engl. J. Med. 2016, 375, 1002–1004. [Google Scholar] [CrossRef]
- Sarno, M.; Sacramento, G.A.; Khouri, R.; do Rosário, M.S.; Costa, F.; Archanjo, G.; Santos, L.A.; Nery, N., Jr.; Vasilakis, N.; Ko, A.I.; et al. Zika virus infection and stillbirths: A case of hydrops fetalis, hydranencephaly and fetal demise. PLoS Negl. Trop. Dis. 2016, 10, e0004517. [Google Scholar] [CrossRef]
- Schaub, B.; Monthieux, A.; Najihoullah, F.; Harte, C.; Césaire, R.; Jolivet, E.; Voluménie, J.L. Late miscarriage: Another zika concern? Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 207, 240–241. [Google Scholar] [CrossRef] [PubMed]
- Leisher, S.H.; Balalian, A.A.; Reinebrant, H.; Shiau, S.; Flenady, V.; Kuhn, L.; Morse, S.S. Systematic review: Fetal death reporting and risk in zika-affected pregnancies. Trop. Med. Int. Health 2020, 26, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, I.R.F.; Frontera, J.A.; Bispo de Filippis, A.M.; Nascimento, O. Neurologic complications associated with the zika virus in brazilian adults. JAMA Neurol. 2017, 74, 1190–1198. [Google Scholar] [CrossRef]
- Rozé, B.; Najioullah, F.; Signate, A.; Apetse, K.; Brouste, Y.; Gourgoudou, S.; Fagour, L.; Abel, S.; Hochedez, P.; Cesaire, R.; et al. Zika virus detection in cerebrospinal fluid from two patients with encephalopathy, martinique, february 2016. Eur. Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2016, 21, 30205. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.S.; Araujo, M.T.; Martins Filho, A.J.; Oliveira, C.S.; Nunes, B.T.; Cruz, A.C.; Nascimento, A.G.; Medeiros, R.C.; Caldas, C.A.; Araujo, F.C.; et al. Zika virus epidemic in brazil. I. Fatal disease in adults: Clinical and laboratorial aspects. J. Clin. Virol. 2016, 85, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Alves-Leon, S.V.; Lima, M.D.R.; Nunes, P.C.G.; Chimelli, L.M.C.; Rabelo, K.; Nogueira, R.M.R.; de Bruycker-Nogueira, F.; de Azeredo, E.L.; Bahia, P.R.; Rueda Lopes, F.C.; et al. Zika virus found in brain tissue of a multiple sclerosis patient undergoing an acute disseminated encephalomyelitis-like episode. Mult. Scler. (Houndmills Basingstoke Engl.) 2019, 25, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Parra, B.; Lizarazo, J.; Jiménez-Arango, J.A.; Zea-Vera, A.F.; González-Manrique, G.; Vargas, J.; Angarita, J.A.; Zuñiga, G.; Lopez-Gonzalez, R.; Beltran, C.L.; et al. Guillain-barré syndrome associated with zika virus infection in colombia. N. Engl. J. Med. 2016, 375, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Brito Ferreira, M.L.; Antunes de Brito, C.A.; Moreira, Á.J.P.; de Morais Machado, M.; Henriques-Souza, A.; Cordeiro, M.T.; de Azevedo Marques, E.T.; Pena, L.J. Guillain-barré syndrome, acute disseminated encephalomyelitis and encephalitis associated with zika virus infection in brazil: Detection of viral rna and isolation of virus during late infection. Am. J. Trop. Med. Hyg. 2017, 97, 1405–1409. [Google Scholar] [CrossRef]
- Muñoz, L.S.; Barreras, P.; Pardo, C.A. Zika virus-associated neurological disease in the adult: Guillain-barré syndrome, encephalitis, and myelitis. Semin. Reprod. Med. 2016, 34, 273–279. [Google Scholar] [CrossRef]
- Soares, C.N.; Brasil, P.; Carrera, R.M.; Sequeira, P.; de Filippis, A.B.; Borges, V.A.; Theophilo, F.; Ellul, M.A.; Solomon, T. Fatal encephalitis associated with zika virus infection in an adult. J. Clin. Virol. 2016, 83, 63–65. [Google Scholar] [CrossRef]
- Schwartzmann, P.V.; Ramalho, L.N.; Neder, L.; Vilar, F.C.; Ayub-Ferreira, S.M.; Romeiro, M.F.; Takayanagui, O.M.; Dos Santos, A.C.; Schmidt, A.; Figueiredo, L.T.; et al. Zika virus meningoencephalitis in an immunocompromised patient. Mayo Clin. Proc. 2017, 92, 460–466. [Google Scholar] [CrossRef]
- Mécharles, S.; Herrmann, C.; Poullain, P.; Tran, T.H.; Deschamps, N.; Mathon, G.; Landais, A.; Breurec, S.; Lannuzel, A. Acute myelitis due to zika virus infection. Lancet 2016, 387, 1481. [Google Scholar] [CrossRef]
- Niemeyer, B.; Niemeyer, R.; Borges, R.; Marchiori, E. Acute disseminated encephalomyelitis following zika virus infection. Eur. Neurol. 2017, 77, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Galliez, R.M.; Spitz, M.; Rafful, P.P.; Cagy, M.; Escosteguy, C.; Germano, C.S.; Sasse, E.; Gonçalves, A.L.; Silveira, P.P.; Pezzuto, P.; et al. Zika virus causing encephalomyelitis associated with immunoactivation. Open Forum Infect. Dis. 2016, 3, ofw203. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.T.; England, J.D.; Lorenzana, I.; Medina-Montoya, M.; Alvarado, D.; De Bastos, M.; Fontiveros, S.; Sierra, M.; Contreras, F. Zika virus associated with sensory polyneuropathy. J. Neurol. Sci. 2016, 369, 271–272. [Google Scholar] [CrossRef]
- Nicastri, E.; Castilletti, C.; Balestra, P.; Galgani, S.; Ippolito, G. Zika virus infection in the central nervous system and female genital tract. Emerg. Infect. Dis. 2016, 22, 2228–2230. [Google Scholar] [CrossRef] [PubMed]
- Bido-Medina, R.; Wirsich, J.; Rodríguez, M.; Oviedo, J.; Miches, I.; Bido, P.; Tusen, L.; Stoeter, P.; Sadaghiani, S. Impact of zika virus on adult human brain structure and functional organization. Ann. Clin. Transl. Neurol. 2018, 5, 752–762. [Google Scholar] [CrossRef]
- Musso, D.; Gubler, D.J. Zika virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef]
- Musso, D.; Baud, D.; Freedman, D.O. Should testing of donors be restricted to active zika virus areas? Lancet Infect. Dis. 2016, 16, 1108–1109. [Google Scholar] [CrossRef]
- McCarthy, M. Four in florida are infected with zika from local mosquitoes. BMJ (Clin. Res. Ed.) 2016, 354, i4235. [Google Scholar] [CrossRef]
- Wikan, N.; Smith, D.R. Zika virus: History of a newly emerging arbovirus. Lancet Infect. Dis. 2016, 16, e119–e126. [Google Scholar] [CrossRef]
- Baud, D.; Gubler, D.J.; Schaub, B.; Lanteri, M.C.; Musso, D. An update on zika virus infection. Lancet 2017, 390, 2099–2109. [Google Scholar] [CrossRef]
- Ferraris, P.; Yssel, H.; Missé, D. Zika virus infection: An update. Microbes Infect. 2019, 21, 353–360. [Google Scholar] [CrossRef]
- Fauci, A.S.; Morens, D.M. Zika virus in the americas—Yet another arbovirus threat. N. Engl. J. Med. 2016, 374, 601–604. [Google Scholar] [CrossRef]
- Meaney-Delman, D.; Hills, S.L.; Williams, C.; Galang, R.R.; Iyengar, P.; Hennenfent, A.K.; Rabe, I.B.; Panella, A.; Oduyebo, T.; Honein, M.A.; et al. Zika virus infection among u.S. Pregnant travelers—August 2015-february 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 211–214. [Google Scholar] [CrossRef]
- Chan, M. Who Director-General Briefs Executive Board on Zika Situation. 2016. Available online: http://www.who.int/dg/speeches/2016/zika-situation/en (accessed on 28 January 2016).
- Rozé, B.; Najioullah, F.; Fergé, J.L.; Apetse, K.; Brouste, Y.; Cesaire, R.; Fagour, C.; Fagour, L.; Hochedez, P.; Jeannin, S.; et al. Zika virus detection in urine from patients with guillain-barré syndrome on martinique, january 2016. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2016, 21, 30154. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, E.M.; Huhtamo, E.; Smura, T.; Kallio-Kokko, H.; Raassina, M.; Vapalahti, O. Zika virus infection in a traveller returning from the maldives, june 2015. Eur. Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2016, 21, 30107. [Google Scholar] [CrossRef]
- Hennessey, M.; Fischer, M.; Staples, J.E. Zika virus spreads to new areas—Region of the americas, may 2015-january 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Morales, A.J. Zika: The new arbovirus threat for latin america. J. Infect. Dev. Ctries. 2015, 9, 684–685. [Google Scholar] [CrossRef][Green Version]
- Martinez-Pulgarin, D.F.; Acevedo-Mendoza, W.F.; Cardona-Ospina, J.A.; Rodríguez-Morales, A.J.; Paniz-Mondolfi, A.E. A bibliometric analysis of global zika research. Travel Med. Infect. Dis. 2016, 14, 55–57. [Google Scholar] [CrossRef]
- Brown, C. Zika virus outbreaks in asia and south america. Cmaj Can. Med. Assoc. J. J. De L’association Med. Can. 2016, 188, E34. [Google Scholar] [CrossRef]
- Cauchemez, S.; Besnard, M.; Bompard, P.; Dub, T.; Guillemette-Artur, P.; Eyrolle-Guignot, D.; Salje, H.; Van Kerkhove, M.D.; Abadie, V.; Garel, C.; et al. Association between zika virus and microcephaly in french polynesia, 2013-2015: A retrospective study. Lancet 2016, 387, 2125–2132. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-barré syndrome outbreak associated with zika virus infection in french polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastere, S.; Valour, F.; Baudouin, L.; Mallet, H.; Musso, D.; Ghawche, F. Zika virus infection complicated by guillain-barre syndrome—Case report, french polynesia, december 2013. Eur. Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2014, 19, 20720. [Google Scholar] [CrossRef]
- Goncé, A.; Martínez, M.J.; Marbán-Castro, E.; Saco, A.; Soler, A.; Alvarez-Mora, M.I.; Peiro, A.; Gonzalo, V.; Hale, G.; Bhatnagar, J.; et al. Spontaneous abortion associated with zika virus infection and persistent viremia. Emerg. Infect. Dis. 2018, 24, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Marques, V.M.; Santos, C.S.; Santiago, I.G.; Marques, S.M.; Nunes Brasil, M.D.G.; Lima, T.T.; Costa, P.S. Neurological complications of congenital zika virus infection. Pediatr. Neurol. 2019, 91, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Martines, R.B.; Bhatnagar, J.; de Oliveira Ramos, A.M.; Davi, H.P.; Iglezias, S.D.; Kanamura, C.T.; Keating, M.K.; Hale, G.; Silva-Flannery, L.; Muehlenbachs, A.; et al. Pathology of congenital zika syndrome in brazil: A case series. Lancet 2016, 388, 898–904. [Google Scholar] [CrossRef]
- Marchette, N.J.; Garcia, R.; Rudnick, A. Isolation of zika virus from aedes aegypti mosquitoes in malaysia. Am. J. Trop. Med. Hyg. 1969, 18, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G.; Chang, G.J. Full-length sequencing and genomic characterization of bagaza, kedougou, and zika viruses. Arch. Virol. 2007, 152, 687–696. [Google Scholar] [CrossRef]
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017, 166, 155–163. [Google Scholar] [CrossRef]
- Diallo, D.; Sall, A.A.; Diagne, C.T.; Faye, O.; Faye, O.; Ba, Y.; Hanley, K.A.; Buenemann, M.; Weaver, S.C.; Diallo, M. Zika virus emergence in mosquitoes in southeastern senegal, 2011. PLoS ONE 2014, 9, e109442. [Google Scholar] [CrossRef]
