Design of Alphavirus Virus-Like Particles Presenting Circumsporozoite Junctional Epitopes That Elicit Protection against Malaria
Abstract
1. Introduction
2. Materials and Methods
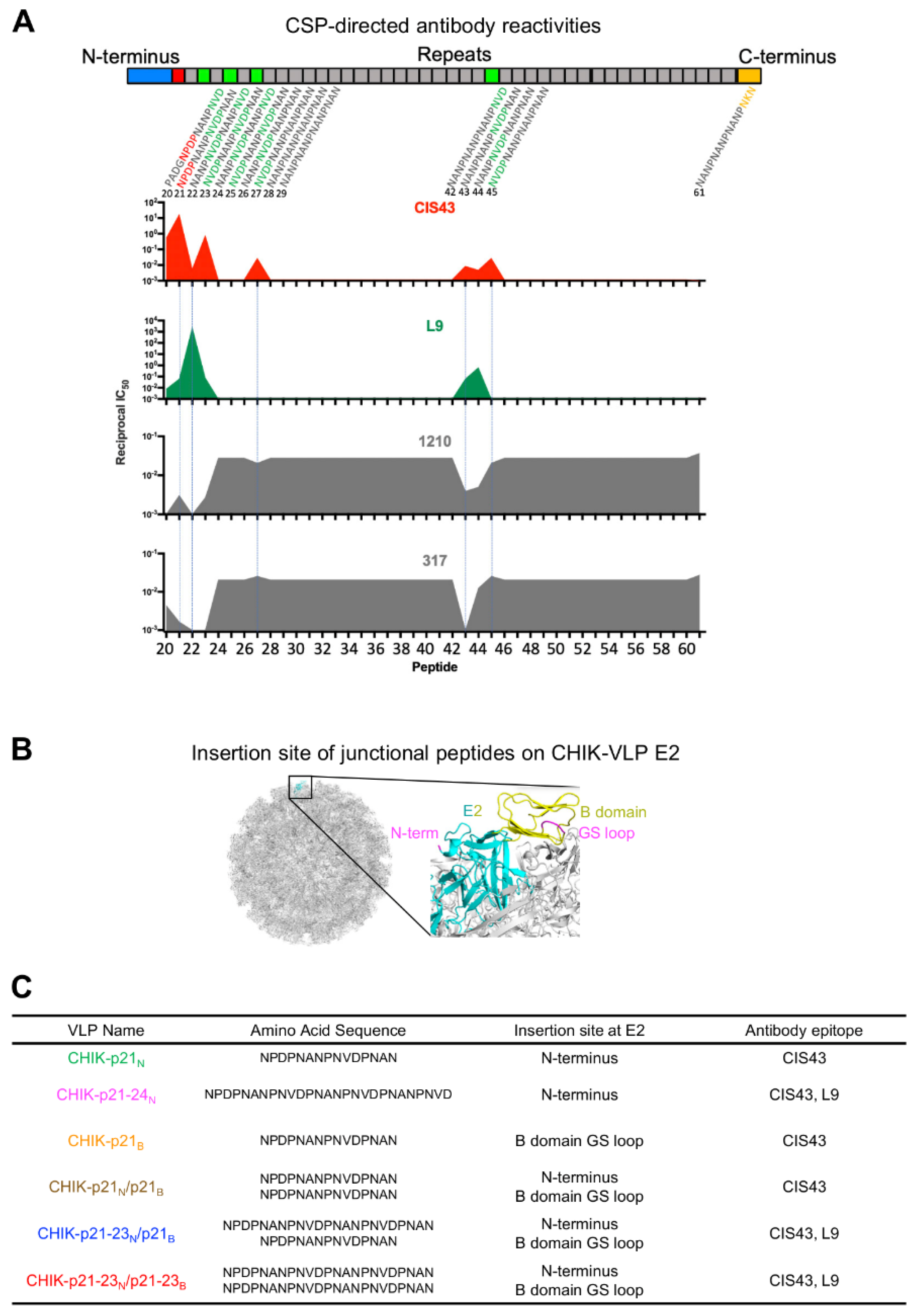
2.1. Epitope Mapping with Overlapping Peptdies
2.2. Production of CHIK-VLP Immunogens
2.3. Purification of CHIK VLP Immunogens
2.4. Production of KLH-p21 Immunogen
