No Such Thing as a Free-Rider? Understanding Drivers of Childhood and Adult Vaccination through a Multicountry Discrete Choice Experiment
Abstract
1. Introduction
2. Material and Methods
2.1. Selection of Study Populations
2.2. Survey Development and Sample Characteristics
2.3. DCE Design
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Health Topics–Immunization. Available online: https://www.who.int/topics/immunization/en/ (accessed on 8 August 2019).
- Godlee, F.; Smith, J.; Marcovitch, H. Wakefield’s article linking MMR vaccine and autism was fraudulent. BMJ 2011, 342, c7452. [Google Scholar] [CrossRef]
- Kata, A. A postmodern Pandora’s box: Anti-vaccination misinformation on the Internet. Vaccine 2010, 28, 1709–1716. [Google Scholar] [CrossRef]
- Ward, J.K.; Peretti-Watel, P.; Larson, H.J.; Raude, J.; Verger, P. Vaccine-criticism on the internet: New insights based on French-speaking websites. Vaccine 2015, 33, 1063–1070. [Google Scholar] [CrossRef]
- Larson, H.J.; de Figueiredo, A.; Xiahong, Z.; Schulz, W.S.; Verger, P.; Johnston, I.G.; Cook, R.A.; Jones, N.J. The state of vaccine confidence 2016: Global insights through a 67-country survey. EBioMedicine 2016, 12, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Hulsey, E.; Bland, T. Immune overload: Parental attitudes toward combination and single antigen vaccines. Vaccine 2015, 33, 2546–2550. [Google Scholar] [CrossRef]
- Ruijs, W.L.; Hautvast, J.L.; van der Velden, K.; de Vos, S.; Knippenberg, H.; Hulscher, M.E. Religious subgroups influencing vaccination coverage in the Dutch Bible belt: An ecological study. BMC Public Health 2011, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Spier, R.E. Perception of risk of vaccine adverse events: A historical perspective. Vaccine 2001, 20, S78–S84. [Google Scholar] [CrossRef]
- Poland, G.A.; Jacobson, R.M. The age-old struggle against the antivaccinationists. N. Engl. J. Med. 2011, 364, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Larson, H.J.; Cooper, L.Z.; Eskola, J.; Katz, S.L.; Ratzan, S. Addressing the vaccine confidence gap. Lancet 2011, 378, 526–535. [Google Scholar] [CrossRef]
- Gallup. Wellcome Global Monitor-First Wave Findings; Wellcome Trust: London, UK, 2019. [Google Scholar]
- Centers for Disease Control and Prevention. Measles, Mumps, and Rubella (MMR) Vaccine Safety. 2018. Available online: https://www.cdc.gov/vaccinesafety/vaccines/mmr-vaccine.html (accessed on 19 December 2019).
- European Centre for Disease Prevention and Control. Who is at Risk for Measles in the EU/EEA? Identifying Susceptible Groups to Close Immunity Gaps Towards Measles Elimination; ECDC: Stockholm, Sweden, 2019.
- Nelson, R. US measles outbreak concentrated among unvaccinated children. Lancet Infect. Dis. 2019, 19, 248. [Google Scholar] [CrossRef]
- Patel, M. Increase in measles cases? United States, January 1–April 26, 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 402–404. [Google Scholar] [CrossRef]
- Verelst, F.; Willem, L.; Beutels, P. Behavioural change models for infectious disease transmission: A systematic review (2010–2015). J. R. Soc. Interface. 2016, 13, 20160820. [Google Scholar] [CrossRef]
- Funk, S.; Salathé, M.; Jansen, V.A. Modelling the influence of human behaviour on the spread of infectious diseases: A review. J. R. Soc. Interface 2010, 7, 1247–1256. [Google Scholar] [CrossRef]
- Funk, S.; Bansal, S.; Bauch, C.T.; Eames, K.T.; Edmunds, W.J.; Galvani, A.P.; Klepac, P. Nine challenges in incorporating the dynamics of behaviour in infectious diseases models. Epidemics 2015, 10, 21–25. [Google Scholar] [CrossRef]
- Hall, J.; Kenny, P.; King, M.; Louviere, J.; Viney, R.; Yeoh, A. Using stated preference discrete choice modelling to evaluate the introduction of varicella vaccination. Health Econ. 2002, 11, 457–465. [Google Scholar] [CrossRef] [PubMed]
- De Bekker-Grob, E.W.; Hofman, R.; Donkers, B.; van Ballegooijen, M.; Helmerhorst, T.J.; Raat, H.; Korfage, I.J. Girls’ preferences for HPV vaccination: A discrete choice experiment. Vaccine 2010, 28, 6692–6697. [Google Scholar] [CrossRef] [PubMed]