- Faye, O.; Faye, O.; Diallo, D.; Diallo, M.; Weidmann, M.; Sall, A.A. Quantitative real-time pcr detection of zika virus and evaluation with field-caught mosquitoes. Virol. J. 2013, 10, 311. [Google Scholar] [CrossRef]
- Weinbren, M.P.; Williams, M.C. Zika virus: Further isolations in the zika area, and some studies on the strains isolated. Trans. R. Soc. Trop. Med. Hyg. 1958, 52, 263–268. [Google Scholar] [CrossRef]
- Haddow, A.J.; Williams, M.C.; Woodall, J.P.; Simpson, D.I.; Goma, L.K. Twelve isolations of zika virus from aedes (stegomyia) africanus (theobald) taken in and above a uganda forest. Bull. World Health Organ. 1964, 31, 57–69. [Google Scholar] [PubMed]
- Berthet, N.; Nakouné, E.; Kamgang, B.; Selekon, B.; Descorps-Declère, S.; Gessain, A.; Manuguerra, J.C.; Kazanji, M. Molecular characterization of three zika flaviviruses obtained from sylvatic mosquitoes in the central african republic. Vector Borne Zoonotic Dis. 2014, 14, 862–865. [Google Scholar] [CrossRef] [PubMed]
- McCrae, A.W.; Kirya, B.G. Yellow fever and zika virus epizootics and enzootics in uganda. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 552–562. [Google Scholar] [CrossRef]
- Grard, G.; Caron, M.; Mombo, I.M.; Nkoghe, D.; Mboui Ondo, S.; Jiolle, D.; Fontenille, D.; Paupy, C.; Leroy, E.M. Zika virus in gabon (central africa)--2007: A new threat from aedes albopictus? PLoS Negl. Trop. Dis. 2014, 8, e2681. [Google Scholar] [CrossRef]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Enfissi, A.; Codrington, J.; Roosblad, J.; Kazanji, M.; Rousset, D. Zika virus genome from the americas. Lancet 2016, 387, 227–228. [Google Scholar] [CrossRef]
- Haddow, A.D.; Woodall, J.P. Distinguishing between zika and spondweni viruses. Bull. World Health Organ. 2016, 94, 711. [Google Scholar] [CrossRef]
- Brès, P. Recent data from serological surveys on the prevalence of arbovirus infections in africa, with special reference to yellow fever. Bull. World Health Organ. 1970, 43, 223–267. [Google Scholar]
- Macnamara, F.N. Zika virus: A report on three cases of human infection during an epidemic of jaundice in nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954, 48, 139–145. [Google Scholar] [CrossRef]
- Olson, J.G.; Ksiazek, T.G.; Suhandiman; Triwibowo. Zika virus, a cause of fever in central java, indonesia. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 389–393. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Lambert, A.J.; Holodniy, M.; Saavedra, S.; Signor Ldel, C. Phylogeny of zika virus in western hemisphere, 2015. Emerg. Infect. Dis. 2016, 22, 933–935. [Google Scholar] [CrossRef]
- Faye, O.; Freire, C.C.; Iamarino, A.; Faye, O.; de Oliveira, J.V.; Diallo, M.; Zanotto, P.M.; Sall, A.A. Molecular evolution of zika virus during its emergence in the 20(th) century. PLoS Negl. Trop. Dis. 2014, 8, e2636. [Google Scholar] [CrossRef]
- Collins, N.D.; Widen, S.G.; Li, L.; Swetnam, D.M.; Shi, P.Y.; Tesh, R.B.; Sarathy, V.V. Inter- and intra-lineage genetic diversity of wild-type zika viruses reveals both common and distinctive nucleotide variants and clusters of genomic diversity. Emerg. Microbes Infect. 2019, 8, 1126–1138. [Google Scholar] [CrossRef]
- Ye, Q.; Liu, Z.Y.; Han, J.F.; Jiang, T.; Li, X.F.; Qin, C.F. Genomic characterization and phylogenetic analysis of zika virus circulating in the americas. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2016, 43, 43–49. [Google Scholar] [CrossRef]
- Pettersson, J.H.; Eldholm, V.; Seligman, S.J.; Lundkvist, Å.; Falconar, A.K.; Gaunt, M.W.; Musso, D.; Nougairède, A.; Charrel, R.; Gould, E.A.; et al. How did zika virus emerge in the pacific islands and latin america? mBio 2016, 7. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. The antigenic structure of zika virus and its relation to other flaviviruses: Implications for infection and immunoprophylaxis. Microbiol. Mol. Biol. Rev. Mmbr 2017, 81, e00055-16. [Google Scholar] [CrossRef]
- Gatherer, D.; Kohl, A. Zika virus: A previously slow pandemic spreads rapidly through the americas. J. Gen. Virol. 2016, 97, 269–273. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on yap island, federated states of micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Musso, D.; Nilles, E.J.; Cao-Lormeau, V.M. Rapid spread of emerging zika virus in the pacific area. Clin. Microbiol. Infect. 2014, 20, O595–O596. [Google Scholar] [CrossRef]
- Brito, C.A.; Cordeiro, M.T. One year after the zika virus outbreak in brazil: From hypotheses to evidence. Rev. Da Soc. Bras. De Med. Trop. 2016, 49, 537–543. [Google Scholar] [CrossRef]
- Brasil, P.; Calvet, G.A.; Siqueira, A.M.; Wakimoto, M.; de Sequeira, P.C.; Nobre, A.; Quintana Mde, S.; Mendonça, M.C.; Lupi, O.; de Souza, R.V.; et al. Zika virus outbreak in rio de janeiro, brazil: Clinical characterization, epidemiological and virological aspects. PLoS Negl. Trop. Dis. 2016, 10, e0004636. [Google Scholar] [CrossRef]
- Ikejezie, J.; Shapiro, C.N.; Kim, J.; Chiu, M.; Almiron, M.; Ugarte, C.; Espinal, M.A.; Aldighieri, S. Zika virus transmission—Region of the americas, 15 may 2015–15 december 2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 329–334. [Google Scholar] [CrossRef]
- White, M.K.; Wollebo, H.S.; David Beckham, J.; Tyler, K.L.; Khalili, K. Zika virus: An emergent neuropathological agent. Ann. Neurol. 2016, 80, 479–489. [Google Scholar] [CrossRef]
- Haddow, A.D.; Nasar, F.; Guzman, H.; Ponlawat, A.; Jarman, R.G.; Tesh, R.B.; Weaver, S.C. Genetic characterization of spondweni and zika viruses and susceptibility of geographically distinct strains of aedes aegypti, aedes albopictus and culex quinquefasciatus (diptera: Culicidae) to spondweni virus. PLoS Negl. Trop. Dis. 2016, 10, e0005083. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.; Calvez, E.; Chouin-Carneiro, T.; Diallo, D.; Failloux, A.B. An overview of mosquito vectors of zika virus. Microbes Infect. 2018, 20, 646–660. [Google Scholar] [CrossRef]
- Weaver, S.C.; Barrett, A.D. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2004, 2, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Dengue, urbanization and globalization: The unholy trinity of the 21(st) century. Trop. Med. Health 2011, 39, 3–11. [Google Scholar] [CrossRef]
- Imperato, P.J. The convergence of a virus, mosquitoes, and human travel in globalizing the zika epidemic. J. Community Health 2016, 41, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Roche, C.; Teissier, A.; Robin, E.; Berry, A.L.; Mallet, H.P.; Sall, A.A.; Musso, D. Zika virus, french polynesia, south pacific, 2013. Emerg. Infect. Dis. 2014, 20, 1085–1086. [Google Scholar] [CrossRef]
- Musso, D. Zika virus transmission from french polynesia to brazil. Emerg. Infect. Dis. 2015, 21, 1887. [Google Scholar] [CrossRef]
- Zanluca, C.; Melo, V.C.; Mosimann, A.L.; Santos, G.I.; Santos, C.N.; Luz, K. First report of autochthonous transmission of zika virus in brazil. Mem. Do Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika virus outbreak, bahia, brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef] [PubMed]
- Paixão, E.S.; Teixeira, M.G.; Rodrigues, L.C. Zika, chikungunya and dengue: The causes and threats of new and re-emerging arboviral diseases. BMJ Glob. Health 2018, 3, e000530. [Google Scholar] [CrossRef] [PubMed]
- Eligio-García, L.; Crisóstomo-Vázquez, M.D.P.; Caballero-García, M.L.; Soria-Guerrero, M.; Méndez-Galván, J.F.; López-Cancino, S.A.; Jiménez-Cardoso, E. Co-infection of dengue, zika and chikungunya in a group of pregnant women from tuxtla gutiérrez, chiapas: Preliminary data. 2019. PLoS Negl. Trop. Dis. 2020, 14, e0008880. [Google Scholar] [CrossRef] [PubMed]
- Villamil-Gómez, W.E.; González-Camargo, O.; Rodriguez-Ayubi, J.; Zapata-Serpa, D.; Rodriguez-Morales, A.J. Dengue, chikungunya and zika co-infection in a patient from colombia. J. Infect. Public Health 2016, 9, 684–686. [Google Scholar] [CrossRef]
- Gautret, P.; Simon, F. Dengue, chikungunya and zika and mass gatherings: What happened in brazil, 2014. Travel Med. Infect. Dis. 2016, 14, 7–8. [Google Scholar] [CrossRef]
- Khandia, R.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Malik, Y.S.; Singh, R.K.; Chaicumpa, W. Modulation of dengue/zika virus pathogenicity by antibody-dependent enhancement and strategies to protect against enhancement in zika virus infection. Front. Immunol. 2018, 9, 597. [Google Scholar] [CrossRef]
- Willis, E.; Hensley, S.E. Characterization of zika virus binding and enhancement potential of a large panel of flavivirus murine monoclonal antibodies. Virology 2017, 508, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, K.; Göhner, C.; Kopp, A.; Schmidt, A.; Merz, W.M.; Markert, U.R.; Junglen, S.; Drosten, C. Zika virus infection in human placental tissue explants is enhanced in the presence of dengue virus antibodies in-vitro. Emerg. Microbes Infect. 2018, 7, 198. [Google Scholar] [CrossRef]
- Bardina, S.V.; Bunduc, P.; Tripathi, S.; Duehr, J.; Frere, J.J.; Brown, J.A.; Nachbagauer, R.; Foster, G.A.; Krysztof, D.; Tortorella, D.; et al. Enhancement of zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017, 356, 175–180. [Google Scholar] [CrossRef]
- Musso, D.; Despres, P. Serological diagnosis of flavivirus-associated human infections. Diagnostics 2020, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.S.; St John, A.L. Cross-reactive immunity among flaviviruses. Front. Immunol. 2020, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Rabe, I.B.; Staples, J.E.; Villanueva, J.; Hummel, K.B.; Johnson, J.A.; Rose, L.; Hills, S.; Wasley, A.; Fischer, M.; Powers, A.M. Interim guidance for interpretation of zika virus antibody test results. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 543–546. [Google Scholar] [CrossRef]
- Terzian, A.C.B.; Schanoski, A.S.; Mota, M.T.O.; da Silva, R.A.; Estofolete, C.F.; Colombo, T.E.; Rahal, P.; Hanley, K.A.; Vasilakis, N.; Kalil, J.; et al. Viral load and cytokine response profile does not support antibody-dependent enhancement in dengue-primed zika virus-infected patients. Clin. Infect. Dis. 2017, 65, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Rossati, A. Global warming and its health impact. Int. J. Occup. Environ. Med. 2017, 8, 7–20. [Google Scholar] [CrossRef]