2.5. Production of Antibody IgGs and Fabs
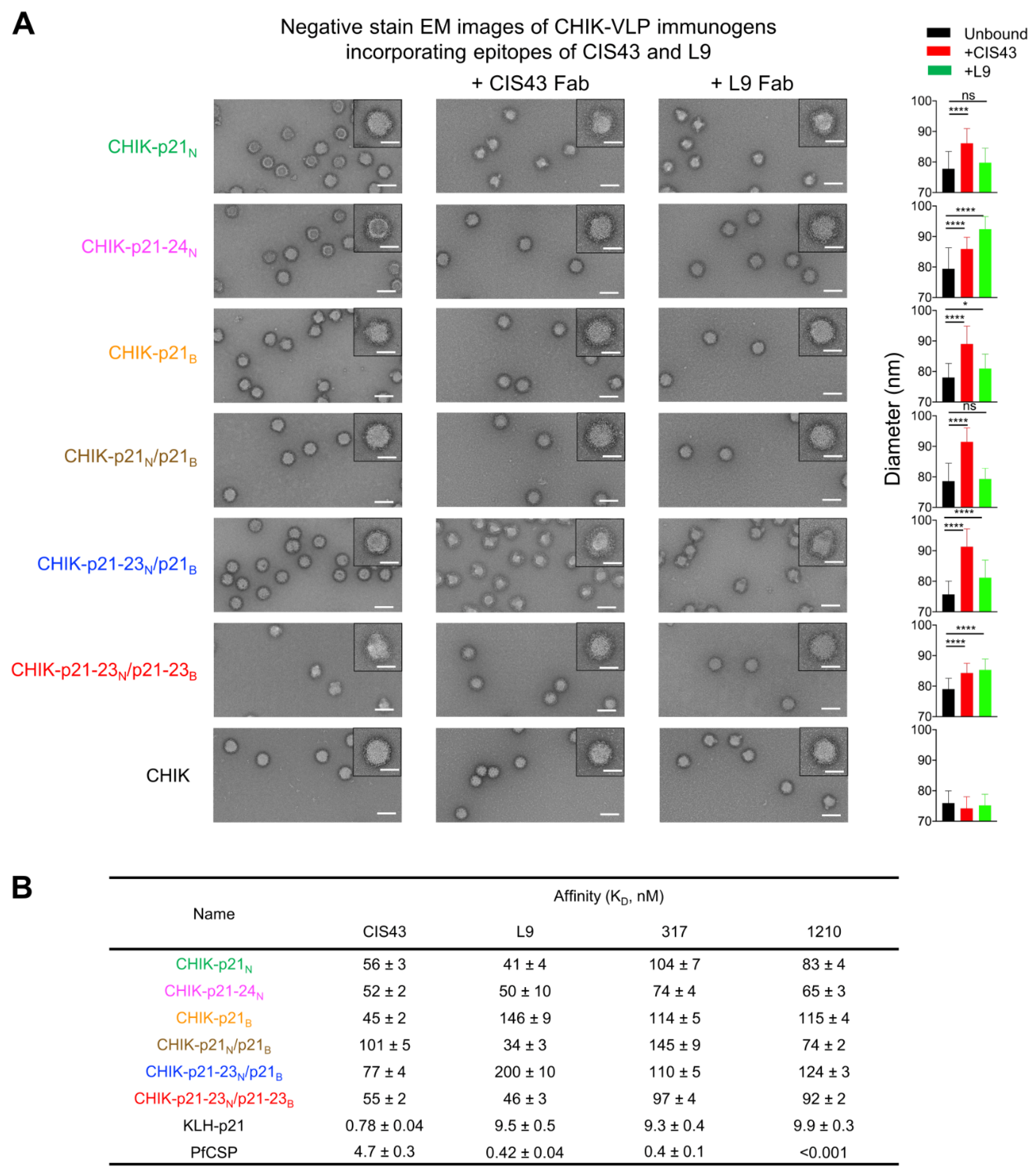
2.6. Antigenic Characterization of Junctional-Epitope–CHIK-VLP Immunogens
2.7. Negative-Stain Electron Microscopy
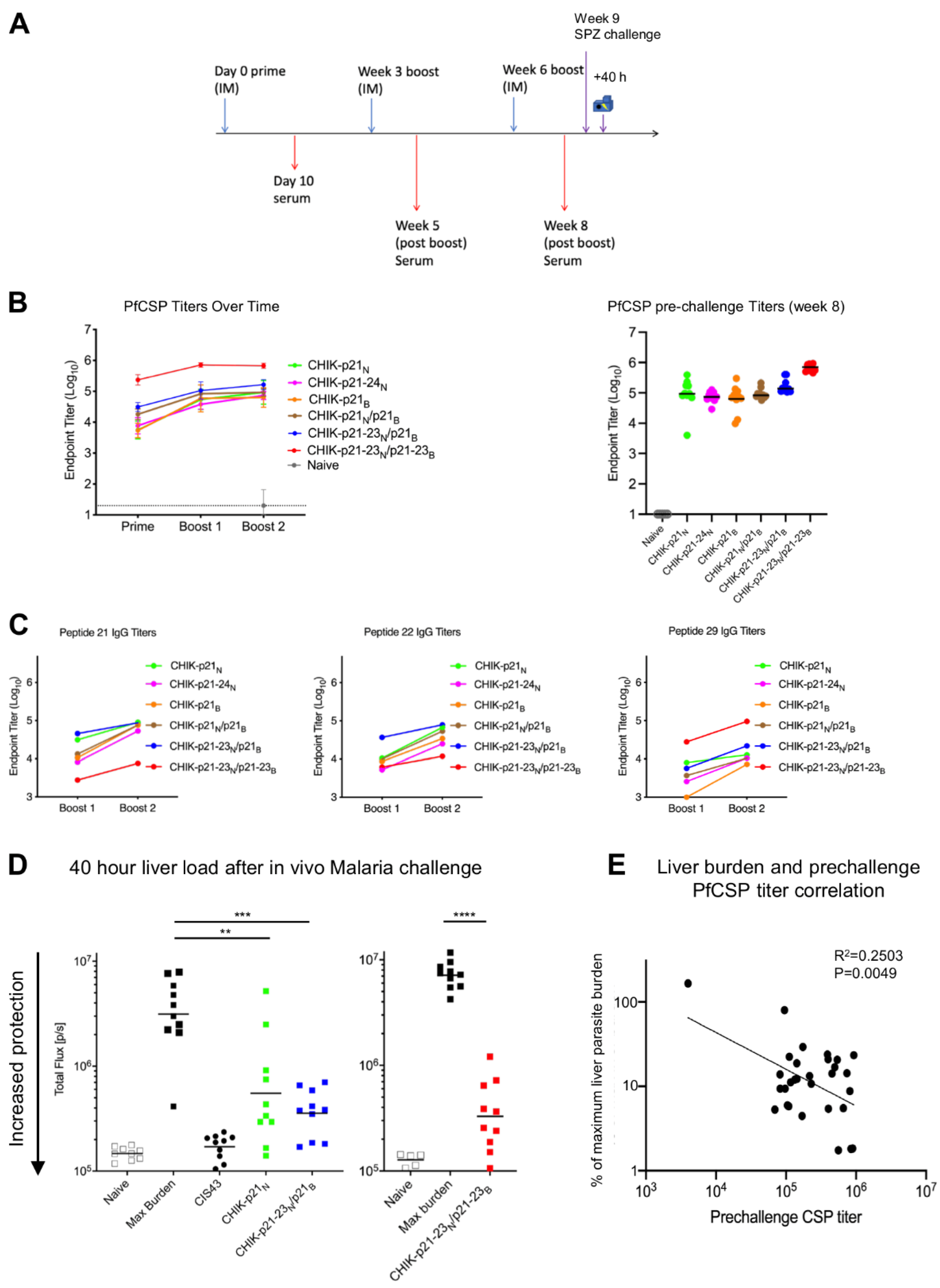
2.8. Immunogenicity and Protection Studies
2.8.1. Mice
2.8.2. Immunizations
2.8.3. Serology
2.8.4. IV Challenge and Quantification of Protection
3. Results
3.1. Design of Immunogens with PfCSP Junctional Residues Displayed on Chikungunya Virus-Like Particles
3.2. Electron Microscopic and Antigenic Characterization of CHIK-VLP-Junctional Peptide Immunogens
3.3. Immunogenicity of CHIK-VLP-Junctional Peptide Immunogens
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Malaria. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 24 November 2020).
