Simultaneous CD8+ T-Cell Immune Response against SARS-Cov-2 S, M, and N Induced by Endogenously Engineered Extracellular Vesicles in Both Spleen and Lungs
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Vector Synthesis
2.2. Cell Cultures and Transfection
2.3. EV Isolation
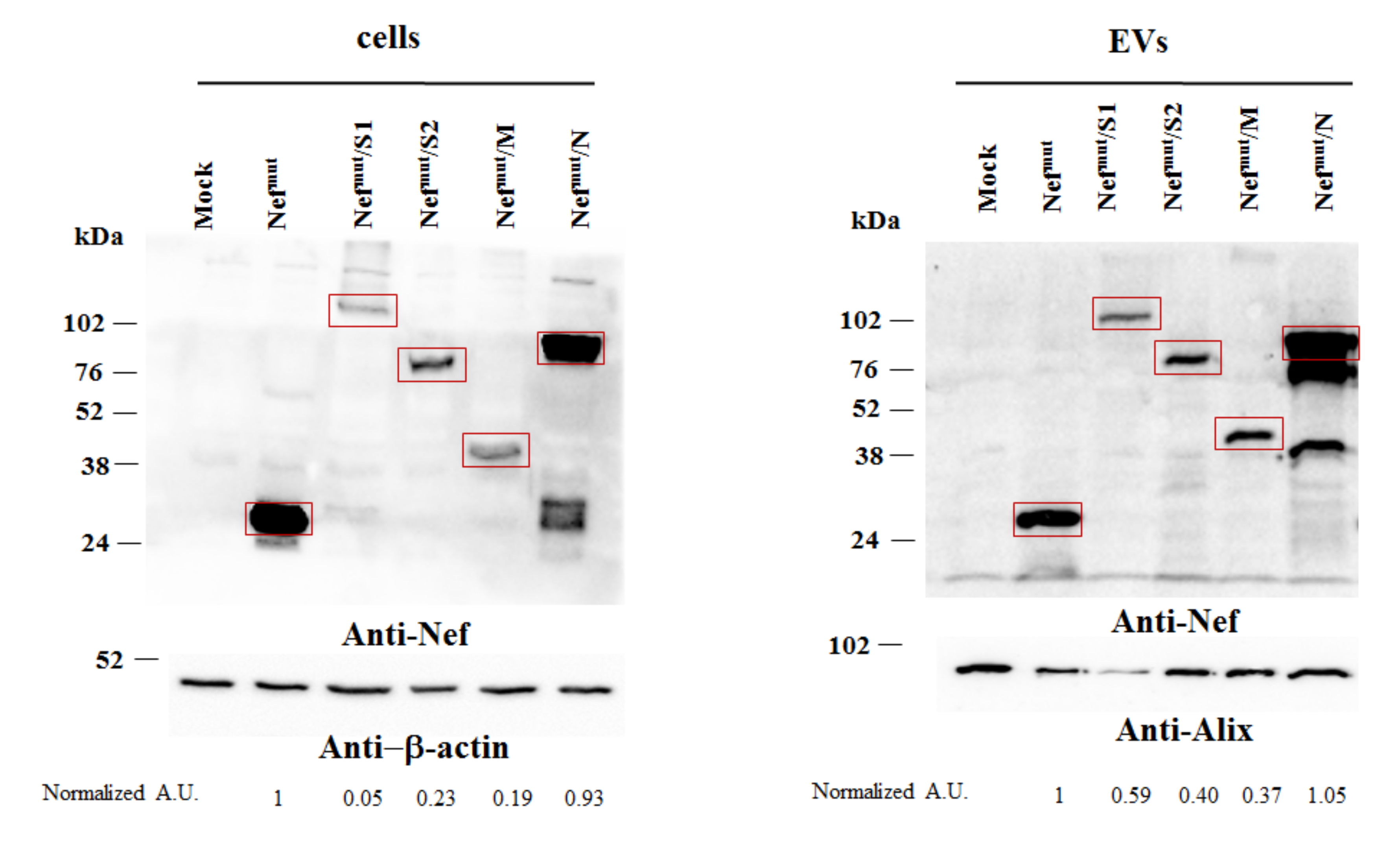
2.4. Western Blot Analysis
2.5. Mice Immunization
2.6. Cell Isolation from Immunized Mice
2.7. IFN-γ EliSpot Analysis
2.8. Cell Staining
2.9. Statistical Analysis
3. Results
3.1. Construction of Vectors Expressing Nefmut Fused with SARS-Cov-2 Antigens
3.2. Uploading of Nefmut-Based Products of Fusion with SARS-Cov-2 Antigens in Evs
3.3. Detection of Virus-Specific CD8+ T Cells in Spleens from Mice Injected with Vectors Expressing Nefmut Fused with Each SARS-Cov-2 Antigen
3.4. Detection of SARS-Cov-2-Specific CD8+ T Lymphocytes in Cells from Bronchoalveolar Lavages of Immunized Mice
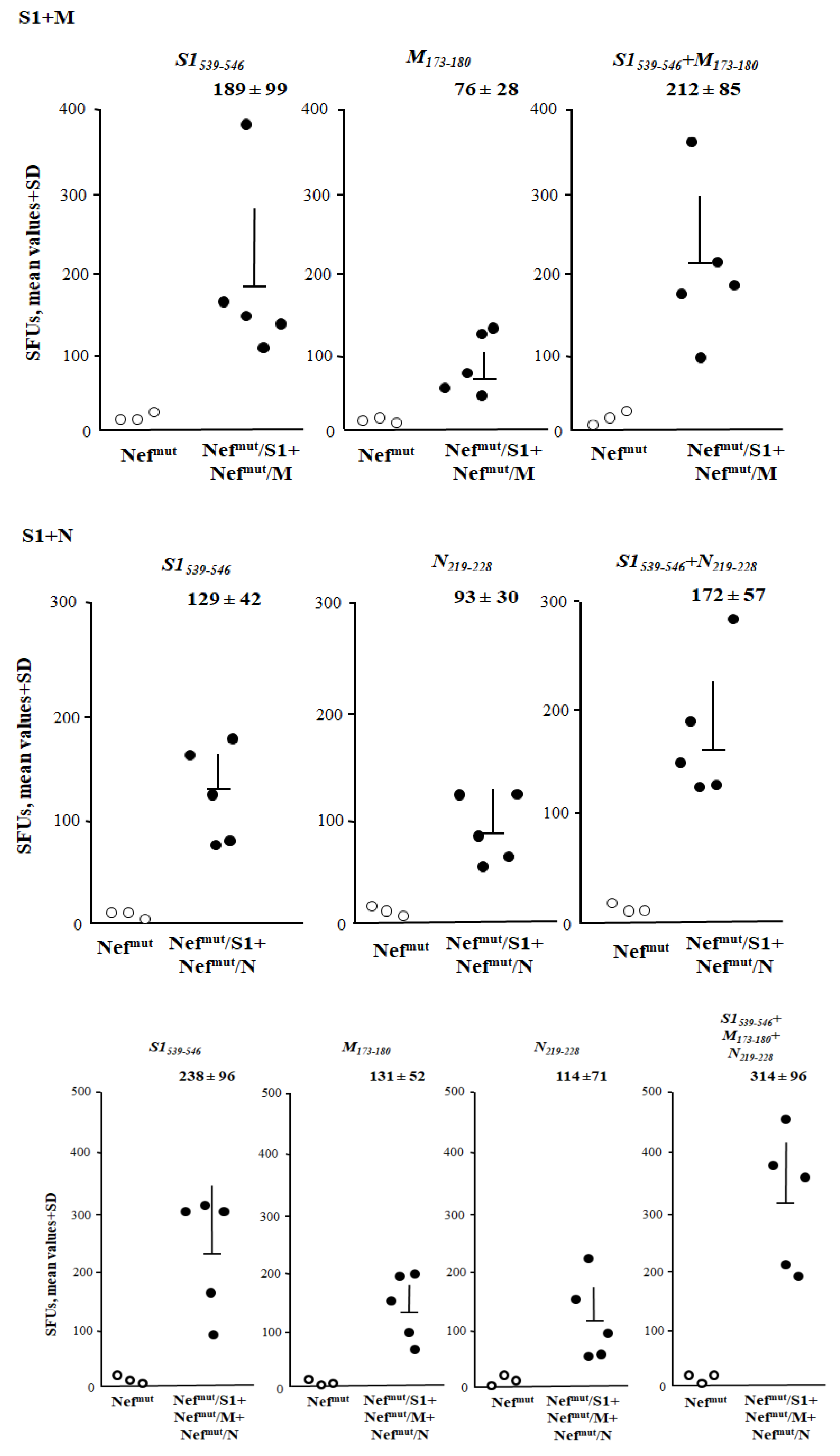
3.5. Additive Immunogenic Effect in Mice Co-Injected with DNA Vectors Expressing Diverse SARS-Cov-2–Based Fusion Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Qun, L.; Xuhua, G.; Peng, W.; Xiaoye, W.; Lei, Z.; Yeqing, T.; Ruiqi, R.; et al. Early transmission dynamics in Wuhan, China, of Novel Coronavirus—Infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S. A Familial Clusterof Pneumonia Associated with the 2019 Novel Coronavirus Indicating Person-to-PersonTransmission: A Study of a Family Cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Johns Hopkins Coronavirus Resource Center. Global Map. Available online: https://coronavirus.jhu.edu/map.html (accessed on 6 October 2020).
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Yuchun, N.; Guangwen, W.; Xuanling, S.; Hong, Z.; Yan, Q.; Zhongping, H.; Wei, W.; Gewei, L.; Xiaolei, Y.; Yin, X.; et al. Neutralizing Antibodies in Patients with Severe Acute Respiratory Syndrome-Associated Coronavirus Infection. J. Infect. Dis. 2004, 190, 1119–1126. [Google Scholar] [CrossRef]