- Bogoch, I.I.; Brady, O.J.; Kraemer, M.U.G.; German, M.; Creatore, M.I.; Kulkarni, M.A.; Brownstein, J.S.; Mekaru, S.R.; Hay, S.I.; Groot, E.; et al. Anticipating the international spread of zika virus from brazil. Lancet 2016, 387, 335–336. [Google Scholar] [CrossRef]
- Ali, S.; Gugliemini, O.; Harber, S.; Harrison, A.; Houle, L.; Ivory, J.; Kersten, S.; Khan, R.; Kim, J.; LeBoa, C.; et al. Environmental and social change drive the explosive emergence of zika virus in the americas. PLoS Negl. Trop. Dis. 2017, 11, e0005135. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Xie, X.; Shi, P.Y. Zika virus vaccine: Progress and challenges. Cell Host Microbe 2018, 24, 12–17. [Google Scholar] [CrossRef]
- Pierson, T.C.; Graham, B.S. Zika virus: Immunity and vaccine development. Cell 2016, 167, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Thomas, S.J.; Michael, N.L. Prospects for a zika virus vaccine. Immunity 2017, 46, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Yip, B.S.; Huang, L.M.; Wu, S.C. Zika virus structural biology and progress in vaccine development. Biotechnol. Adv. 2018, 36, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Morabito, K.M.; Graham, B.S. Zika virus vaccine development. J. Infect. Dis. 2017, 216, S957–S963. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Guida, J.P.; Costa Do Nascimento, M.L.; Mysorekar, I.U. Host and viral mechanisms of congenital zika syndrome. Virulence 2019, 10, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Nhan, T.; Robin, E.; Roche, C.; Bierlaire, D.; Zisou, K.; Shan Yan, A.; Cao-Lormeau, V.M.; Broult, J. Potential for zika virus transmission through blood transfusion demonstrated during an outbreak in french polynesia, november 2013 to february 2014. Eur. Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2014, 19, 20761. [Google Scholar] [CrossRef] [PubMed]
- Wiwanitkit, S.; Wiwanitkit, V. Afebrile, asymptomatic and non-thrombocytopenic zika virus infection: Don’t miss it! Asian Pac. J. Trop. Med. 2016, 9, 513. [Google Scholar] [CrossRef]
- Wikan, N.; Suputtamongkol, Y.; Yoksan, S.; Smith, D.R.; Auewarakul, P. Immunological evidence of zika virus transmission in thailand. Asian Pac. J. Trop. Med. 2016, 9, 141–144. [Google Scholar] [CrossRef]
- Oduyebo, T.; Petersen, E.E.; Rasmussen, S.A.; Mead, P.S.; Meaney-Delman, D.; Renquist, C.M.; Ellington, S.R.; Fischer, M.; Staples, J.E.; Powers, A.M.; et al. Update: Interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible zika virus exposure—United states, 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Haby, M.M.; Pinart, M.; Elias, V.; Reveiz, L. Prevalence of asymptomatic zika virus infection: A systematic review. Bull. World Health Organ. 2018, 96, 402–413. [Google Scholar] [CrossRef]
- Grossi-Soyster, E.N.; LaBeaud, A.D. Clinical aspects of zika virus. Curr. Opin. Pediatr. 2017, 29, 102–106. [Google Scholar] [CrossRef]
- Plourde, A.R.; Bloch, E.M. A literature review of zika virus. Emerg. Infect. Dis. 2016, 22, 1185–1192. [Google Scholar] [CrossRef]
- Mercado-Reyes, M.; Acosta-Reyes, J.; Navarro-Lechuga, E.; Corchuelo, S.; Rico, A.; Parra, E.; Tolosa, N.; Pardo, L.; González, M.; Martìn-Rodriguez-Hernández, J.; et al. Dengue, chikungunya and zika virus coinfection: Results of the national surveillance during the zika epidemic in colombia. Epidemiol. Infect. 2019, 147, e77. [Google Scholar] [CrossRef]
- Chaves, B.A.; Orfano, A.S.; Nogueira, P.M.; Rodrigues, N.B.; Campolina, T.B.; Nacif-Pimenta, R.; Pires, A.; Júnior, A.B.V.; Paz, A.D.C.; Vaz, E.; et al. Coinfection with zika virus (zikv) and dengue virus results in preferential zikv transmission by vector bite to vertebrate host. J. Infect. Dis. 2018, 218, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.; Guimarães, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The brazilian zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef]
- Halai, U.A.; Nielsen-Saines, K.; Moreira, M.L.; de Sequeira, P.C.; Junior, J.P.P.; de Araujo Zin, A.; Cherry, J.; Gabaglia, C.R.; Gaw, S.L.; Adachi, K.; et al. Maternal zika virus disease severity, virus load, prior dengue antibodies, and their relationship to birth outcomes. Clin. Infect. Dis. 2017, 65, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Herrlinger, S.; Zhu, Y.N.; Yang, M.; Goodfellow, F.; Stice, S.L.; Qi, X.P.; Brindley, M.A.; Chen, J.F. The african zika virus mr-766 is more virulent and causes more severe brain damage than current asian lineage and dengue virus. Development 2017, 144, 4114–4124. [Google Scholar] [CrossRef]
- Esser-Nobis, K.; Aarreberg, L.D.; Roby, J.A.; Fairgrieve, M.R.; Green, R.; Gale, M., Jr. Comparative analysis of african and asian lineage-derived zika virus strains reveals differences in activation of and sensitivity to antiviral innate immunity. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Dowall, S.D.; Graham, V.A.; Hewson, R. Lineage-dependent differences of zika virus infection in a susceptible mouse model are associated with different profiles of cytokines, chemokines, growth factors and acute phase proteins. Cytokine 2020, 125, 154864. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Vergara, P.A.; Trujillo-Correa, A.; Gómez-Suárez, L.A.; Ricardo-Caldera, D.; Soto-De León, S.C.; Brango, H.; Tovar Acero, C. Denv and zikv detection in patients with acute febrile syndrome in córdoba, colombia. Int. J. Infect. Dis. 2020, 99, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, M.; Manet, C.; Montagutelli, X. Host genetic susceptibility to viral infections: The role of type i interferon induction. Genes Immun. 2020, 21, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Sultan, N.; Bukhari, S.A.; Ali, I.; Asif, M.; Umar, Z.; Akash, M.S.H. Zika virus: A critical analysis and pharmaceutical perspectives. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 357–371. [Google Scholar] [CrossRef]
- Osuna, C.E.; Lim, S.Y.; Deleage, C.; Griffin, B.D.; Stein, D.; Schroeder, L.T.; Omange, R.W.; Best, K.; Luo, M.; Hraber, P.T.; et al. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat. Med. 2016, 22, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, M.C.; Ribeiro, I.P.; Lima, N.S.; Dos Santos, A.A.; Menezes, L.S.; da Cruz, S.O.; de Mello, I.S.; Furtado, N.D.; de Moura, E.E.; Damasceno, L.; et al. Isolation of infective zika virus from urine and saliva of patients in brazil. PLoS Negl. Trop. Dis. 2016, 10, e0004816. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, J.R.; Sotelo, A.B.; Sotelo, F.J.B.; Doi, A.M.; Pinho, J.R.R.; Oliveira, R.C.; Bezerra, A.; Deutsch, A.D.; Villas-Boas, L.S.; Felix, A.C.; et al. Persistence of zika virus in breast milk after infection in late stage of pregnancy. Emerg. Infect. Dis. 2017, 23, 856–857. [Google Scholar] [CrossRef]
- Aid, M.; Abbink, P.; Larocca, R.A.; Boyd, M.; Nityanandam, R.; Nanayakkara, O.; Martinot, A.J.; Moseley, E.T.; Blass, E.; Borducchi, E.N.; et al. Zika virus persistence in the central nervous system and lymph nodes of rhesus monkeys. Cell 2017, 169, 610–620.e614. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Armstrong, N.; Obwolo, L.A.; Thomas, M.; Pang, X.; Jones, K.S.; Tang, Q. Determination of the cell permissiveness spectrum, mode of rna replication, and rna-protein interaction of zika virus. BMC Infect. Dis. 2017, 17, 239. [Google Scholar] [CrossRef]
- Retallack, H.; Di Lullo, E.; Arias, C.; Knopp, K.A.; Laurie, M.T.; Sandoval-Espinosa, C.; Mancia Leon, W.R.; Krencik, R.; Ullian, E.M.; Spatazza, J.; et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc. Natl. Acad. Sci. USA 2016, 113, 14408–14413. [Google Scholar] [CrossRef] [PubMed]
- Meertens, L.; Labeau, A.; Dejarnac, O.; Cipriani, S.; Sinigaglia, L.; Bonnet-Madin, L.; Le Charpentier, T.; Hafirassou, M.L.; Zamborlini, A.; Cao-Lormeau, V.M.; et al. Axl mediates zika virus entry in human glial cells and modulates innate immune responses. Cell Rep. 2017, 18, 324–333. [Google Scholar] [CrossRef]
- Aagaard, K.M.; Lahon, A.; Suter, M.A.; Arya, R.P.; Seferovic, M.D.; Vogt, M.B.; Hu, M.; Stossi, F.; Mancini, M.A.; Harris, R.A.; et al. Primary human placental trophoblasts are permissive for zika virus (zikv) replication. Sci. Rep. 2017, 7, 41389. [Google Scholar] [CrossRef]
- Tabata, T.; Petitt, M.; Puerta-Guardo, H.; Michlmayr, D.; Wang, C.; Fang-Hoover, J.; Harris, E.; Pereira, L. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 2016, 20, 155–166. [Google Scholar] [CrossRef]
- Chen, J.C.; Wang, Z.; Huang, H.; Weitz, S.H.; Wang, A.; Qiu, X.; Baumeister, M.A.; Uzgiris, A. Infection of human uterine fibroblasts by zika virus in vitro: Implications for viral transmission in women. Int. J. Infect. Dis. 2016, 51, 139–140. [Google Scholar] [CrossRef]
- Miner, J.J.; Diamond, M.S. Zika virus pathogenesis and tissue tropism. Cell Host Microbe 2017, 21, 134–142. [Google Scholar] [CrossRef]
- Ngono, A.E.; Shresta, S. Immune response to dengue and zika. Annu. Rev. Immunol. 2018, 36, 279–308. [Google Scholar] [CrossRef] [PubMed]
- Wiwanitkit, V. Eye problem in zika virus infection. Eur. J. Intern. Med. 2018, 47, e17. [Google Scholar] [CrossRef]
- Carson, M.J.; Doose, J.M.; Melchior, B.; Schmid, C.D.; Ploix, C.C. Cns immune privilege: Hiding in plain sight. Immunol. Rev. 2006, 213, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.B.; Jungmann, P.; Beltrão-Braga, P.C.B. Zika infection and the development of neurological defects. Cell. Microbiol. 2017, 19, e12744. [Google Scholar] [CrossRef]
- Serman, T.M.; Gack, M.U. Evasion of innate and intrinsic antiviral pathways by the zika virus. Viruses 2019, 11, 970. [Google Scholar] [CrossRef]
- Tonnerre, P.; Melgaço, J.G.; Torres-Cornejo, A.; Pinto, M.A.; Yue, C.; Blümel, J.; de Sousa, P.S.F.; de Mello, V.D.M.; Moran, J.; de Filippis, A.M.B.; et al. Evolution of the innate and adaptive immune response in women with acute zika virus infection. Nat. Microbiol. 2020, 5, 76–83. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Meylan, E.; Tschopp, J.; Karin, M. Intracellular pattern recognition receptors in the host response. Nature 2006, 442, 39–44. [Google Scholar] [CrossRef]