- Snow, R.W.; Guerra, C.A.; Noor, A.M.; Myint, H.Y.; Hay, S.I. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 2005, 434, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Programme, G.M. World Malria Report; World Health Organization: Geneva, Switzerland, 2019; p. 232. [Google Scholar]
- Cissé, B.; Ba, E.H.; Sokhna, C.; NDiaye, J.L.; Gomis, J.F.; Dial, Y.; Pitt, C.; Ndiaye, M.; Cairns, M.; Faye, E.; et al. Effectiveness of Seasonal Malaria Chemoprevention in Children under Ten Years of Age in Senegal: A Stepped-Wedge Cluster-Randomised Trial. PLoS Med. 2016, 13, e1002175. [Google Scholar] [CrossRef] [PubMed]
- Mendis, K.; Rietveld, A.; Warsame, M.; Bosman, A.; Greenwood, B.; Wernsdorfer, W.H. From malaria control to eradication: The WHO perspective. Trop. Med. Int. Health 2009, 14, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Strode, C.; Donegan, S.; Garner, P.; Enayati, A.A.; Hemingway, J. The impact of pyrethroid resistance on the efficacy of insecticide-treated bed nets against African anopheline mosquitoes: Systematic review and meta-analysis. PLoS Med. 2014, 11, e1001619. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, I.A.; Seder, R.A. Malaria prevention: From immunological concepts to effective vaccines and protective antibodies. Nat. Immunol. 2018, 19, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.A.; Snaith, R.; Cottingham, M.G.; Gilbert, S.C.; Hill, A.V.S. Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci. Rep. 2017, 7, 46621. [Google Scholar] [CrossRef]
- Seder, R.A.; Chang, L.J.; Enama, M.E.; Zephir, K.L.; Sarwar, U.N.; Gordon, I.J.; Holman, L.A.; James, E.R.; Billingsley, P.F.; Gunasekera, A.; et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013, 341, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, R.; DeBot, M.; Porter, M.; Nikki, J.; Rein, L.; Spaccapelo, R.; Crisanti, A.; Wightman, P.D.; Ockenhouse, C.F.; Dutta, S. IgG2 antibodies against a clinical grade Plasmodium falciparum CSP vaccine antigen associate with protection against transgenic sporozoite challenge in mice. PLoS ONE 2014, 9, e111020. [Google Scholar]
- Illingworth, J.J.; Alanine, D.G.; Brown, R.; Marshall, J.M.; Bartlett, H.E.; Silk, S.E.; Labbe, G.M.; Quinkert, D.; Cho, J.S.; Wendler, J.P.; et al. Functional Comparison of Blood-Stage Plasmodium falciparum Malaria Vaccine Candidate Antigens. Front. Immunol. 2019, 10, 1254. [Google Scholar] [CrossRef]
- Payne, R.O.; Silk, S.E.; Elias, S.C.; Miura, K.; Diouf, A.; Galaway, F.; de Graaf, H.; Brendish, N.J.; Poulton, I.D.; Griffiths, O.J.; et al. Human vaccination against RH5 induces neutralizing antimalarial antibodies that inhibit RH5 invasion complex interactions. JCI Insight 2017, 2, e96381. [Google Scholar] [CrossRef]
- Kaslow, D.C.; Shiloach, J. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. Biotechnology 1994, 12, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Shimp, R.L., Jr.; Rowe, C.; Reiter, K.; Chen, B.; Nguyen, V.; Aebig, J.; Rausch, K.M.; Kumar, K.; Wu, Y.; Jin, A.J.; et al. Development of a Pfs25-EPA malaria transmission blocking vaccine as a chemically conjugated nanoparticle. Vaccine 2013, 31, 2954–2962. [Google Scholar] [CrossRef][Green Version]