- Liu, W.; Fontanet, A.; Zhang, P.; Zhan, L.; Xin, Z.; Baril, L.; Tang, F.; Lv, H.; Cao, W. Two-Year Prospective Study of the Humoral Immune Response of Patients with Severe Acute Respiratory Syndrome. J. Infect. Dis. 2006, 193, 792–795. [Google Scholar] [CrossRef]
- Mo, H.; Zeng, G.; Ren, X.; Li, H.; Ke, C.; Tan, Y.; Cai, C.; Lai, K.; Chen, R.; Chan-Yeung, M.; et al. Longitudinal Profile of Antibodies against SARS-Coronavirus in SARS Patientsand Their Clinical Significance. Respirology 2006, 11, 49–53. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Wong, B.H.L.; Chan, K.-H.; Chu, C.-M.; Tsoi, H.-W.; Huang, Y.; Peiris, J.S.M.; Yuen, K.-Y. Longitudinal Profile of Immunoglobulin G (IgG), IgM, and IgA Antibodies against the Severe Acute Respiratory Syndrome (SARS) Coronavirus Nucleocapsid Protein in Patients with Pneumonia Due to the SARS Coronavirus. Clin. Diagn. Lab. Immunol. 2004, 11, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; Wang, J.-T.; Huang, L.-M.; Chen, Y.-C.; Fang, C.-T.; Sheng, W.-H.; Wang, J.-L.; Yu, C.-J.; Yang, P.-C. Longitudinal Analysis of Severe Acute Respiratory Syndrome(SARS) Coronavirus-Specific Antibody in SARS Patients. Clin. Diagn. Lab. Immunol. 2005, 12, 1455–1457. [Google Scholar] [CrossRef]
- Temperton, N.J.; Chan, P.K.; Simmons, G.; Zambon, M.C.; Tedder, R.S.; Takeuchi, Y.; Weiss, R.A. Longitudinally Profiling Neutralizing Antibody Response to SARS Coronavirus with Pseudotypes. Emerg. Infect. Dis. 2005, 11, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-P.; Wang, N.-C.; Chang, Y.-H.; Tian, X.-Y.; Na, D.-Y.; Zhang, L.-Y.; Zheng, L.; Lan, T.; Wang, L.-F.; Liang, G.-D. Duration of Antibody Responses after Severe Acute Respiratory Syndrome. Emerg. Infect. Dis. 2007, 13, 1562–1564. [Google Scholar] [CrossRef]
- Tang, F.; Quan, Y.; Xin, Z.-T.; Wrammert, J.; Ma, M.-J.; Lv, H.; Wang, T.-B.; Yang, H.; Richardus, J.H.; Liu, W.; et al. Lack of Peripheral Memory B Cell Responses in Recovered Patients with Severe Acute Respiratory Syndrome: A Six-Year Follow-Up Study. J. Immunol. 2011, 186, 7264–7268. [Google Scholar] [CrossRef]