- Suthar, M.S.; Aguirre, S.; Fernandez-Sesma, A. Innate immune sensing of flaviviruses. PLoS Pathog. 2013, 9, e1003541. [Google Scholar] [CrossRef]
- Sirohi, D.; Kuhn, R.J. Zika virus structure, maturation, and receptors. J. Infect. Dis. 2017, 216, S935–s944. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.P.; Moraes, A.H. Zika virus proteins at an atomic scale: How does structural biology help us to understand and develop vaccines and drugs against zika virus infection? J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190013. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.L.; Jones, C.T.; Rice, C.M. Architects of assembly: Roles of flaviviridae non-structural proteins in virion morphogenesis. Nat. Rev. Microbiol. 2008, 6, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Agrelli, A.; de Moura, R.R.; Crovella, S.; Brandão, L.A.C. Zika virus entry mechanisms in human cells. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2019, 69, 22–29. [Google Scholar] [CrossRef]
- Best, S.M. The many faces of the flavivirus ns5 protein in antagonism of type i interferon signaling. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Rastogi, M.; Sharma, N.; Singh, S.K. Flavivirus ns1: A multifaceted enigmatic viral protein. Virol. J. 2016, 13, 131. [Google Scholar] [CrossRef]
- Nunes, B.T.D.; Fontes-Garfias, C.R.; Shan, C.; Muruato, A.E.; Nunes, J.G.C.; Burbano, R.M.R.; Vasconcelos, P.F.C.; Shi, P.Y.; Medeiros, D.B.A. Zika structural genes determine the virulence of african and asian lineages. Emerg. Microbes Infect. 2020, 9, 1023–1033. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, M.; Chi, X.; Liu, X.; Zhou, J.; Lin, T.; Yang, W. High-throughput screening identifies mixed-lineage kinase 3 as a key host regulatory factor in zika virus infection. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Shan, C.; Xie, X.; Muruato, A.E.; Rossi, S.L.; Roundy, C.M.; Azar, S.R.; Yang, Y.; Tesh, R.B.; Bourne, N.; Barrett, A.D.; et al. An infectious cdna clone of zika virus to study viral virulence, mosquito transmission, and antiviral inhibitors. Cell Host Microbe 2016, 19, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liao, H.M.; Li, B.; Tsai, S.; Hung, G.C.; Lo, S.C. Comparative genomics, infectivity and cytopathogenicity of american isolates of zika virus that developed persistent infections in human embryonic kidney (hek293) cells. Int. J. Mol. Sci. 2019, 20, 3035. [Google Scholar] [CrossRef]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of mda5 and rig-i helicases in the recognition of rna viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of zika virus infection in human skin cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef] [PubMed]
- Hiscott, J.; Grandvaux, N.; Sharma, S.; Tenoever, B.R.; Servant, M.J.; Lin, R. Convergence of the nf-kappab and interferon signaling pathways in the regulation of antiviral defense and apoptosis. Ann. N. Y. Acad. Sci. 2003, 1010, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef]
- Chevaliez, S.; Pawlotsky, J.M. Interferons and their use in persistent viral infections. Handb. Exp. Pharmacol. 2009, 189, 203–241. [Google Scholar]
- De Veer, M.J.; Holko, M.; Frevel, M.; Walker, E.; Der, S.; Paranjape, J.M.; Silverman, R.H.; Williams, B.R. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 2001, 69, 912–920. [Google Scholar]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type i interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, S.; Farr, D.; Kumar, A. Interferon-stimulated gene 15 (isg15) restricts zika virus replication in primary human corneal epithelial cells. Ocul. Surf. 2019, 17, 551–559. [Google Scholar] [CrossRef]
- Dukhovny, A.; Lamkiewicz, K.; Chen, Q.; Fricke, M.; Jabrane-Ferrat, N.; Marz, M.; Jung, J.U.; Sklan, E.H. A crispr activation screen identifies genes that protect against zika virus infection. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Wichit, S.; Hamel, R.; Yainoy, S.; Gumpangseth, N.; Panich, S.; Phuadraksa, T.; Saetear, P.; Monteil, A.; Morales Vargas, R.; Missé, D. Interferon-inducible protein (ifi) 16 regulates chikungunya and zika virus infection in human skin fibroblasts. EXCLI J. 2019, 18, 467–476. [Google Scholar] [PubMed]
- Zhang, D.; Zhang, D.E. Interferon-stimulated gene 15 and the protein isgylation system. J. Interferon Cytokine Res. 2011, 31, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, K.; Li, S.; Yang, C.; Chen, L. Interferon stimulated gene 15 promotes zika virus replication through regulating jak/stat and isgylation pathways. Virus Res. 2020, 287, 198087. [Google Scholar] [CrossRef]
- Ojha, C.R.; Rodriguez, M.; Karuppan, M.K.M.; Lapierre, J.; Kashanchi, F.; El-Hage, N. Toll-like receptor 3 regulates zika virus infection and associated host inflammatory response in primary human astrocytes. PLoS ONE 2019, 14, e0208543. [Google Scholar] [CrossRef] [PubMed]
- Vanwalscappel, B.; Tada, T.; Landau, N.R. Toll-like receptor agonist r848 blocks zika virus replication by inducing the antiviral protein viperin. Virology 2018, 522, 199–208. [Google Scholar] [CrossRef]
- Khan, S.; Lew, I.; Wu, F.; Fritts, L.; Fontaine, K.A.; Tomar, S.; Trapecar, M.; Shehata, H.M.; Ott, M.; Miller, C.J.; et al. Low expression of rna sensors impacts zika virus infection in the lower female reproductive tract. Nat. Commun. 2019, 10, 4344. [Google Scholar] [CrossRef] [PubMed]
- Ledur, P.F.; Karmirian, K.; Pedrosa, C.; Souza, L.R.Q.; Assis-de-Lemos, G.; Martins, T.M.; Ferreira, J.; de Azevedo Reis, G.F.; Silva, E.S.; Silva, D.; et al. Zika virus infection leads to mitochondrial failure, oxidative stress and DNA damage in human ipsc-derived astrocytes. Sci. Rep. 2020, 10, 1218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, Q.; Wu, Y.; Ma, L.; Zhang, Z.; Liu, T.; Jin, S.; She, Y.; Li, Y.P.; Cui, J. Zika virus elicits inflammation to evade antiviral response by cleaving cgas via ns1-caspase-1 axis. Embo J. 2018, 37, e99347. [Google Scholar] [CrossRef]
- Coldbeck-Shackley, R.C.; Eyre, N.S.; Beard, M.R. The molecular interactions of zikv and denv with the type-i ifn response. Vaccines 2020, 8, 530. [Google Scholar] [CrossRef]
- Bowen, J.R.; Zimmerman, M.G.; Suthar, M.S. Taking the defensive: Immune control of zika virus infection. Virus Res. 2018, 254, 21–26. [Google Scholar] [CrossRef] [PubMed]
- De Weerd, N.A.; Nguyen, T. The interferons and their receptors--distribution and regulation. Immunol. Cell Biol. 2012, 90, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type i interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Stark, G.R.; Darnell, J.E., Jr. The jak-stat pathway at twenty. Immunity 2012, 36, 503–514. [Google Scholar] [CrossRef]
- MacMicking, J.D. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol. 2012, 12, 367–382. [Google Scholar] [CrossRef]
- Schoggins, J.W.; Wilson, S.J.; Panis, M.; Murphy, M.Y.; Jones, C.T.; Bieniasz, P.; Rice, C.M. A diverse range of gene products are effectors of the type i interferon antiviral response. Nature 2011, 472, 481–485. [Google Scholar] [CrossRef]
- Rusinova, I.; Forster, S.; Yu, S.; Kannan, A.; Masse, M.; Cumming, H.; Chapman, R.; Hertzog, P.J. Interferome v2.0: An updated database of annotated interferon-regulated genes. Nucleic Acids Res. 2013, 41, D1040–D1046. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A mouse model of zika virus pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef]
- Frumence, E.; Roche, M.; Krejbich-Trotot, P.; El-Kalamouni, C.; Nativel, B.; Rondeau, P.; Missé, D.; Gadea, G.; Viranaicken, W.; Desprès, P. The south pacific epidemic strain of zika virus replicates efficiently in human epithelial a549 cells leading to ifn-β production and apoptosis induction. Virology 2016, 493, 217–226. [Google Scholar] [CrossRef]
- Bayer, A.; Lennemann, N.J.; Ouyang, Y.; Bramley, J.C.; Morosky, S.; Marques, E.T., Jr.; Cherry, S.; Sadovsky, Y.; Coyne, C.B. Type iii interferons produced by human placental trophoblasts confer protection against zika virus infection. Cell Host Microbe 2016, 19, 705–712. [Google Scholar] [CrossRef]
- Savidis, G.; Perreira, J.M.; Portmann, J.M.; Meraner, P.; Guo, Z.; Green, S.; Brass, A.L. The ifitms inhibit zika virus replication. Cell Rep. 2016, 15, 2323–2330. [Google Scholar] [CrossRef]
- Goubau, D.; Deddouche, S.; Reis e Sousa, C. Cytosolic sensing of viruses. Immunity 2013, 38, 855–869. [Google Scholar] [CrossRef]
- Bowen, J.R.; Quicke, K.M.; Maddur, M.S.; O’Neal, J.T.; McDonald, C.E.; Fedorova, N.B.; Puri, V.; Shabman, R.S.; Pulendran, B.; Suthar, M.S. Zika virus antagonizes type i interferon responses during infection of human dendritic cells. PLoS Pathog. 2017, 13, e1006164. [Google Scholar] [CrossRef]
- Marzi, A.; Emanuel, J.; Callison, J.; McNally, K.L.; Arndt, N.; Chadinha, S.; Martellaro, C.; Rosenke, R.; Scott, D.P.; Safronetz, D.; et al. Lethal zika virus disease models in young and older interferon α/β receptor knock out mice. Front. Cell. Infect. Microbiol. 2018, 8, 117. [Google Scholar] [CrossRef]
- Metz, S.W.; Thomas, A.; Brackbill, A.; Forsberg, J.; Miley, M.J.; Lopez, C.A.; Lazear, H.M.; Tian, S.; de Silva, A.M. Oligomeric state of the zikv e protein defines protective immune responses. Nat. Commun. 2019, 10, 4606. [Google Scholar] [CrossRef]
- Kim, S.Y.; Zhao, J.; Liu, X.; Fraser, K.; Lin, L.; Zhang, X.; Zhang, F.; Dordick, J.S.; Linhardt, R.J. Interaction of zika virus envelope protein with glycosaminoglycans. Biochemistry 2017, 56, 1151–1162. [Google Scholar] [CrossRef]
- Strange, D.P.; Jiyarom, B.; Pourhabibi Zarandi, N.; Xie, X.; Baker, C.; Sadri-Ardekani, H.; Shi, P.Y.; Verma, S. Axl promotes zika virus entry and modulates the antiviral state of human sertoli cells. mBio 2019, 10. [Google Scholar] [CrossRef]
- Di Scipio, R.G.; Hermodson, M.A.; Yates, S.G.; Davie, E.W. A comparison of human prothrombin, factor ix (christmas factor), factor x (stuart factor), and protein s. Biochemistry 1977, 16, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Stitt, T.N.; Conn, G.; Gore, M.; Lai, C.; Bruno, J.; Radziejewski, C.; Mattsson, K.; Fisher, J.; Gies, D.R.; Jones, P.F.; et al. The anticoagulation factor protein s and its relative, gas6, are ligands for the tyro 3/axl family of receptor tyrosine kinases. Cell 1995, 80, 661–670. [Google Scholar] [CrossRef]