- Chaturvedi, N.; Bharti, P.K.; Tiwari, A.; Singh, N. Strategies & recent development of transmission-blocking vaccines against Plasmodium falciparum. Indian J. Med. Res. 2016, 143, 696–711. [Google Scholar] [PubMed]
- Epstein, J.E.; Paolino, K.M.; Richie, T.L.; Sedegah, M.; Singer, A.; Ruben, A.J.; Chakravarty, S.; Stafford, A.; Ruck, R.C.; Eappen, A.G.; et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight 2017, 2, e89154. [Google Scholar] [CrossRef]
- Sissoko, M.S.; Healy, S.A.; Katile, A.; Omaswa, F.; Zaidi, I.; Gabriel, E.E.; Kamate, B.; Samake, Y.; Guindo, M.A.; Dolo, A.; et al. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: A randomised, double-blind phase 1 trial. Lancet Infect. Dis. 2017, 17, 498–509. [Google Scholar] [CrossRef]
- Jongo, S.A.; Shekalaghe, S.A.; Church, L.W.P.; Ruben, A.J.; Schindler, T.; Zenklusen, I.; Rutishauser, T.; Rothen, J.; Tumbo, A.; Mkindi, C.; et al. Safety, Immunogenicity, and Protective Efficacy against Controlled Human Malaria Infection of Plasmodium falciparum Sporozoite Vaccine in Tanzanian Adults. Am. J. Trop. Med. Hyg. 2018, 99, 338–349. [Google Scholar] [CrossRef]
- Gordon, D.M.; McGovern, T.W.; Krzych, U.; Cohen, J.C.; Schneider, I.; LaChance, R.; Heppner, D.G.; Yuan, G.; Hollingdale, M.; Slaoui, M.; et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J. Infect. Dis. 1995, 171, 1576–1585. [Google Scholar] [CrossRef]
- Garcon, N.; van Mechelen, M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev. Vaccines 2011, 10, 471–486. [Google Scholar] [CrossRef]
- Rts, S.C.T.P. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar]
- Rts, S.C.T.P. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 2011, 365, 1863–1875. [Google Scholar]
- White, M.T.; Bejon, P.; Olotu, A.; Griffin, J.T.; Riley, E.M.; Kester, K.E.; Ockenhouse, C.F.; Ghani, A.C. The relationship between RTS,S vaccine-induced antibodies, CD4(+) T cell responses and protection against Plasmodium falciparum infection. PLoS ONE 2013, 8, e61395. [Google Scholar] [CrossRef] [PubMed]
- White, M.T.; Bejon, P.; Olotu, A.; Griffin, J.T.; Bojang, K.; Lusingu, J.; Salim, N.; Abdulla, S.; Otsyula, N.; Agnandji, S.T. A combined analysis of immunogenicity, antibody kinetics and vaccine efficacy from phase 2 trials of the RTS,S malaria vaccine. BMC Med. 2014, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- White, M.T.; Verity, R.; Griffin, J.T.; Asante, K.P.; Owusu-Agyei, S.; Greenwood, B.; Drakeley, C.; Gesase, S.; Lusingu, J.; Ansong, D.; et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: Secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect. Dis. 2015, 15, 1450–1458. [Google Scholar] [CrossRef]
- Oyen, D.; Torres, J.L.; Wille-Reece, U.; Ockenhouse, C.F.; Emerling, D.; Glanville, J.; Volkmuth, W.; Flores-Garcia, Y.; Zavala, F.; Ward, A.B.; et al. Structural basis for antibody recognition of the NANP repeats in Plasmodium falciparum circumsporozoite protein. Proc. Natl. Acad. Sci. USA 2017, 114, E10438–E10445. [Google Scholar] [CrossRef] [PubMed]