- Sadique, M.Z.; Devlin, N.; Edmunds, W.J.; Parkin, D. The effect of perceived risks on the demand for vaccination: Results from a discrete choice experiment. PLoS ONE 2013, 8, e54149. [Google Scholar] [CrossRef] [PubMed]
- Determann, D.; Korfage, I.J.; Lambooij, M.S.; Bliemer, M.; Richardus, J.H.; Steyerberg, E.W.; de Bekker-Grob, E.W. Acceptance of vaccinations in pandemic outbreaks: A discrete choice experiment. PLoS ONE 2014, 9, e102505. [Google Scholar] [CrossRef]
- Verelst, F.; Willem, L.; Kessels, R.; Beutels, P. Individual decisions to vaccinate one’s child or oneself: A discrete choice experiment rejecting free-riding motives. Soc. Sci. Med. 2018, 207, 106–116. [Google Scholar] [CrossRef]
- Verelst, F.; Kessels, R.; Delva, W.; Beutels, P.; Willem, L. Drivers of vaccine decision-making in South Africa: A discrete choice experiment. Vaccine 2019, 37, 2079–2089. [Google Scholar] [CrossRef]
- Bishai, D.; Brice, R.; Girod, I.; Saleh, A.; Ehreth, J. Conjoint analysis of French and German parents’ willingness to pay for meningococcal vaccine. Pharmacoeconomics 2007, 25, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Gidengil, C.; Lieu, T.A.; Payne, K.; Rusinak, D.; Messonnier, M.; Prosser, L.A. Parental and societal values for the risks and benefits of childhood combination vaccines. Vaccine 2012, 30, 3445–3452. [Google Scholar] [CrossRef] [PubMed]
- de Bekker-Grob, E.W.; Ryan, M.; Gerard, K. Discrete choice experiments in health economics: A review of the literature. Health Econ. 2012, 21, 145–172. [Google Scholar] [CrossRef]
- Kessels, R.; Jones, B.; Goos, P.; Vandebroek, M. The usefulness of Bayesian optimal designs for discrete choice experiments. Appl. Stoch. Models Bus. Ind. 2011, 27, 173–188. [Google Scholar] [CrossRef]
- Oteng, B.; Marra, F.; Lynd, L.D.; Ogilvie, G.; Patrick, D.; Marra, C.A. Evaluating societal preferences for human papillomavirus vaccine and cervical smear test screening programme. Sex. Transm. Infect. 2011, 87, 52–57. [Google Scholar] [CrossRef] [PubMed]
- de Bekker-Grob, E.W.; Swait, J.D.; Kassahun, H.T.; Bliemer, M.C.; Jonker, M.F.; Veldwijk, J.; Cong, K.; Rose, J.M.; Donkers, B. Are healthcare choices predictable? The impact of discrete choice experiment designs and models. Value Health 2019, 22, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Hoogink, J.; Verelst, F.; Kessels, R.; van Hoek, A.J.; Timen, A.; Willem, L.; Beutels, P.; Wallinga, J.; de Wit, A. Preferential differences in vaccination decision-making for oneself or one’s child in the Netherlands: A discrete choice experiment. BMC Public Health 2020, 20, 828. [Google Scholar] [CrossRef] [PubMed]
- Peretti-Watel, P.; Verger, P.; Raude, J.; Constant, A.; Gautier, A.; Jestin, C.; Beck, F. Dramatic change in public attitudes towards vaccination during the 2009 influenza A (H1N1) pandemic in France. Eurosurveillance 2013, 18, 20623. [Google Scholar] [CrossRef]
- Amirthalingam, G.; Gupta, S.; Campbell, H. Pertussis immunisation and control in England and Wales, 1957 to 2012: A historical review. Eurosurveillance 2013, 18, 20587. [Google Scholar] [CrossRef]
- Vandermeulen, C.; Braeckman, T.; Roelants, M.; Blaizot, S.; Maertens, K.; Van Damme, P.; Theeten, H.; Hoppenbrouwers, K. Vaccinatiegraad in Vlaanderen in 2016. Vlaams infectieziektebulletin 2017, 2, 6–17. [Google Scholar]
- Tjalma, W.; Brasseur, C.; Top, G.; Ribesse, N.; Morales, I.; Van Damme, P. HPV vaccination coverage in the federal state of Belgium according to regions and their impact. Facts Views Vis. ObGyn. 2018, 10, 101. [Google Scholar]
- Bults, M.; Beaujean, D.J.; de Zwart, O.; Kok, G.; van Empelen, P.; van Steenbergen, J.E.; Richardus, J.H.; Voeten, H.A.C.M. Perceived risk, anxiety, and behavioural responses of the general public during the early phase of the InfluenzaA (H1N1) pandemic in the Netherlands: Results of three consecutive online surveys. BMC Public Health 2011, 11, 2. [Google Scholar] [CrossRef]
- Chew, L.D.; Bradley, K.A.; Boyko, E.J. Brief questions to identify patients with inadequate health literacy. Fam. Med. 2004, 36, 588–594. [Google Scholar] [PubMed]
- European Statistical System (ESS). 2011 Census Hub. 2011. Available online: https://ec.europa.eu/eurostat/web/population-and-housing-census/census-data/2011-census. (accessed on 12 February 2021).