- Amara, A.; Mercer, J. Viral apoptotic mimicry. Nat. Rev. Microbiol. 2015, 13, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.S.; Shim, B.S.; Kwon, Y.C.; Zhang, R.; Otsuka, Y.; Schmitt, K.; Berri, F.; Diamond, M.S.; Choe, H. Axl-dependent infection of human fetal endothelial cells distinguishes zika virus from other pathogenic flaviviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 2024–2029. [Google Scholar] [CrossRef] [PubMed]
- Lemke, G. Biology of the tam receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a009076. [Google Scholar] [CrossRef]
- In, H.J.; Lee, Y.H.; Jang, S.; Lim, H.J.; Kim, M.Y.; Kim, J.A.; Yoo, J.S.; Chung, G.T.; Kim, Y.J. Enhanced effect of modified zika virus e antigen on the immunogenicity of DNA vaccine. Virology 2020, 549, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Larocca, R.A.; Abbink, P.; Peron, J.P.; Zanotto, P.M.; Iampietro, M.J.; Badamchi-Zadeh, A.; Boyd, M.; Ng’ang’a, D.; Kirilova, M.; Nityanandam, R.; et al. Vaccine protection against zika virus from brazil. Nature 2016, 536, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Larocca, R.A.; De La Barrera, R.A.; Bricault, C.A.; Moseley, E.T.; Boyd, M.; Kirilova, M.; Li, Z.; Ng’ang’a, D.; Nanayakkara, O.; et al. Protective efficacy of multiple vaccine platforms against zika virus challenge in rhesus monkeys. Science 2016, 353, 1129–1132. [Google Scholar] [CrossRef]
- López-Camacho, C.; Abbink, P.; Larocca, R.A.; Dejnirattisai, W.; Boyd, M.; Badamchi-Zadeh, A.; Wallace, Z.R.; Doig, J.; Velazquez, R.S.; Neto, R.D.L.; et al. Rational zika vaccine design via the modulation of antigen membrane anchors in chimpanzee adenoviral vectors. Nat. Commun. 2018, 9, 2441. [Google Scholar] [CrossRef] [PubMed]
- Sager, G.; Gabaglio, S.; Sztul, E.; Belov, G.A. Role of host cell secretory machinery in zika virus life cycle. Viruses 2018, 10, 559. [Google Scholar] [CrossRef]
- Xia, H.; Luo, H.; Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Medeiros, D.B.A.; Zou, J.; Xie, X.; Giraldo, M.I.; Vasconcelos, P.F.C.; et al. An evolutionary ns1 mutation enhances zika virus evasion of host interferon induction. Nat. Commun. 2018, 9, 414. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Zhou, J.; Xie, W.; Chen, C.; Wang, Z.; Yang, H.; Cui, J. Zika virus evades interferon-mediated antiviral response through the co-operation of multiple nonstructural proteins in vitro. Cell Discov. 2017, 3, 17006. [Google Scholar] [CrossRef]
- Ngueyen, T.T.N.; Kim, S.J.; Lee, J.Y.; Myoung, J. Zika virus proteins ns2a and ns4a are major antagonists that reduce ifn-β promoter activity induced by the mda5/rig-i signaling pathway. J. Microbiol. Biotechnol. 2019, 29, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.M.; Gale, M., Jr. Immune signaling by rig-i-like receptors. Immunity 2011, 34, 680–692. [Google Scholar] [CrossRef]
- Ding, Q.; Gaska, J.M.; Douam, F.; Wei, L.; Kim, D.; Balev, M.; Heller, B.; Ploss, A. Species-specific disruption of sting-dependent antiviral cellular defenses by the zika virus ns2b3 protease. Proc. Natl. Acad. Sci. USA 2018, 115, E6310–e6318. [Google Scholar] [CrossRef] [PubMed]
- Riedl, W.; Acharya, D.; Lee, J.H.; Liu, G.; Serman, T.; Chiang, C.; Chan, Y.K.; Diamond, M.S.; Gack, M.U. Zika virus ns3 mimics a cellular 14-3-3-binding motif to antagonize rig-i- and mda5-mediated innate immunity. Cell Host Microbe 2019, 26, 493–503.e496. [Google Scholar] [CrossRef]
- Lin, J.P.; Fan, Y.K.; Liu, H.M. The 14-3-3η chaperone protein promotes antiviral innate immunity via facilitating mda5 oligomerization and intracellular redistribution. PLoS Pathog. 2019, 15, e1007582. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Loo, Y.M.; Horner, S.M.; Zornetzer, G.A.; Katze, M.G.; Gale, M., Jr. The mitochondrial targeting chaperone 14-3-3ε regulates a rig-i translocon that mediates membrane association and innate antiviral immunity. Cell Host Microbe 2012, 11, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Salazar, M.; Gutiérrez-Escolano, A.L.; Reyes-Ruiz, J.M.; del Angel, R.M. The nonstructural proteins 3 and 5 from flavivirus modulate nuclear-cytoplasmic transport and innate immune response targeting nuclear proteins. bioRxiv 2018, 375899. [Google Scholar] [CrossRef]
- Ma, J.; Ketkar, H.; Geng, T.; Lo, E.; Wang, L.; Xi, J.; Sun, Q.; Zhu, Z.; Cui, Y.; Yang, L.; et al. Zika virus non-structural protein 4a blocks the rlr-mavs signaling. Front. Microbiol. 2018, 9, 1350. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hou, S.; Airo, A.M.; Limonta, D.; Mancinelli, V.; Branton, W.; Power, C.; Hobman, T.C. Zika virus inhibits type-i interferon production and downstream signaling. Embo Rep. 2016, 17, 1766–1775. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, X.; He, Z.; Wu, Y.; Zhang, S.; Lin, J.; Yang, Y.; Chen, J.; An, S.; Yin, Y.; et al. Zika virus antagonizes interferon response in patients and disrupts rig-i-mavs interaction through its card-tm domains. Cell Biosci. 2019, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chan, J.F.; Tee, K.M.; Choi, G.K.; Lau, S.K.; Woo, P.C.; Tse, H.; Yuen, K.Y. Comparative genomic analysis of pre-epidemic and epidemic zika virus strains for virological factors potentially associated with the rapidly expanding epidemic. Emerg. Microbes Infect. 2016, 5, e22. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Thurmond, S.; Hai, R.; Song, J. Structure and function of zika virus ns5 protein: Perspectives for drug design. Cell. Mol. Life Sci. 2018, 75, 1723–1736. [Google Scholar] [CrossRef]
- Elshahawi, H.; Syed Hassan, S.; Balasubramaniam, V. Importance of zika virus ns5 protein for viral replication. Pathogens 2019, 8, 169. [Google Scholar] [CrossRef]
- Ng, I.H.W.; Chan, K.W.; Tan, M.J.A.; Gwee, C.P.; Smith, K.M.; Jeffress, S.J.; Saw, W.G.; Swarbrick, C.M.D.; Watanabe, S.; Jans, D.A.; et al. Zika virus ns5 forms supramolecular nuclear bodies that sequester importin-α and modulate the host immune and pro-inflammatory response in neuronal cells. Acs Infect. Dis. 2019, 5, 932–948. [Google Scholar] [CrossRef]
- Tan, M.J.A.; Chan, K.W.K.; Ng, I.H.W.; Kong, S.Y.Z.; Gwee, C.P.; Watanabe, S.; Vasudevan, S.G. The potential role of the zikv ns5 nuclear spherical-shell structures in cell type-specific host immune modulation during zikv infection. Cells 2019, 8, 1519. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, W.; Wang, Y.; Chen, K.; Xiao, F.; Hu, D.; Hui, L.; Liu, W.; Feng, Y.; Li, G.; et al. Ns5 conservative site is required for zika virus to restrict the rig-i signaling. Front. Immunol. 2020, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, R.; Melén, K.; Westenius, V.; Jiang, M.; Österlund, P.; Khan, H.; Vapalahti, O.; Julkunen, I.; Kakkola, L. Zika virus non-structural protein ns5 inhibits the rig-i pathway and interferon lambda 1 promoter activation by targeting ikk epsilon. Viruses 2019, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, S.; He, J.; Guest, J.D.; Ma, Z.; Yang, L.; Pierce, B.G.; Tang, Q.; Zhang, Y.J. Zika virus ns5 protein antagonizes type i interferon production via blocking tbk1 activation. Virology 2019, 527, 180–187. [Google Scholar] [CrossRef]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sánchez-Seco, M.P.; Evans, M.J.; Best, S.M.; et al. Zika virus targets human stat2 to inhibit type i interferon signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef]
- Hertzog, J.; Dias Junior, A.G.; Rigby, R.E.; Donald, C.L.; Mayer, A.; Sezgin, E.; Song, C.; Jin, B.; Hublitz, P.; Eggeling, C.; et al. Infection with a brazilian isolate of zika virus generates rig-i stimulatory rna and the viral ns5 protein blocks type i ifn induction and signaling. Eur. J. Immunol. 2018, 48, 1120–1136. [Google Scholar] [CrossRef]
- Zhao, Z.; Tao, M.; Han, W.; Fan, Z.; Imran, M.; Cao, S.; Ye, J. Nuclear localization of zika virus ns5 contributes to suppression of type i interferon production and response. J. Gen. Virol. 2019. [Google Scholar] [CrossRef]
- Conde, J.N.; Schutt, W.R.; Mladinich, M.; Sohn, S.Y.; Hearing, P.; Mackow, E.R. Ns5 sumoylation directs nuclear responses that permit zika virus to persistently infect human brain microvascular endothelial cells. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Göertz, G.P.; Abbo, S.R.; Fros, J.J.; Pijlman, G.P. Functional rna during zika virus infection. Virus Res. 2018, 254, 41–53. [Google Scholar] [CrossRef]
- Kümmerer, B.M. Establishment and application of flavivirus replicons. Adv. Exp. Med. Biol. 2018, 1062, 165–173. [Google Scholar] [PubMed]
- Pijlman, G.P.; Funk, A.; Kondratieva, N.; Leung, J.; Torres, S.; van der Aa, L.; Liu, W.J.; Palmenberg, A.C.; Shi, P.Y.; Hall, R.A.; et al. A highly structured, nuclease-resistant, noncoding rna produced by flaviviruses is required for pathogenicity. Cell Host Microbe 2008, 4, 579–591. [Google Scholar] [CrossRef]
- Silva, P.A.; Pereira, C.F.; Dalebout, T.J.; Spaan, W.J.; Bredenbeek, P.J. An rna pseudoknot is required for production of yellow fever virus subgenomic rna by the host nuclease xrn1. J. Virol. 2010, 84, 11395–11406. [Google Scholar] [CrossRef]
- Moon, S.L.; Anderson, J.R.; Kumagai, Y.; Wilusz, C.J.; Akira, S.; Khromykh, A.A.; Wilusz, J. A noncoding rna produced by arthropod-borne flaviviruses inhibits the cellular exoribonuclease xrn1 and alters host mrna stability. RNA 2012, 18, 2029–2040. [Google Scholar] [CrossRef] [PubMed]
- Funk, A.; Truong, K.; Nagasaki, T.; Torres, S.; Floden, N.; Balmori Melian, E.; Edmonds, J.; Dong, H.; Shi, P.Y.; Khromykh, A.A. Rna structures required for production of subgenomic flavivirus rna. J. Virol. 2010, 84, 11407–11417. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, B.M.; Laurence, H.M.; Massey, A.R.; Costantino, D.A.; Xie, X.; Yang, Y.; Shi, P.Y.; Nix, J.C.; Beckham, J.D.; Kieft, J.S. Zika virus produces noncoding rnas using a multi-pseudoknot structure that confounds a cellular exonuclease. Science 2016, 354, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Filomatori, C.V.; Carballeda, J.M.; Villordo, S.M.; Aguirre, S.; Pallarés, H.M.; Maestre, A.M.; Sánchez-Vargas, I.; Blair, C.D.; Fabri, C.; Morales, M.A.; et al. Dengue virus genomic variation associated with mosquito adaptation defines the pattern of viral non-coding rnas and fitness in human cells. PLoS Pathog. 2017, 13, e1006265. [Google Scholar] [CrossRef] [PubMed]