- Dobano, C.; Sanz, H.; Sorgho, H.; Dosoo, D.; Mpina, M.; Ubillos, I.; Aguilar, R.; Ford, T.; Diez-Padrisa, N.; Williams, N.A.; et al. Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy. Nat. Commun. 2019, 10, 2174. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Ockenhouse, C.F.; Regules, J.A.; Dutta, S.; Wallqvist, A.; Jongert, E.; Waters, N.C.; Lemiale, F.; Bergmann-Leitner, E. The biological function of antibodies induced by the RTS,S/AS01 malaria vaccine candidate is determined by their fine specificity. Malar. J. 2016, 15, 301. [Google Scholar] [CrossRef]
- Regules, J.A.; Cicatelli, S.B.; Bennett, J.W.; Paolino, K.M.; Twomey, P.S.; Moon, J.E.; Kathcart, A.K.; Hauns, K.D.; Komisar, J.L.; Qabar, A.N.; et al. Fractional Third and Fourth Dose of RTS,S/AS01 Malaria Candidate Vaccine: A Phase 2a Controlled Human Malaria Parasite Infection and Immunogenicity Study. J. Infect. Dis. 2016, 214, 762–771. [Google Scholar] [CrossRef]
- Thompson, H.A.; Hogan, A.B.; Walker, P.G.T.; White, M.T.; Cunnington, A.J.; Ockenhouse, C.F.; Ghani, A.C. Modelling the roles of antibody titre and avidity in protection from Plasmodium falciparum malaria infection following RTS,S/AS01 vaccination. Vaccine 2020, 38, 7498–7507. [Google Scholar] [CrossRef]
- Kisalu, N.K.; Idris, A.H.; Weidle, C.; Flores-Garcia, Y.; Flynn, B.J.; Sack, B.K.; Murphy, S.; Schon, A.; Freire, E.; Francica, J.R.; et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat. Med. 2018, 24, 408–416. [Google Scholar] [CrossRef]
- Tan, J.; Sack, B.K.; Oyen, D.; Zenklusen, I.; Piccoli, L.; Barbieri, S.; Foglierini, M.; Fregni, C.S.; Marcandalli, J.; Jongo, S.; et al. A public antibody lineage that potently inhibits malaria infection through dual binding to the circumsporozoite protein. Nat. Med. 2018, 24, 401–407. [Google Scholar] [CrossRef]
- Oyen, D.; Torres, J.L.; Aoto, P.C.; Flores-Garcia, Y.; Binter, S.; Pholcharee, T.; Carroll, S.; Reponen, S.; Wash, R.; Liang, Q.; et al. Structure and mechanism of monoclonal antibody binding to the junctional epitope of Plasmodium falciparum circumsporozoite protein. PLoS Pathog. 2020, 16, e1008373. [Google Scholar] [CrossRef]
- Wang, L.T.; Pereira, L.S.; Flores-Garcia, Y.; O’Connor, J.; Flynn, B.J.; Schon, A.; Hurlburt, N.K.; Dillon, M.; Yang, A.S.P.; Fabra-Garcia, A.; et al. A Potent Anti-Malarial Human Monoclonal Antibody Targets Circumsporozoite Protein Minor Repeats and Neutralizes Sporozoites in the Liver. Immunity 2020, 53, 733–744. [Google Scholar] [CrossRef]
- Xu, K.; Acharya, P.; Kong, R.; Cheng, C.; Chuang, G.Y.; Liu, K.; Louder, M.K.; O’Dell, S.; Rawi, R.; Sastry, M.; et al. Epitope-based vaccine design yields fusion peptide-directed antibodies that neutralize diverse strains of HIV-1. Nat. Med. 2018, 24, 857–867. [Google Scholar] [CrossRef]
- Kong, R.; Duan, H.; Sheng, Z.; Xu, K.; Acharya, P.; Chen, X.; Cheng, C.; Dingens, A.S.; Gorman, J.; Sastry, M.; et al. Antibody Lineages with Vaccine-Induced Antigen-Binding Hotspots Develop Broad HIV Neutralization. Cell 2019, 178, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Xu, K.; Kong, R.; Chuang, G.Y.; Corrigan, A.R.; Geng, H.; Hill, K.R.; Jafari, A.J.; O’Dell, S.; Ou, L.; et al. Consistent elicitation of cross-clade HIV-neutralizing responses achieved in guinea pigs after fusion peptide priming by repetitive envelope trimer boosting. PLoS ONE 2019, 14, e0215163. [Google Scholar]