- Peng, H.; Yang, L.-T.; Wang, L.-Y.; Li, J.; Huang, J.; Lu, Z.-Q.; Koup, R.A.; Bailer, R.T.; Wu, C.-Y. Long-lived memory T lymphocyte responses against SARS coronavirus nucleocapsid protein in SARS-recovered patients. Virology 2006, 351, 466–475. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Huang, Z.-T.; Li, L.; Wu, M.-H.; Yu, T.; Koup, R.A.; Bailer, R.T.; Wu, C.-Y. Characterization of SARS-CoV-Specific Memory T Cells from Recovered Individuals 4Years after Infection. Arch. Virol. 2009, 154, 1093–1099. [Google Scholar] [CrossRef]
- Ng, O.-W.; Chia, A.; Tan, A.T.; Jadi, R.S.; Leong, H.N.; Bertoletti, A.; Tan, Y.-J. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine 2016, 34, 2008–2014. [Google Scholar] [CrossRef]
- Da Guan, W.; Mok, C.K.P.; Chen, Z.L.; Feng, L.Q.; Li, Z.T.; Huang, J.C.; Ke, C.W.; Deng, X.; Ling, Y.; Wu, S.G.; et al. Characteristics of Traveler with Middle East Respiratory Syndrome, China, 2015. Emerg. Infect. Dis. 2015, 21, 2278–2280. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-L.J.; Chia, A.; Chang, C.X.L.; Leong, H.N.; Ling, K.L.; Grotenbreg, G.M.; Gehring, A.J.; Tan, Y.J.; Bertoletti, A. Engineering T Cells Specific for a Dominant SevereAcute Respiratory Syndrome Coronavirus CD8 T Cell Epitope. J. Virol. 2011, 85, 10464–10471. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501. [Google Scholar] [CrossRef]
- Ferretti, A.P.; Kula, T.; Wang, Y.; Nguyen, D.M.; Weinheimer, A.; Dunlap, G.S.; Xu, Q.; Nabilsi, N.; Perullo, C.R.; Cristofaro, A.W.; et al. Unbiased Screens Show CD8+ T Cells of COVID-19 Patients Recognize Shared Epitopes in SARS-CoV-2 that Largely Reside outside the Spike Protein. Immunity 2020, 53, 1095–1107. [Google Scholar] [CrossRef]
- Ni, L.; Ye, F.; Cheng, M.-L.; Feng, Y.; Deng, Y.-Q.; Zhao, H.; Wei, P.; Ge, J.; Gou, M.; Li, X.; et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 2020, 52, 971–977. [Google Scholar] [CrossRef]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingleday, M.; Kruse, B.; Fauchere, F.; et al. Presence of SARS-CoV-2 Reactive T Cells inCOVID-19 Patients and Healthy Donors. medRxiv 2020. [Google Scholar] [CrossRef]