- Villordo, S.M.; Carballeda, J.M.; Filomatori, C.V.; Gamarnik, A.V. Rna structure duplications and flavivirus host adaptation. Trends Microbiol. 2016, 24, 270–283. [Google Scholar] [CrossRef]
- Donald, C.L.; Brennan, B.; Cumberworth, S.L.; Rezelj, V.V.; Clark, J.J.; Cordeiro, M.T.; Freitas de Oliveira França, R.; Pena, L.J.; Wilkie, G.S.; Da Silva Filipe, A.; et al. Full genome sequence and sfrna interferon antagonist activity of zika virus from recife, brazil. PLoS Negl. Trop. Dis. 2016, 10, e0005048. [Google Scholar] [CrossRef]
- Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Luo, H.; Xie, X.; Medeiros, D.B.A.; Wakamiya, M.; Tesh, R.B.; Barrett, A.D.; Wang, T.; et al. A live-attenuated zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat. Med. 2017, 23, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Caine, E.A.; Walker, E.C.; Larkin, K.E.; Camacho, E.; Osorio, J.E. Characterization of lethal zika virus infection in ag129 mice. PLoS Negl. Trop. Dis. 2016, 10, e0004682. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Shin, O.S. Advances in zika virus–host cell interaction: Current knowledge and future perspectives. Int. J. Mol. Sci. 2019, 20, 1101. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Balasubramaniam, V.R.; Brown, J.A.; Mena, I.; Grant, A.; Bardina, S.V.; Maringer, K.; Schwarz, M.C.; Maestre, A.M.; Sourisseau, M.; et al. A novel zika virus mouse model reveals strain specific differences in virus pathogenesis and host inflammatory immune responses. PLoS Pathog. 2017, 13, e1006258. [Google Scholar] [CrossRef]
- Smith, D.R.; Hollidge, B.; Daye, S.; Zeng, X.; Blancett, C.; Kuszpit, K.; Bocan, T.; Koehler, J.W.; Coyne, S.; Minogue, T.; et al. Neuropathogenesis of zika virus in a highly susceptible immunocompetent mouse model after antibody blockade of type i interferon. PLoS Negl. Trop. Dis. 2017, 11, e0005296. [Google Scholar] [CrossRef]
- Jagger, B.W.; Miner, J.J.; Cao, B.; Arora, N.; Smith, A.M.; Kovacs, A.; Mysorekar, I.U.; Coyne, C.B.; Diamond, M.S. Gestational stage and ifn-λ signaling regulate zikv infection in utero. Cell Host Microbe 2017, 22, 366–376.e363. [Google Scholar] [CrossRef]
- Casazza, R.L.; Lazear, H.M. Antiviral immunity backfires: Pathogenic effects of type i interferon signaling in fetal development. Sci. Immunol. 2018, 3, 19. [Google Scholar] [CrossRef]
- Pallarés, H.M.; Costa Navarro, G.S.; Villordo, S.M.; Merwaiss, F.; de Borba, L.; Gonzalez Lopez Ledesma, M.M.; Ojeda, D.S.; Henrion-Lacritick, A.; Morales, M.A.; Fabri, C.; et al. Zika virus subgenomic flavivirus rna generation requires cooperativity between duplicated rna structures that are essential for productive infection in human cells. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Slonchak, A.; Hugo, L.E.; Freney, M.E.; Hall-Mendelin, S.; Amarilla, A.A.; Torres, F.J.; Setoh, Y.X.; Peng, N.Y.G.; Sng, J.D.J.; Hall, R.A.; et al. Zika virus noncoding rna suppresses apoptosis and is required for virus transmission by mosquitoes. Nat. Commun. 2020, 11, 2205. [Google Scholar] [CrossRef]
- Fensterl, V.; Sen, G.C. Interferon-induced ifit proteins: Their role in viral pathogenesis. J. Virol. 2015, 89, 2462–2468. [Google Scholar] [CrossRef]
- Daffis, S.; Szretter, K.J.; Schriewer, J.; Li, J.; Youn, S.; Errett, J.; Lin, T.Y.; Schneller, S.; Zust, R.; Dong, H.; et al. 2’-o methylation of the viral mrna cap evades host restriction by ifit family members. Nature 2010, 468, 452–456. [Google Scholar] [CrossRef]
- Szretter, K.J.; Daniels, B.P.; Cho, H.; Gainey, M.D.; Yokoyama, W.M.; Gale, M., Jr.; Virgin, H.W.; Klein, R.S.; Sen, G.C.; Diamond, M.S. 2′-o methylation of the viral mrna cap by west nile virus evades ifit1-dependent and -independent mechanisms of host restriction in vivo. PLoS Pathog. 2012, 8, e1002698. [Google Scholar] [CrossRef] [PubMed]
- Züst, R.; Dong, H.; Li, X.F.; Chang, D.C.; Zhang, B.; Balakrishnan, T.; Toh, Y.X.; Jiang, T.; Li, S.H.; Deng, Y.Q.; et al. Rational design of a live attenuated dengue vaccine: 2’-o-methyltransferase mutants are highly attenuated and immunogenic in mice and macaques. PLoS Pathog. 2013, 9, e1003521. [Google Scholar] [CrossRef] [PubMed]
- Coutard, B.; Barral, K.; Lichière, J.; Selisko, B.; Martin, B.; Aouadi, W.; Lombardia, M.O.; Debart, F.; Vasseur, J.J.; Guillemot, J.C.; et al. Zika virus methyltransferase: Structure and functions for drug design perspectives. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Fink, K.; Züst, R.; Lim, S.P.; Qin, C.F.; Shi, P.Y. Flavivirus rna methylation. J. Gen. Virol. 2014, 95, 763–778. [Google Scholar] [CrossRef]
- Egloff, M.P.; Benarroch, D.; Selisko, B.; Romette, J.L.; Canard, B. An rna cap (nucleoside-2′-o-)-methyltransferase in the flavivirus rna polymerase ns5: Crystal structure and functional characterization. Embo J. 2002, 21, 2757–2768. [Google Scholar] [CrossRef] [PubMed]
- Coloma, J.; Jain, R.; Rajashankar, K.R.; García-Sastre, A.; Aggarwal, A.K. Structures of ns5 methyltransferase from zika virus. Cell Rep. 2016, 16, 3097–3102. [Google Scholar] [CrossRef]
- Duan, W.; Song, H.; Wang, H.; Chai, Y.; Su, C.; Qi, J.; Shi, Y.; Gao, G.F. The crystal structure of zika virus ns5 reveals conserved drug targets. Embo J. 2017, 36, 919–933. [Google Scholar] [CrossRef]
- Stephen, P.; Baz, M.; Boivin, G.; Lin, S.X. Structural insight into ns5 of zika virus leading to the discovery of mtase inhibitors. J. Am. Chem. Soc. 2016, 138, 16212–16215. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Chang, D.C.; Xie, X.; Toh, Y.X.; Chung, K.Y.; Zou, G.; Lescar, J.; Lim, S.P.; Shi, P.Y. Biochemical and genetic characterization of dengue virus methyltransferase. Virology 2010, 405, 568–578. [Google Scholar] [CrossRef]
- Li, S.H.; Dong, H.; Li, X.F.; Xie, X.; Zhao, H.; Deng, Y.Q.; Wang, X.Y.; Ye, Q.; Zhu, S.Y.; Wang, H.J.; et al. Rational design of a flavivirus vaccine by abolishing viral rna 2’-o methylation. J. Virol. 2013, 87, 5812–5819. [Google Scholar] [CrossRef]
- Ray, D.; Shah, A.; Tilgner, M.; Guo, Y.; Zhao, Y.; Dong, H.; Deas, T.S.; Zhou, Y.; Li, H.; Shi, P.Y. West nile virus 5’-cap structure is formed by sequential guanine n-7 and ribose 2‘-o methylations by nonstructural protein 5. J. Virol. 2006, 80, 8362–8370. [Google Scholar] [CrossRef]
- Zhou, Y.; Ray, D.; Zhao, Y.; Dong, H.; Ren, S.; Li, Z.; Guo, Y.; Bernard, K.A.; Shi, P.Y.; Li, H. Structure and function of flavivirus ns5 methyltransferase. J. Virol. 2007, 81, 3891–3903. [Google Scholar] [CrossRef] [PubMed]
- Desvignes, T.; Batzel, P.; Berezikov, E.; Eilbeck, K.; Eppig, J.T.; McAndrews, M.S.; Singer, A.; Postlethwait, J.H. Mirna nomenclature: A view incorporating genetic origins, biosynthetic pathways, and sequence variants. Trends Genet. 2015, 31, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, M.A. Micrornas and the immune response. Trends Immunol. 2008, 29, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Micrornas: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- He, L.; Hannon, G.J. Micrornas: Small rnas with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Dang, J.W.; Tiwari, S.K.; Qin, Y.; Rana, T.M. Genome-wide integrative analysis of zika-virus-infected neuronal stem cells reveals roles for micrornas in cell cycle and stemness. Cell Rep. 2019, 27, 3618–3628.e3615. [Google Scholar] [CrossRef]
- Baril, M.; Racine, M.E.; Penin, F.; Lamarre, D. Mavs dimer is a crucial signaling component of innate immunity and the target of hepatitis c virus ns3/4a protease. J. Virol. 2009, 83, 1299–1311. [Google Scholar] [CrossRef]
- Icardi, L.; Mori, R.; Gesellchen, V.; Eyckerman, S.; De Cauwer, L.; Verhelst, J.; Vercauteren, K.; Saelens, X.; Meuleman, P.; Leroux-Roels, G.; et al. The sin3a repressor complex is a master regulator of stat transcriptional activity. Proc. Natl. Acad. Sci. USA 2012, 109, 12058–12063. [Google Scholar] [CrossRef]
- Kozak, R.A.; Majer, A.; Biondi, M.J.; Medina, S.J.; Goneau, L.W.; Sajesh, B.V.; Slota, J.A.; Zubach, V.; Severini, A.; Safronetz, D.; et al. Microrna and mrna dysregulation in astrocytes infected with zika virus. Viruses 2017, 9, 297. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Sen, U.; Vrati, S. Regulated ire1-dependent decay pathway is activated during japanese encephalitis virus-induced unfolded protein response and benefits viral replication. J. Gen. Virol. 2014, 95, 71–79. [Google Scholar] [CrossRef]
- Liang, Q.; Luo, Z.; Zeng, J.; Chen, W.; Foo, S.S.; Lee, S.A.; Ge, J.; Wang, S.; Goldman, S.A.; Zlokovic, B.V.; et al. Zika virus ns4a and ns4b proteins deregulate akt-mtor signaling in human fetal neural stem cells to inhibit neurogenesis and induce autophagy. Cell Stem Cell 2016, 19, 663–671. [Google Scholar] [CrossRef]
- Hetz, C.; Papa, F.R. The unfolded protein response and cell fate control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhang, W.; Sun, J.; Fu, Z.; Ke, X.; Zheng, C.; Zhang, Y.; Li, P.; Liu, Y.; Hu, Q.; et al. Zikv infection activates the ire1-xbp1 and atf6 pathways of unfolded protein response in neural cells. J. Neuroinflammation 2018, 15, 275. [Google Scholar] [CrossRef]
- Azouz, F.; Arora, K.; Krause, K.; Nerurkar, V.R.; Kumar, M. Integrated microrna and mrna profiling in zika virus-infected neurons. Viruses 2019, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Pareek, S.; Roy, S.; Kumari, B.; Jain, P.; Banerjee, A.; Vrati, S. Mir-155 induction in microglial cells suppresses japanese encephalitis virus replication and negatively modulates innate immune responses. J. Neuroinflammation 2014, 11, 97. [Google Scholar] [CrossRef]
- Jiang, M.; Broering, R.; Trippler, M.; Wu, J.; Zhang, E.; Zhang, X.; Gerken, G.; Lu, M.; Schlaak, J.F. Microrna-155 controls toll-like receptor 3- and hepatitis c virus-induced immune responses in the liver. J. Viral Hepat. 2014, 21, 99–110. [Google Scholar] [CrossRef]
- Dickey, L.L.; Hanley, T.M.; Huffaker, T.B.; Ramstead, A.G.; O’Connell, R.M.; Lane, T.E. Microrna 155 and viral-induced neuroinflammation. J. Neuroimmunol. 2017, 308, 17–24. [Google Scholar] [CrossRef]