- Chuang, G.Y.; Lai, Y.T.; Boyington, J.C.; Cheng, C.; Geng, H.; Narpala, S.; Rawi, R.; Schmidt, S.D.; Tsybovsky, Y.; Verardi, R.; et al. Development of a 3Mut-Apex-Stabilized Envelope Trimer That Expands HIV-1 Neutralization Breadth When Used To Boost Fusion Peptide-Directed Vaccine-Elicited Responses. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Akahata, W.; Yang, Z.Y.; Andersen, H.; Sun, S.; Holdaway, H.A.; Kong, W.P.; Lewis, M.G.; Higgs, S.; Rossmann, M.G.; Rao, S.; et al. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat. Med. 2010, 16, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Coates, E.E.; Plummer, S.H.; Carter, C.A.; Berkowitz, N.; Conan-Cibotti, M.; Cox, J.H.; Beck, A.; O’Callahan, M.; Andrews, C.; et al. Effect of a Chikungunya Virus-Like Particle Vaccine on Safety and Tolerability Outcomes: A Randomized Clinical Trial. JAMA 2020, 323, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Urakami, A.; Sakurai, A.; Ishikawa, M.; Yap, M.L.; Flores-Garcia, Y.; Haseda, Y.; Aoshi, T.; Zavala, F.P.; Rossmann, M.G.; Kuno, S.; et al. Development of a Novel Virus-Like Particle Vaccine Platform That Mimics the Immature Form of Alphavirus. Clin. Vaccine Immunol. 2017, 24. [Google Scholar] [CrossRef]
- Tang, G.; Peng, L.; Baldwin, P.R.; Mann, D.S.; Jiang, W.; Rees, I.; Ludtke, S.J. EMAN2: An extensible image processing suite for electron microscopy. J. Struct. Biol. 2007, 157, 38–46. [Google Scholar] [CrossRef]
- Alving, C.R.; Peachman, K.K.; Matyas, G.R.; Rao, M.; Beck, Z. Army Liposome Formulation (ALF) family of vaccine adjuvants. Expert Rev. Vaccines 2020, 19, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Flores-Garcia, Y.; Herrera, S.M.; Jhun, H.; Perez-Ramos, D.W.; King, C.R.; Locke, E.; Raghunandan, R.; Zavala, F. Optimization of an in vivo model to study immunity to Plasmodium falciparum pre-erythrocytic stages. Malar. J. 2019, 18, 426. [Google Scholar] [CrossRef]
- Voss, J.E.; Vaney, M.C.; Duquerroy, S.; Vonrhein, C.; Girard-Blanc, C.; Crublet, E.; Thompson, A.; Bricogne, G.; Rey, F.A. Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature 2010, 468, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Cawlfield, A.; Genito, C.J.; Beck, Z.; Bergmann-Leitner, E.S.; Bitzer, A.A.; Soto, K.; Zou, X.; Hadiwidjojo, S.H.; Gerbasi, R.V.; Mullins, A.B.; et al. Safety, toxicity and immunogenicity of a malaria vaccine based on the circumsporozoite protein (FMP013) with the adjuvant army liposome formulation containing QS21 (ALFQ). Vaccine 2019, 37, 3793–3803. [Google Scholar] [CrossRef]
- Genito, C.J.; Beck, Z.; Phares, T.W.; Kalle, F.; Limbach, K.J.; Stefaniak, M.E.; Patterson, N.B.; Bergmann-Leitner, E.S.; Waters, N.C.; Matyas, G.R.; et al. Liposomes containing monophosphoryl lipid A and QS-21 serve as an effective adjuvant for soluble circumsporozoite protein malaria vaccine FMP013. Vaccine 2017, 35, 3865–3874. [Google Scholar] [CrossRef] [PubMed]