- Weiskopf, D.; Schmitz, K.S.; Raadsen, M.P.; Grifoni, A.; Okba, N.M.A.; Endeman, H.; Akker, J.P.C.V.D.; Molenkamp, R.; Koopmans, M.P.G.; Van Gorp, E.C.M.; et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020, 5, eabd2071. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Rome, S.; Forterre, A.; Mizgier, M.L.; Bouzakri, K. Skeletal Muscle-Released Extracellular Vesicles: State of the Art. Front. Physiol. 2019, 10, 929. [Google Scholar] [CrossRef]
- Li, J.; Liu, K.; Liu, Y.; Xu, Y.; Zhang, F.; Yang, H.; Liu, J.; Pan, T.; Chen, J.; Wu, M.; et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat. Immunol. 2013, 14, 793–803. [Google Scholar] [CrossRef]
- Théry, C.; Duban, L.; Segura, E.; Véron, P.; Lantz, O.; Amigorena, S. Indirect activation of naïve CD4+ T cells by dendritic cell–derived exosomes. Nat. Immunol. 2002, 3, 1156–1162. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Muratori, C.; Cavallin, L.E.; Krätzel, K.; Tinari, A.; De Milito, A.; Fais, S.; D’Aloja, P.; Federico, M.; Vullo, V.; Fomina, A.; et al. Massive Secretion by T Cells Is Caused by HIV Nef in Infected Cells and by Nef Transfer to Bystander Cells. Cell Host Microbe 2009, 6, 218–230. [Google Scholar] [CrossRef]
- Anticoli, S.; Manfredi, F.; Chiozzini, C.; Arenaccio, C.; Olivetta, E.; Ferrantelli, F.; Capocefalo, A.; Falcone, E.; Ruggieri, A.; Federico, M. An Exosome-Based Vaccine Platform Imparts Cytotoxic T Lymphocyte Immunity against Viral Antigens. Biotechnol. J. 2018, 13, e1700443. [Google Scholar] [CrossRef]
- Anticoli, S.; Aricò, E.; Arenaccio, C.; Manfredi, F.; Chiozzini, C.; Olivetta, E.; Ferrantelli, F.; Lattanzi, L.; D’Urso, M.T.; Proietti, E.; et al. Engineered exosomes emerging from muscle cells break immune tolerance to HER2 in transgenic mice and induce anti-gen-specific CTLs upon challenge by human dendritic cells. J. Mol. Med. 2018, 96, 211–221. [Google Scholar] [CrossRef]
- D’Aloja, P.; Santarcangelo, A.C.; Arold, S.; Baur, A.; Federico, M. Genetic and FunctionalAnalysis of the Human Immunodeficiency Virus (HIV) Type 1-Inhibiting F12-HIVnefAllele. J. Gen. Virol. 2001, 82, 2735–2745. [Google Scholar] [CrossRef]