- Thounaojam, M.C.; Kaushik, D.K.; Kundu, K.; Basu, A. Microrna-29b modulates japanese encephalitis virus-induced microglia activation by targeting tumor necrosis factor alpha-induced protein 3. J. Neurochem. 2014, 129, 143–154. [Google Scholar] [CrossRef]
- Buggele, W.A.; Horvath, C.M. Microrna profiling of sendai virus-infected a549 cells identifies mir-203 as an interferon-inducible regulator of ifit1/isg56. J. Virol. 2013, 87, 9260–9270. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Li, J.; Yang, Y.; Kang, X.; Li, Y.; Wu, X.; Zhu, Q.; Zhou, Y.; Hu, Y. Up-regulation of microrna-203 in influenza a virus infection inhibits viral replication by targeting dr1. Sci. Rep. 2018, 8, 6797. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.N.; Holanda, G.M.; Pinto Silva, E.V.; Casseb, S.M.M.; Melo, K.F.L.; Carvalho, C.A.M.; Lima, J.A.; Vasconcelos, P.F.C.; Cruz, A.C.R. Zika virus alters the expression profile of microrna-related genes in liver, lung, and kidney cell lineages. Viral Immunol. 2018, 31, 583–588. [Google Scholar] [CrossRef]
- Xu, Y.P.; Qiu, Y.; Zhang, B.; Chen, G.; Chen, Q.; Wang, M.; Mo, F.; Xu, J.; Wu, J.; Zhang, R.R.; et al. Zika virus infection induces rnai-mediated antiviral immunity in human neural progenitors and brain organoids. Cell Res. 2019, 29, 265–273. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, C.; Xi, Z. Enhancement of rnai by a small molecule antibiotic enoxacin. Cell Res. 2008, 18, 1077–1079. [Google Scholar] [CrossRef]
- Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Sandoval-Espinosa, C.; Bershteyn, M.; Kriegstein, A.R. Expression analysis highlights axl as a candidate zika virus entry receptor in neural stem cells. Cell Stem Cell 2016, 18, 591–596. [Google Scholar] [CrossRef]
- Tang, H.; Hammack, C.; Ogden, S.C.; Wen, Z.; Qian, X.; Li, Y.; Yao, B.; Shin, J.; Zhang, F.; Lee, E.M.; et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 2016, 18, 587–590. [Google Scholar] [CrossRef]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-region-specific organoids using mini-bioreactors for modeling zikv exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Netea, M.G.; Schlitzer, A.; Placek, K.; Joosten, L.A.B.; Schultze, J.L. Innate and adaptive immune memory: An evolutionary continuum in the host’s response to pathogens. Cell Host Microbe 2019, 25, 13–26. [Google Scholar] [CrossRef]
- Gourbal, B.; Pinaud, S.; Beckers, G.J.M.; Van Der Meer, J.W.M.; Conrath, U.; Netea, M.G. Innate immune memory: An evolutionary perspective. Immunol. Rev. 2018, 283, 21–40. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef]
- Maloney, B.E.; Perera, K.D.; Saunders, D.R.D.; Shadipeni, N.; Fleming, S.D. Interactions of viruses and the humoral innate immune response. Clin. Immunol. 2020, 212, 108351. [Google Scholar] [CrossRef]
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal center b cell dynamics. Immunity 2016, 45, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Elong Ngono, A.; Vizcarra, E.A.; Tang, W.W.; Sheets, N.; Joo, Y.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Mapping and role of the cd8(+) t cell response during primary zika virus infection in mice. Cell Host Microbe 2017, 21, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Pardy, R.D.; Rajah, M.M.; Condotta, S.A.; Taylor, N.G.; Sagan, S.M.; Richer, M.J. Analysis of the t cell response to zika virus and identification of a novel cd8+ t cell epitope in immunocompetent mice. PLoS Pathog. 2017, 13, e1006184. [Google Scholar] [CrossRef]
- Hassert, M.; Harris, M.G.; Brien, J.D.; Pinto, A.K. Identification of protective cd8 t cell responses in a mouse model of zika virus infection. Front. Immunol. 2019, 10, 1678. [Google Scholar] [CrossRef]
- Grifoni, A.; Pham, J.; Sidney, J.; O’Rourke, P.H.; Paul, S.; Peters, B.; Martini, S.R.; de Silva, A.D.; Ricciardi, M.J.; Magnani, D.M.; et al. Prior dengue virus exposure shapes t cell immunity to zika virus in humans. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Weiskopf, D.; Angelo, M.A.; de Azeredo, E.L.; Sidney, J.; Greenbaum, J.A.; Fernando, A.N.; Broadwater, A.; Kolla, R.V.; De Silva, A.D.; de Silva, A.M.; et al. Comprehensive analysis of dengue virus-specific responses supports an hla-linked protective role for cd8+ t cells. Proc. Natl. Acad. Sci. USA 2013, 110, E2046–E2053. [Google Scholar] [CrossRef]
- Weiskopf, D.; Angelo, M.A.; Sidney, J.; Peters, B.; Shresta, S.; Sette, A. Immunodominance changes as a function of the infecting dengue virus serotype and primary versus secondary infection. J. Virol. 2014, 88, 11383–11394. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Angelo, M.A.; Grifoni, A.; O’Rourke, P.H.; Sidney, J.; Paul, S.; De Silva, A.D.; Phillips, E.; Mallal, S.; Premawansa, S.; et al. Hla-drb1 alleles are associated with different magnitudes of dengue virus-specific cd4+ t-cell responses. J. Infect. Dis. 2016, 214, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Elong Ngono, A.; Shresta, S. Cross-reactive t cell immunity to dengue and zika viruses: New insights into vaccine development. Front. Immunol. 2019, 10, 1316. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Sammon, M.; Garg, M. Dengue, zika and chikungunya: Emerging arboviruses in the new world. West. J. Emerg. Med. 2016, 17, 671–679. [Google Scholar] [CrossRef]
- Zimmerman, M.G.; Wrammert, J.; Suthar, M.S. Cross-reactive antibodies during zika virus infection: Protection, pathogenesis, and placental seeding. Cell Host Microbe 2020, 27, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef]
- Ades, A.E.; Thorne, C.; Soriano-Arandes, A.; Peckham, C.S.; Brown, D.W.; Lang, D.; Morris, J.G.; Christie, C.D.C.; Giaquinto, C. Researching zika in pregnancy: Lessons for global preparedness. Lancet Infect. Dis. 2020, 20, e61–e68. [Google Scholar] [CrossRef]
- Erbelding, E.; Cassetti, C. Zika virus and future research directions. J. Infect. Dis. 2017, 216, S991–S994. [Google Scholar] [CrossRef]
- Slon Campos, J.L.; Mongkolsapaya, J.; Screaton, G.R. The immune response against flaviviruses. Nat. Immunol. 2018, 19, 1189–1198. [Google Scholar] [CrossRef]
- Wen, J.; Tang, W.W.; Sheets, N.; Ellison, J.; Sette, A.; Kim, K.; Shresta, S. Identification of zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive cd8(+) t cells. Nat. Microbiol. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Wen, J.; Elong Ngono, A.; Regla-Nava, J.A.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Dengue virus-reactive cd8(+) t cells mediate cross-protection against subsequent zika virus challenge. Nat. Commun. 2017, 8, 1459. [Google Scholar] [CrossRef]
- Regla-Nava, J.A.; Elong Ngono, A.; Viramontes, K.M.; Huynh, A.T.; Wang, Y.T.; Nguyen, A.T.; Salgado, R.; Mamidi, A.; Kim, K.; Diamond, M.S.; et al. Cross-reactive dengue virus-specific cd8(+) t cells protect against zika virus during pregnancy. Nat. Commun. 2018, 9, 3042. [Google Scholar] [CrossRef]
- Herrera, B.B.; Tsai, W.Y.; Chang, C.A.; Hamel, D.J.; Wang, W.K.; Lu, Y.; Mboup, S.; Kanki, P.J. Sustained specific and cross-reactive t cell responses to zika and dengue virus ns3 in west africa. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Lim, M.Q.; Kumaran, E.A.P.; Tan, H.C.; Lye, D.C.; Leo, Y.S.; Ooi, E.E.; MacAry, P.A.; Bertoletti, A.; Rivino, L. Cross-reactivity and anti-viral function of dengue capsid and ns3-specific memory t cells toward zika virus. Front. Immunol. 2018, 9, 2225. [Google Scholar] [CrossRef]
- Huber, R.G.; Lim, X.N.; Ng, W.C.; Sim, A.Y.L.; Poh, H.X.; Shen, Y.; Lim, S.Y.; Sundstrom, K.B.; Sun, X.; Aw, J.G.; et al. Structure mapping of dengue and zika viruses reveals functional long-range interactions. Nat. Commun. 2019, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Gresh, L.; Ojeda, S.; Katzelnick, L.C.; Sanchez, N.; Mercado, J.C.; Chowell, G.; Lopez, B.; Elizondo, D.; Coloma, J.; et al. Prior dengue virus infection and risk of zika: A pediatric cohort in nicaragua. PLoS Med. 2019, 16, e1002726. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Barraquer, I.; Costa, F.; Nascimento, E.J.M.; Nery, N.J.; Castanha, P.M.S.; Sacramento, G.A.; Cruz, J.; Carvalho, M.; De Olivera, D.; Hagan, J.E.; et al. Impact of preexisting dengue immunity on zika virus emergence in a dengue endemic region. Science 2019, 363, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, C.; Fischer, C.; Feldmann, M.; Sarno, M.; Luz, E.; Moreira-Soto, A.; Cabral, R.; Netto, E.M.; Brites, C.; Kümmerer, B.M.; et al. Cross-protection of dengue virus infection against congenital zika syndrome, northeastern brazil. Emerg. Infect. Dis. 2019, 25, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Hassert, M.; Wolf, K.J.; Schwetye, K.E.; DiPaolo, R.J.; Brien, J.D.; Pinto, A.K. Cd4+t cells mediate protection against zika associated severe disease in a mouse model of infection. PLoS Pathog. 2018, 14, e1007237. [Google Scholar] [CrossRef] [PubMed]
- Elong Ngono, A.; Young, M.P.; Bunz, M.; Xu, Z.; Hattakam, S.; Vizcarra, E.; Regla-Nava, J.A.; Tang, W.W.; Yamabhai, M.; Wen, J.; et al. Cd4+ t cells promote humoral immunity and viral control during zika virus infection. PLoS Pathog. 2019, 15, e1007474. [Google Scholar]
- Nancy, P.; Tagliani, E.; Tay, C.S.; Asp, P.; Levy, D.E.; Erlebacher, A. Chemokine gene silencing in decidual stromal cells limits t cell access to the maternal-fetal interface. Science 2012, 336, 1317–1321. [Google Scholar] [CrossRef]
- Shan, C.; Xie, X.; Luo, H.; Muruato, A.E.; Liu, Y.; Wakamiya, M.; La, J.H.; Chung, J.M.; Weaver, S.C.; Wang, T.; et al. Maternal vaccination and protective immunity against zika virus vertical transmission. Nat. Commun. 2019, 10, 5677. [Google Scholar] [CrossRef] [PubMed]
- Elong Ngono, A.; Syed, T.; Nguyen, A.V.; Regla-Nava, J.A.; Susantono, M.; Spasova, D.; Aguilar, A.; West, M.; Sparks, J.; Gonzalez, A.; et al. Cd8(+) t cells mediate protection against zika virus induced by an ns3-based vaccine. Sci. Adv. 2020, 6, eabb2154. [Google Scholar] [CrossRef] [PubMed]
- Sariol, C.A.; Nogueira, M.L.; Vasilakis, N. A tale of two viruses: Does heterologous flavivirus immunity enhance zika disease? Trends Microbiol. 2018, 26, 186–190. [Google Scholar] [CrossRef]