- JMP®, Version Pro 14; SAS Institute Inc.: Cary, NC, USA, 2019.
- RStudio Team. RStudio: Integrated Development Environment for R. 2015. Available online: http://www.rstudio.com/ (accessed on 12 February 2021).
- Skea, Z.C.; Entwistle, V.A.; Watt, I.; Russell, E. ’Avoiding harm to others’ considerations in relation to parental measles, mumps and rubella (MMR) vaccination discussions–An analysis of an online chat forum. Soc. Sci. Med. 2008, 67, 1382–1390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hakim, H.; Gaur, A.H.; McCullers, J.A. Motivating factors for high rates of influenza vaccination among healthcare workers. Vaccine 2011, 29, 5963–5969. [Google Scholar] [CrossRef] [PubMed]
- Shim, E.; Chapman, G.B.; Townsend, J.P.; Galvani, A.P. The influence of altruism on influenza vaccination decisions. J. R. Soc. Interface 2012, 9, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Vietri, J.T.; Li, M.; Galvani, A.P.; Chapman, G.B. Vaccinating to help ourselves and others. Med. Decis. Mak. 2012, 32, 447–458. [Google Scholar] [CrossRef]
- Leask, J.; Kinnersley, P.; Jackson, C.; Cheater, F.; Bedford, H.; Rowles, G. Communicating with parents about vaccination: A framework for health professionals. BMC Pediatrics 2012, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Man, K.K.; Ip, P.; Kwan, M.; McGhee, S.M. Mothers’ preferences and willingness to pay for human papillomavirus vaccination for their daughters: A discrete choice experiment in Hong Kong. Value Health 2018, 21, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Poulos, C.; Curran, D.; Anastassopoulou, A.; De Moerlooze, L. German travelers’ preferences for travel vaccines assessed by a discrete choice experiment. Vaccine 2018, 36, 969–978. [Google Scholar] [CrossRef]
- Lefevere, E.; Hens, N.; De Smet, F.; Beutels, P. The impact of non-financial and financial encouragements on participation in non school-based human papillomavirus vaccination: A retrospective cohort study. Eur. J. Health Econ. 2016, 17, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Tafforeau, J. Vaccinatie. In Gezondheidsenquête 2013. Rapport 5: Preventie; Demarest, S., Charafeddine, R., Eds.; WIV-ISP: Brussel, Belgium, 2015. [Google Scholar]
- Kuylen, E.; Willem, L.; Hens, N.; Broeckhove, J. Future Ramifications of Age-Dependent Immunity Levels for Measles: Explorations in an Individual-Based Model. In International Conference on Computational Science; Springer: Cham, Switzerland, 2019; pp. 456–467. [Google Scholar]
- Funk, S.; Camacho, A.; Kucharski, A.J.; Eggo, R.M.; Edmunds, W.J. Real-time forecasting of infectious disease dynamics with a stochastic semi-mechanistic model. Epidemics 2018, 22, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Trentini, F.; Poletti, P.; Merler, S.; Melegaro, A. Measles immunity gaps and the progress towards elimination: A multi-country modelling analysis. Lancet Infect. Dis. 2017, 17, 1089–1097. [Google Scholar] [CrossRef]
- World Health Organization. Global Vaccine Safety. Adverse events Following Immunization (AEFI). 2019. Available online: https://www.who.int/vaccine_safety/initiative/detection/AEFI/en/ (accessed on 3 August 2020).