- Green, L.A.; Liu, Y.; He, J.J. Inhibition of HIV-1 Infection and Replication by Enhancing Viral Incorporation of Innate Anti-HIV-1 Protein A3G. J. Biol. Chem. 2009, 284, 13363–13372. [Google Scholar] [CrossRef]
- Lattanzi, L.; Federico, M. A strategy of antigen incorporation into exosomes: Comparing cross-presentation levels of antigens delivered by engineered exosomes and by lentiviral virus-like particles. Vaccine 2012, 30, 7229–7237. [Google Scholar] [CrossRef]
- Ferrantelli, F.; Manfredi, F.; Chiozzini, C.; Anticoli, S.; Olivetta, E.; Arenaccio, C.; Federico, M. DNA Vectors Generating Engineered Exosomes Potential CTL Vaccine CandidatesAgainst AIDS, Hepatitis B, and Tumors. Mol. Biotechnol. 2018, 60, 773–782. [Google Scholar] [CrossRef]
- Di Bonito, P.; Chiozzini, C.; Arenaccio, C.; Anticoli, S.; Manfredi, F.; Olivetta, E.; Ferrantelli, F.; Falcone, E.; Ruggieri, A.; Federico, M. Antitumor HPV E7-specific CTL activity elicited by in vivo engineered exosomes produced through DNA inoculation. Int. J. Nanomed. 2017, 12, 4579–4591. [Google Scholar] [CrossRef]
- Di Bonito, P.; Ridolfi, B.; Columba-Cabezas, S.; Giovannelli, A.; Chiozzini, C.; Manfredi, F.; Anticoli, S.; Arenaccio, C.; Federico, M. HPV-E7 Delivered by Engineered Exosomes Elicits a Protective CD8+ T Cell-Mediated Immune Response. Viruses 2015, 7, 1079–1099. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef]
- Sun, F.; Xiao, G.; Qu, Z. Murine Bronchoalveolar Lavage. BIO-PROTOCOL 2017, 7, e2287. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Job, E.R.; Saelens, X.; Roose, K. Bronchoalveolar Lavage of Murine Lungs to Analyze Inflammatory Cell Infiltration. J. Vis. Exp. 2017, 55398. [Google Scholar] [CrossRef]
- Bauer, S.; Heeg, K.; Wagner, H.; Lipford, G.B. Identification of H-2Kb Binding and Immunogenic Peptides from Human Papilloma Virus Tumour Antigens E6 and E7. Scand. J. Immunol. 1995, 42, 317–323. [Google Scholar] [CrossRef]