- Rathore, A.P.S.; Saron, W.A.A.; Lim, T.; Jahan, N.; St John, A.L. Maternal immunity and antibodies to dengue virus promote infection and zika virus-induced microcephaly in fetuses. Sci. Adv. 2019, 5, eaav3208. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Singh, G.; Acklin, J.A.; Lee, S.; Duehr, J.E.; Chokola, A.N.; Frere, J.J.; Hoffman, K.W.; Foster, G.A.; Krysztof, D.; et al. Dengue virus immunity increases zika virus-induced damage during pregnancy. Immunity 2019, 50, 751–762.e755. [Google Scholar] [CrossRef]
- Zimmerman, M.G.; Quicke, K.M.; O’Neal, J.T.; Arora, N.; Machiah, D.; Priyamvada, L.; Kauffman, R.C.; Register, E.; Adekunle, O.; Swieboda, D.; et al. Cross-reactive dengue virus antibodies augment zika virus infection of human placental macrophages. Cell Host Microbe 2018, 24, 731–742.e736. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Olsen, P.C.; Costa, F.; Wang, Q.; Oliveira, T.Y.; Nery, N., Jr.; Aromolaran, A.; do Rosário, M.S.; Sacramento, G.A.; Cruz, J.S.; et al. Risk of zika microcephaly correlates with features of maternal antibodies. J. Exp. Med. 2019, 216, 2302–2315. [Google Scholar] [CrossRef]
- Diamond, M.S.; Ledgerwood, J.E.; Pierson, T.C. Zika virus vaccine development: Progress in the face of new challenges. Annu. Rev. Med. 2019, 70, 121–135. [Google Scholar] [CrossRef]
- Grubor-Bauk, B.; Wijesundara, D.K.; Masavuli, M.; Abbink, P.; Peterson, R.L.; Prow, N.A.; Larocca, R.A.; Mekonnen, Z.A.; Shrestha, A.; Eyre, N.S.; et al. Ns1 DNA vaccination protects against zika infection through t cell-mediated immunity in immunocompetent mice. Sci. Adv. 2019, 5, eaax2388. [Google Scholar] [CrossRef] [PubMed]
- Bullard, B.L.; Corder, B.N.; Gorman, M.J.; Diamond, M.S.; Weaver, E.A. Efficacy of a t cell-biased adenovirus vector as a zika virus vaccine. Sci. Rep. 2018, 8, 18017. [Google Scholar] [CrossRef] [PubMed]
- Counotte, M.J.; Kim, C.R.; Wang, J.; Bernstein, K.; Deal, C.D.; Broutet, N.J.N.; Low, N. Sexual transmission of zika virus and other flaviviruses: A living systematic review. PLoS Med. 2018, 15, e1002611. [Google Scholar] [CrossRef] [PubMed]
- Spadoni, I.; Fornasa, G.; Rescigno, M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat. Rev. Immunol. 2017, 17, 761–773. [Google Scholar] [CrossRef] [PubMed]
- El Costa, H.; Gouilly, J.; Mansuy, J.M.; Chen, Q.; Levy, C.; Cartron, G.; Veas, F.; Al-Daccak, R.; Izopet, J.; Jabrane-Ferrat, N. Zika virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci. Rep. 2016, 6, 35296. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.R.; Roby, J.A.; Dobyns, W.B.; Rajagopal, L.; Gale, M., Jr.; Adams Waldorf, K.M. Immune evasion strategies used by zika virus to infect the fetal eye and brain. Viral Immunol. 2020, 33, 22–37. [Google Scholar] [CrossRef]
- Adams Waldorf, K.M.; Stencel-Baerenwald, J.E.; Kapur, R.P.; Studholme, C.; Boldenow, E.; Vornhagen, J.; Baldessari, A.; Dighe, M.K.; Thiel, J.; Merillat, S.; et al. Fetal brain lesions after subcutaneous inoculation of zika virus in a pregnant nonhuman primate. Nat. Med. 2016, 22, 1256–1259. [Google Scholar] [CrossRef]
- Chiu, C.F.; Chu, L.W.; Liao, I.C.; Simanjuntak, Y.; Lin, Y.L.; Juan, C.C.; Ping, Y.H. The mechanism of the zika virus crossing the placental barrier and the blood-brain barrier. Front. Microbiol. 2020, 11, 214. [Google Scholar] [CrossRef]
- Wu, K.Y.; Zuo, G.L.; Li, X.F.; Ye, Q.; Deng, Y.Q.; Huang, X.Y.; Cao, W.C.; Qin, C.F.; Luo, Z.G. Vertical transmission of zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res. 2016, 26, 645–654. [Google Scholar] [CrossRef]
- Onorati, M.; Li, Z.; Liu, F.; Sousa, A.M.M.; Nakagawa, N.; Li, M.; Dell’Anno, M.T.; Gulden, F.O.; Pochareddy, S.; Tebbenkamp, A.T.N.; et al. Zika virus disrupts phospho-tbk1 localization and mitosis in human neuroepithelial stem cells and radial glia. Cell Rep. 2016, 16, 2576–2592. [Google Scholar] [CrossRef]
- Adams Waldorf, K.M.; Nelson, B.R.; Stencel-Baerenwald, J.E.; Studholme, C.; Kapur, R.P.; Armistead, B.; Walker, C.L.; Merillat, S.; Vornhagen, J.; Tisoncik-Go, J.; et al. Congenital zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat. Med. 2018, 24, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Coffey, L.L.; Keesler, R.I.; Pesavento, P.A.; Woolard, K.; Singapuri, A.; Watanabe, J.; Cruzen, C.; Christe, K.L.; Usachenko, J.; Yee, J.; et al. Intraamniotic zika virus inoculation of pregnant rhesus macaques produces fetal neurologic disease. Nat. Commun. 2018, 9, 2414. [Google Scholar] [CrossRef] [PubMed]
- Coletti, A.M.; Singh, D.; Kumar, S.; Shafin, T.N.; Briody, P.J.; Babbitt, B.F.; Pan, D.; Norton, E.S.; Brown, E.C.; Kahle, K.T.; et al. Characterization of the ventricular-subventricular stem cell niche during human brain development. Development 2018, 145, dev170100. [Google Scholar] [CrossRef]
- Satterfield-Nash, A.; Kotzky, K.; Allen, J.; Bertolli, J.; Moore, C.A.; Pereira, I.O.; Pessoa, A.; Melo, F.; Santelli, A.; Boyle, C.A.; et al. Health and development at age 19–24 months of 19 children who were born with microcephaly and laboratory evidence of congenital zika virus infection during the 2015 zika virus outbreak—Brazil, 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.G.; Werner, H.; Lopes, F.; Hygino da Cruz, L.C., Jr.; Fazecas, T.M.; Daltro, P.A.N.; Nogueira, R.A. Central nervous system effects of intrauterine zika virus infection: A pictorial review. Radiographics 2017, 37, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Martins-Filho, P.R.; Souza Tavares, C.S.; Araújo Carvalho, A.C.; Reis, M.; Santos, H.P., Jr.; Santos, V.S. Association between arthrogryposis and mortality in infants with congenital zika syndrome: A systematic review and meta-analysis. Pediatr. Neurol. 2020, 110, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Garcez, P.P.; Loiola, E.C.; Madeiro da Costa, R.; Higa, L.M.; Trindade, P.; Delvecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818. [Google Scholar] [CrossRef]
- Simonin, Y.; Loustalot, F.; Desmetz, C.; Foulongne, V.; Constant, O.; Fournier-Wirth, C.; Leon, F.; Molès, J.P.; Goubaud, A.; Lemaitre, J.M.; et al. Zika virus strains potentially display different infectious profiles in human neural cells. EBioMedicine 2016, 12, 161–169. [Google Scholar] [CrossRef]
- Li, H.; Saucedo-Cuevas, L.; Regla-Nava, J.A.; Chai, G.; Sheets, N.; Tang, W.; Terskikh, A.V.; Shresta, S.; Gleeson, J.G. Zika virus infects neural progenitors in the adult mouse brain and alters proliferation. Cell Stem Cell 2016, 19, 593–598. [Google Scholar] [CrossRef]
- Figueiredo, C.P.; Barros-Aragão, F.G.Q.; Neris, R.L.S.; Frost, P.S.; Soares, C.; Souza, I.N.O.; Zeidler, J.D.; Zamberlan, D.C.; de Sousa, V.L.; Souza, A.S.; et al. Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nat. Commun. 2019, 10, 3890. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, A. Ocular manifestations of emerging flaviviruses and the blood-retinal barrier. Viruses 2018, 10, 530. [Google Scholar] [CrossRef]
- De Paula Freitas, B.; Zin, A.; Ko, A.; Maia, M.; Ventura, C.V.; Belfort, R., Jr. Anterior-segment ocular findings and microphthalmia in congenital zika syndrome. Ophthalmology 2017, 124, 1876–1878. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, F.S.Y.; Pietrobon, A.J.; Branco, A.; Pereira, N.Z.; Oliveira, L.; Machado, C.M.; Duarte, A.; Sato, M.N. Zika virus infects newborn monocytes without triggering a substantial cytokine response. J. Infect. Dis. 2019, 220, 32–40. [Google Scholar] [CrossRef]
- Sinigaglia, A.; Riccetti, S.; Trevisan, M.; Barzon, L. In silico approaches to zika virus drug discovery. Expert Opin. Drug Discov. 2018, 13, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Devillers, J. Repurposing drugs for use against zika virus infection. Sar Qsar Environ. Res. 2018, 29, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Bernatchez, J.A.; Tran, L.T.; Li, J.; Luan, Y.; Siqueira-Neto, J.L.; Li, R. Drugs for the treatment of zika virus infection. J. Med. Chem. 2020, 63, 470–489. [Google Scholar] [CrossRef] [PubMed]
- Hazlewood, J.E.; Rawle, D.J.; Tang, B.; Yan, K.; Vet, L.J.; Nakayama, E.; Hobson-Peters, J.; Hall, R.A.; Suhrbier, A. A zika vaccine generated using the chimeric insect-specific binjari virus platform protects against fetal brain infection in pregnant mice. Vaccines 2020, 8, 496. [Google Scholar] [CrossRef]
- Hurtado-Monzón, A.M.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Osuna-Ramos, J.F.; De Jesús-González, L.A.; Reyes-Ruiz, J.M.; Del Ángel, R.M. The role of anti-flavivirus humoral immune response in protection and pathogenesis. Rev. Med. Virol. 2020, 30, e2100. [Google Scholar] [CrossRef]
- Pattnaik, A.; Sahoo, B.R.; Pattnaik, A.K. Current status of zika virus vaccines: Successes and challenges. Vaccines 2020, 8, 266. [Google Scholar] [CrossRef] [PubMed]
- Martín-Acebes, M.A.; Saiz, J.C.; Jiménez de Oya, N. Antibody-dependent enhancement and zika: Real threat or phantom menace? Front. Cell. Infect. Microbiol. 2018, 8, 44. [Google Scholar] [CrossRef]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef] [PubMed]
- Rossant, J.; Cross, J.C. Placental development: Lessons from mouse mutants. Nat. Rev. Genet. 2001, 2, 538–548. [Google Scholar] [CrossRef] [PubMed]
- França, G.V.; Schuler-Faccini, L.; Oliveira, W.K.; Henriques, C.M.; Carmo, E.H.; Pedi, V.D.; Nunes, M.L.; Castro, M.C.; Serruya, S.; Silveira, M.F.; et al. Congenital zika virus syndrome in brazil: A case series of the first 1501 livebirths with complete investigation. Lancet 2016, 388, 891–897. [Google Scholar] [CrossRef]
- Coyne, C.B.; Lazear, H.M. Zika virus—Reigniting the torch. Nat. Rev. Microbiol. 2016, 14, 707–715. [Google Scholar] [CrossRef]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the pattern of anomalies in congenital zika syndrome for pediatric clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Vhp, L.; Aragão, M.M.; Pinho, R.S.; Hazin, A.N.; Paciorkowski, A.R.; Penalva de Oliveira, A.C.; Masruha, M.R. Congenital zika virus infection: A review with emphasis on the spectrum of brain abnormalities. Curr. Neurol. Neurosci. Rep. 2020, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Stephenson, K.E.; Barouch, D.H. Zika virus vaccines. Nat. Rev. Microbiol. 2018, 16, 594–600. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).