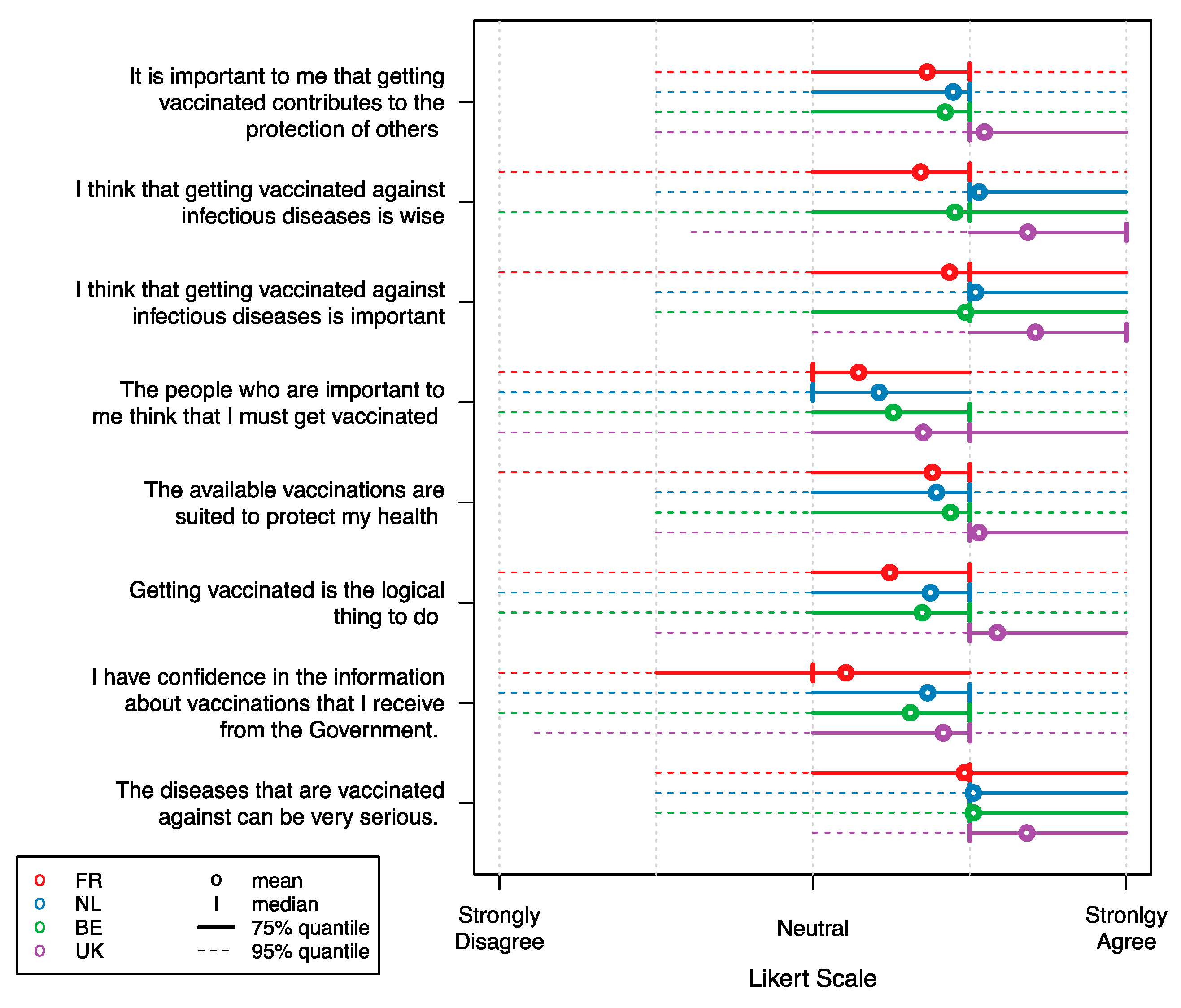
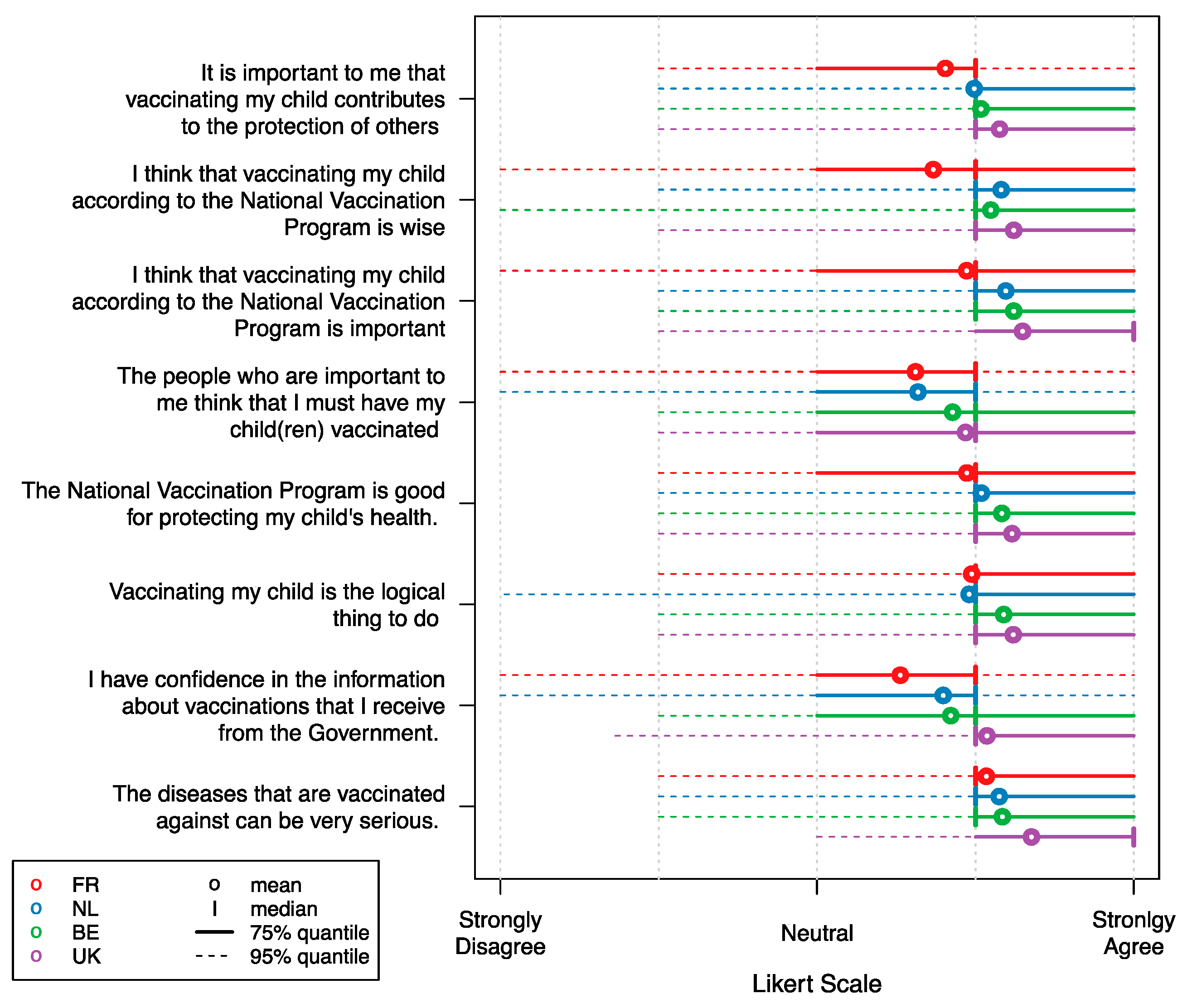
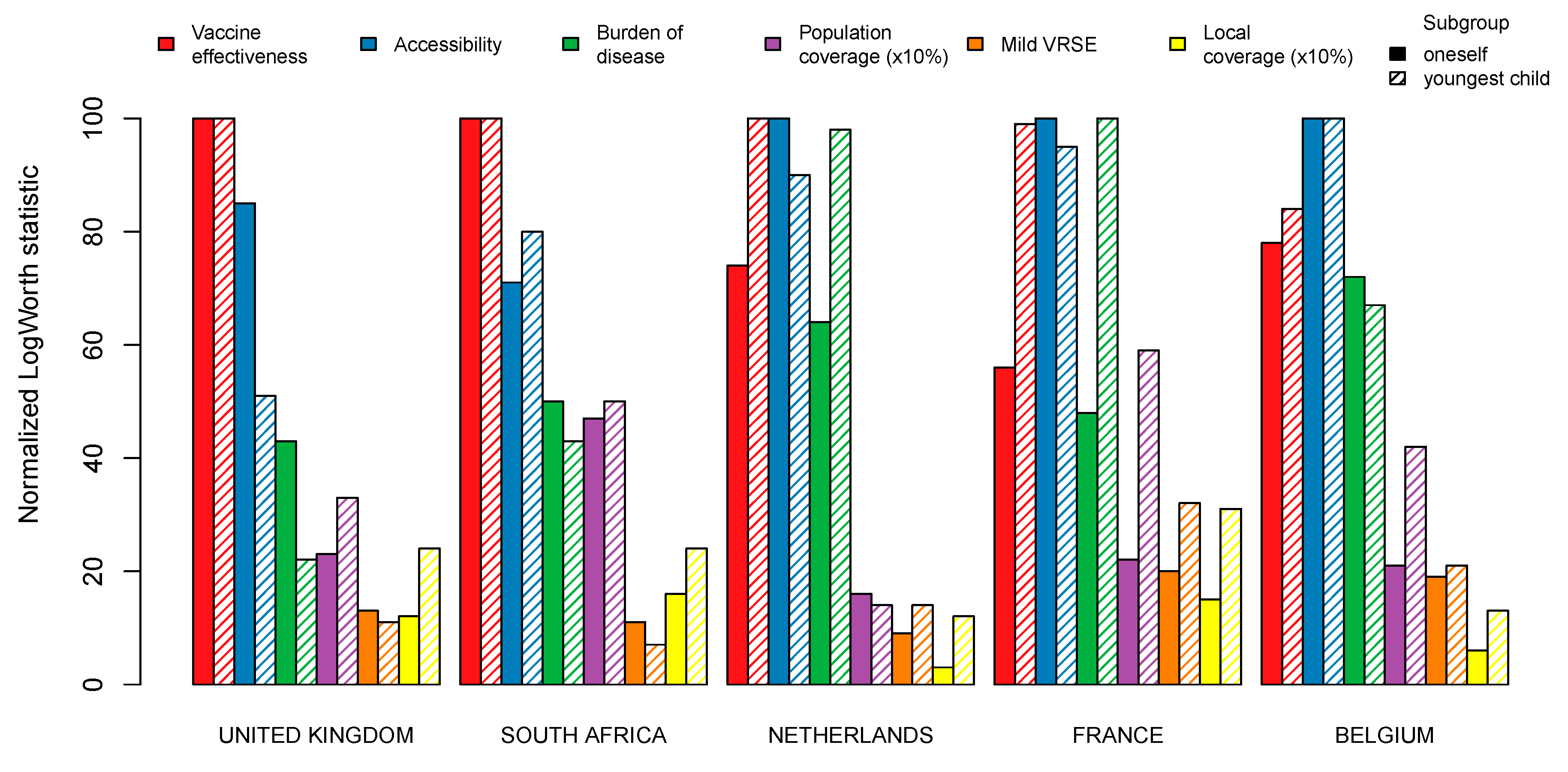
| Characteristic | Belgium | United Kingdom | France | |||
|---|---|---|---|---|---|---|
| Sample (%) | Population (%) | Sample (%) | Population (%) | Sample (%) | Population (%) | |
| Gender | ||||||
| Male | 50.2 | 49.1 | 45.8 | 49.1 | 40.7 | 48.4 |
| Female | 49.8 | 50.9 | 54.2 | 50.9 | 59.3 | 51.6 |
| Age group * | ||||||
| 18–34 | 26.6 | 22.9 | 24.8 | 24.9 | 27.1 | 23.5 |
| 35–49 | 26.4 | 34.7 | 36.4 | 34.7 | 39.6 | 34.2 |
| 50–65 | 25.4 | 24.0 | 26.9 | 22.7 | 24.6 | 24.2 |
| 66–85 | 21.6 | 18.4 | 12.0 | 17.7 | 8.8 | 18.1 |
| Educational attainment | ||||||
| Primary education (ISCED 1) or lower | 8.3 | 14.6 | <1 | <1 | 1.1 | 17.5 |
| Secondary education (ISCED 2 + 3) | 55.6 | 49.6 | 58.4 | 70.0 | 72.2 | 58.3 |
| Post-secondary or (post-)university education (ISCED 4 or higher) | 33.7 | 26.3 | 39.3 | 30.0 | 25.8 | 24.2 |
| Other | 2.4 | 9.5 | 1.8 | <1 | <1 | <1 |
| NUTS † 1 region | ||||||
| Belgium | ||||||
| Flanders | 57.4 | 57.5 | ||||
| Walloon region | 30.2 | 32.2 | ||||
| Brussels Capital Region | 12.3 | 10.3 | ||||
| United Kingdom | ||||||
| North East | 5.4 | 4.1 | ||||
| North West | 10.0 | 11.2 | ||||
| Yorkshire and the Humber | 8.6 | 8.4 | ||||
| East Midlands | 6.6 | 7.2 | ||||
| West Midlands | 9.7 | 8.9 | ||||
| East of England | 8.9 | 9.3 | ||||
| London | 11.2 | 12.9 | ||||
| South East | 12.6 | 13.7 | ||||
| South West | 8.3 | 8.4 | ||||
| Wales | 6.3 | 4.8 | ||||
| Scotland | 8.6 | 8.4 | ||||
| Northern Ireland | 3.9 | 2.9 | ||||
| France | ||||||
| Région parisienne | 16.3 | 18.3 | ||||
| Bassin parisien | 22.1 | 16.6 | ||||
| Nord | 5.6 | 6.2 | ||||
| Est | 10.8 | 8.3 | ||||
| Ouest | 12.9 | 13.2 | ||||
| Sud-Ouest | 11.3 | 10.6 | ||||
| Centre-Est | 10.0 | 11.8 | ||||
| Méditerranée | 10.8 | 12.2 | ||||
| Départements d’Outre Mer | 0.2 | 2.9 | ||||
| Sample size | N = 1602 | N = 1600 | N = 1600 | |||