- Jelinkova, L.; Jhun, H.; Eaton, A.; Petrovsky, N.; Zavala, F.; Chackerian, B. An epitope-based malaria vaccine targeting the junctional region of circumsporozoite protein. NPJ Vaccines 2021, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Calle, J.M.; Mitchell, R.; Altszuler, R.; Othoro, C.; Nardin, E. Identification of a neutralizing epitope within minor repeat region of Plasmodium falciparum CS protein. NPJ Vaccines 2021, 6, 1–8. [Google Scholar] [CrossRef]
- Murugan, R.; Scally, S.W.; Costa, G.; Mustafa, G.; Thai, E.; Decker, T.; Bosch, A.; Prieto, K.; Levashina, E.A.; Julien, J.P.; et al. Evolution of protective human antibodies against Plasmodium falciparum circumsporozoite protein repeat motifs. Nat. Med. 2020, 26, 1135–1145. [Google Scholar] [CrossRef]
- Pholcharee, T.; Oyen, D.; Torres, J.L.; Flores-Garcia, Y.; Martin, G.M.; Gonzalez-Paez, G.E.; Emerling, D.; Volkmuth, W.; Locke, E.; King, C.R.; et al. Diverse Antibody Responses to Conserved Structural Motifs in Plasmodium falciparum Circumsporozoite Protein. J. Mol. Biol. 2020, 432, 1048–1063. [Google Scholar] [CrossRef]
- Langowski, M.D.; Khan, F.A.; Bitzer, A.A.; Genito, C.J.; Schrader, A.J.; Martin, M.L.; Soto, K.; Zou, X.; Hadiwidjojo, S.; Beck, Z.; et al. Optimization of a Plasmodium falciparum circumsporozoite protein repeat vaccine using the tobacco mosaic virus platform. Proc. Natl. Acad. Sci. USA 2020, 117, 3114–3122. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francica, J.R.; Shi, W.; Chuang, G.-Y.; Chen, S.J.; Da Silva Pereira, L.; Farney, S.K.; Flynn, B.J.; Ou, L.; Stephens, T.; Tsybovsky, Y.; et al. Design of Alphavirus Virus-Like Particles Presenting Circumsporozoite Junctional Epitopes That Elicit Protection against Malaria. Vaccines 2021, 9, 272. https://doi.org/10.3390/vaccines9030272
Francica JR, Shi W, Chuang G-Y, Chen SJ, Da Silva Pereira L, Farney SK, Flynn BJ, Ou L, Stephens T, Tsybovsky Y, et al. Design of Alphavirus Virus-Like Particles Presenting Circumsporozoite Junctional Epitopes That Elicit Protection against Malaria. Vaccines. 2021; 9(3):272. https://doi.org/10.3390/vaccines9030272
Chicago/Turabian StyleFrancica, Joseph R., Wei Shi, Gwo-Yu Chuang, Steven J. Chen, Lais Da Silva Pereira, S. Katie Farney, Barbara J. Flynn, Li Ou, Tyler Stephens, Yaroslav Tsybovsky, and et al. 2021. "Design of Alphavirus Virus-Like Particles Presenting Circumsporozoite Junctional Epitopes That Elicit Protection against Malaria" Vaccines 9, no. 3: 272. https://doi.org/10.3390/vaccines9030272
APA StyleFrancica, J. R., Shi, W., Chuang, G.-Y., Chen, S. J., Da Silva Pereira, L., Farney, S. K., Flynn, B. J., Ou, L., Stephens, T., Tsybovsky, Y., Wang, L. T., Anderson, A., Beck, Z., Dillon, M., Idris, A. H., Hurlburt, N., Liu, T., Zhang, B., Alving, C. R., ... Seder, R. A. (2021). Design of Alphavirus Virus-Like Particles Presenting Circumsporozoite Junctional Epitopes That Elicit Protection against Malaria. Vaccines, 9(3), 272. https://doi.org/10.3390/vaccines9030272