| ‘Oneself’ | N = 1001 | N = 850 | N = 850 | |||
| ‘Youngest child’ | N = 601 | N = 750 | N = 750 | |||
| Attribute | Level Description |
|---|---|
| 1. Vaccine effectiveness | (a) Protects 50% of vaccinated persons (b) Protects 90% of vaccinated persons |
| 2. Burden of disease | (a) The disease, against which the vaccine protects is rare and often mild: hospitalization is exceptional and the disease is not life-threatening (b) The disease, against which the vaccine protects is rare and often severe: often with hospitalization and the disease is life-threatening (c) The disease, against which the vaccine protects is common and often mild: hospitalization is exceptional and the disease is not life-threatening (d) The disease, against which the vaccine protects is common and often severe: often with hospitalization and the disease is life-threatening |
| 3. Vaccine related side-effects | (a) Mild side-effects commonly occur and severe side-effects are highly unlikely (b) Mild side-effects rarely occur and severe side-effects are highly unlikely |
| 4. Accessibility | (a) The vaccine is provided for free and is directly available at the vaccinator (GP, well-baby clinic, school or occupational physician) (b) The vaccine is not reimbursed and is only available with a prescription |
| 5. Local coverage | (a) 30% of your acquaintances (friends and family) is vaccinated (b) 60% of your acquaintances (friends and family) is vaccinated (c) 90% of your acquaintances (friends and family) is vaccinated |
| 6. Population coverage | (a) 30% of the population in general is vaccinated (b) 60% of the population in general is vaccinated (c) 90% of the population in general is vaccinated |
| Term | Mean Estimate (Std Dev; Subject Std Dev) | LR Chi-Square | DF | p-Value |
|---|---|---|---|---|
| ‘Oneself’ Model | ||||
| Accessibility | ||||
| Copayment & prescription | −0.403 (0.020; 0.334) | 326.606 | 1 | <0.0001 |
| Free & accessible | 0.403 (0.020; 0.316) | |||
| Vaccine Effectiveness | ||||
| 50% | −0.465 (0.024; 0.244) | 252.171 | 1 | <0.0001 |
| 90% | 0.465 (0.025; 0.210) | |||
| Burden of Disease | ||||
| Rare & mild | −0.436 (0.045; 0.615) | 243.682 | 3 | <0.0001 |
| Common & mild | −0.481 (0.047; 0.361) | |||
| Rare & severe | 0.324 (0.037; 0.128) | |||
| Common & severe | 0.593 (0.043; 0.174) | |||
| Population Coverage (×10%) | 0.081 (0.008; 0.099) | 65.749 | 1 | <0.0001 |
| Mild VRSE | ||||
| Common | −0.184 (0.019; 0.098) | 57.931 | 1 | <0.0001 |
| Rare | 0.184 (0.020; 0.091) | |||
| Local coverage (×10%) | 0.043 (0.008; 0.079) | 17.977 | 1 | <0.0001 |
| ‘Youngest child’ Model | ||||
| Accessibility | ||||
| Copayment & prescription | −0.472 (0.031; 0.346) | 228.127 | 1 | <0.0001 |
| Free & accessible | 0.472 (0.029; 0.337) | |||
| Vaccine Effectiveness | ||||
| 50% | −0.571 (0.037; 0.245) | 191.508 | 1 | <0.0001 |
| 90% | 0.571 (0.039; 0.226) | |||
| Burden of Disease | ||||
| Rare & mild | −0.370 (0.058; 0.463) | 161.86 | 3 | <0.0001 |
| Common & mild | −0.613 (0.062; 0.418) | |||
| Rare & severe | 0.307 (0.056; 0.284) | |||
| Common & severe | 0.676 (0.061; 0.348) | |||
| Population Coverage (× 10%) | 0.128 (0.012; 0.126) | 93.449 | 1 | <0.0001 |
| Mild VRSE | ||||
| Common | −0.234 (0.028; 0.137) | 45.28 | 1 | <0.0001 |
| Rare | 0.234 (0.031; 0.129) | |||
| Local Coverage (× 10%) | 0.071 (0.013; 0.123) | 27.429 | 1 | <0.0001 |
| Term | Mean Estimate (Std Dev; Subject Std Dev) | LR Chi-Square | DF | p-Value |
|---|---|---|---|---|
| ‘Oneself’ Model | ||||
| Vaccine Effectiveness | ||||
| 50% | −0.683 (0.035; 0.275) | 425.353 | 1 | <0.0001 |
| 90% | 0.683 (0.031; 0.277) | |||
| Accessibility | ||||
| Copayment & prescription | −0.486 (0.023; 0.316) | 360.26 | 1 | <0.0001 |
| Free & accessible | 0.486 (0.027; 0.292) | |||
| Burden of Disease | ||||
| Rare & mild | −0.517 (0.049; 0.277) | 189.172 | 3 | <0.0001 |
| Common & mild | −0.351 (0.051; 0.430) | |||
| Rare & severe | 0.307 (0.045; 0.206) | |||
| Common & severe | 0.561 (0.051; 0.239) | |||
| Population Coverage (×10%) | 0.096 (0.010; 0.118) | 94.33 | 1 | <0.0001 |
| Mild VRSE | ||||
| Common | −0.180 (0.024; 0.124) | 50.29 | 1 | <0.0001 |
| Rare | 0.180 (0.025; 0.123) | |||
| Local Coverage (×10%) | 0.080 (0.010; 0.078) | 47.291 | 1 | <0.0001 |
| ‘Youngest child’ Model | ||||
| Vaccine Effectiveness | ||||
| 50% | −0.591 (0.033; 0.243) | 297.13 | 1 | <0.0001 |
| 90% | 0.591 (0.033; 0.246) | |||
| Accessibility | ||||
| Copayment & prescription | −0.309 (0.024; 0.233) | 149.559 | 1 | <0.0001 |
| Free & accessible | 0.309 (0.024; 0.218) | |||
| Population Coverage (×10%) | 0.107 (0.009; 0.101) | 94.979 | 1 | <0.0001 |
| Local Coverage (×10%) | 0.097 (0.010; 0.094) | 67.461 | 1 | <0.0001 |
| Burden of Disease | ||||
| Rare & mild | −0.198 (0.055; 0.266) | 70.146 | 3 | <0.0001 |
| Common & mild | −0.344 (0.042; 0.305) | |||
| Rare & severe | 0.187 (0.045; 0.178) | |||
| Common & severe | 0.355 (0.053; 0.216) | |||
| Mild VRSE | ||||
| Common | −0.143 (0.026; 0.087) | 30.732 | 1 | <0.0001 |
| Rare | 0.143 (0.026; 0.085) | |||
| Term | Mean Estimate (Std Dev; Subject Std Dev) | LR Chi-Square | DF | p-Value |
|---|---|---|---|---|
| ‘Oneself’ Model | ||||
| Accessibility | ||||
| Copayment & prescription | −0.389 (0.026; 0.410) | 238.254 | 1 | <0.0001 |
| Free & accessible | 0.389 (0.025; 0.383) | |||
| Vaccine Effectiveness | ||||
| 50% | −0.375 (0.030; 0.222) | 131.38 | 1 | <0.0001 |
| 90% | 0.375 (0.032; 0.218) | |||
| Burden of Disease | ||||
| Rare & mild | −0.364 (0.052; 0.318) | 122.873 | 3 | <0.0001 |
| Common & mild | −0.358 (0.042; 0.416) | |||
| Rare & severe | 0.273 (0.048; 0.224) | |||
| Common & severe | 0.449 (0.053; 0.187) | |||
| Population Coverage (×10%) | 0.079 (0.010; 0.131) | 48.157 | 1 | <0.0001 |
| Mild VRSE | ||||
| Common | −0.164 (0.027; 0.092) | 44.45 | 1 | <0.0001 |
| Rare | 0.164 (0.025; 0.093) | |||
| Local Coverage (×10%) | 0.064 (0.010; 0.093) | 33.48 | 1 | <0.0001 |
| ‘Youngest child’ Model | ||||
| Burden of Disease | ||||
| Rare & mild | −0.369 (0.048; 0.300) | 163.809 | 3 | <0.0001 |
| Common & mild | −0.474 (0.051; 0.323) | |||
| Rare & severe | 0.331 (0.049; 0.202) | |||
| Common & severe | 0.512 (0.048; 0.190) | |||
| Vaccine effectiveness | ||||
| 50% | −0.430 (0.029; 0.231) | 152.182 | 1 | <0.0001 |
| 90% | 0.430 (0.034; 0.237) | |||
| Accessibility | ||||
| Copayment & prescription | −0.314 (0.023; 0.278) | 144.967 | 1 | <0.0001 |
| Free & accessible | 0.314 (0.025; 0.260) | |||
| Population Coverage (×10%) | 0.108 (0.010; 0.135) | 88.489 | 1 | <0.0001 |
| Mild VRSE | ||||
| Common | −0.180 (0.026; 0.098) | 46.913 | 1 | <0.0001 |
| Rare | 0.180 (0.022; 0.095) | |||
| Local Coverage (×10%) | 0.078 (0.010; 0.086) | 44.981 | 1 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verelst, F.; Kessels, R.; Willem, L.; Beutels, P. No Such Thing as a Free-Rider? Understanding Drivers of Childhood and Adult Vaccination through a Multicountry Discrete Choice Experiment. Vaccines 2021, 9, 264. https://doi.org/10.3390/vaccines9030264
Verelst F, Kessels R, Willem L, Beutels P. No Such Thing as a Free-Rider? Understanding Drivers of Childhood and Adult Vaccination through a Multicountry Discrete Choice Experiment. Vaccines. 2021; 9(3):264. https://doi.org/10.3390/vaccines9030264
Chicago/Turabian StyleVerelst, Frederik, Roselinde Kessels, Lander Willem, and Philippe Beutels. 2021. "No Such Thing as a Free-Rider? Understanding Drivers of Childhood and Adult Vaccination through a Multicountry Discrete Choice Experiment" Vaccines 9, no. 3: 264. https://doi.org/10.3390/vaccines9030264
APA StyleVerelst, F., Kessels, R., Willem, L., & Beutels, P. (2021). No Such Thing as a Free-Rider? Understanding Drivers of Childhood and Adult Vaccination through a Multicountry Discrete Choice Experiment. Vaccines, 9(3), 264. https://doi.org/10.3390/vaccines9030264