- De Oliveira, L.M.F.; Morale, M.G.; Chaves, A.A.M.; Cavalher, A.M.; Lopes, A.S.; Diniz, M.D.O.; Schanoski, A.S.; De Melo, R.L.; Ferreira, L.C.D.S.; De Oliveira, M.L.S.; et al. Design, Immune Responses and Anti-Tumor Potential of an HPV16 E6E7 Multi-Epitope Vaccine. PLoS ONE 2015, 10, e0138686. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Kobinger, G.P.; Jordan, H.; Suchma, K.; Weiss, S.R.; Shen, H.; Schumer, G.; Gao, G.; Boyer, J.L.; Crystal, R.G.; et al. Identification of murine CD8 T cell epitopes in codon-optimized SARS-associated coronavirus spike protein. Virology 2005, 335, 34–45. [Google Scholar] [CrossRef]
- Huang, J.; Cao, Y.; Du, J.; Bu, X.; Ma, R.; Wu, C. Priming with SARS CoV S DNA and boosting with SARS CoV S epitopes specific for CD4+ and CD8+ T cells promote cellular immune responses. Vaccine 2007, 25, 6981–6991. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, J.; Perlman, S. T Cell Responses Are Required for Protection from Clinical Disease and for Virus Clearance in Severe Acute Respiratory Syndrome Corona-virus-Infected Mice. J. Virol. 2010, 84, 9318–9325. [Google Scholar] [CrossRef] [PubMed]
- Hartenian, E.; Nandakumar, D.; Lari, A.; Ly, M.; Tucker, J.M.; Glaunsinger, B.A. The molecular virology of coronaviruses. J. Biol. Chem. 2020, 295, 12910–12934. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 183, 1735. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Chiozzini, C.; Manfredi, F.; Arenaccio, C.; Ferrantelli, F.; Leone, P.; Federico, M. N-Terminal Fatty Acids of NEFMUT Are Required for the CD8+ T-Cell Immunogenicity of In Vivo Engineered Extracellular Vesicles. Vaccines 2020, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, J.E.; Woodland, D.L. Immunity to Respiratory Viruses. Annu. Rev. Immunol. 2009, 27, 61–82. [Google Scholar] [CrossRef]
- Takamura, S.; Kato, S.; Motozono, C.; Shimaoka, T.; Ueha, S.; Matsuo, K.; Miyauchi, K.; Masumoto, T.; Katsushima, A.; Nakayama, T.; et al. Interstitial-resident memory CD8+ T cells sustain frontline epithelial memory in the lung. J. Exp. Med. 2019, 216, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Zhao, M.; Liu, K.; Xu, K.; Wong, G.; Tan, W.; Gao, G.F. T-cell immunity of SARS-CoV: Implications for vaccine development against MERS-CoV. Antivir. Res. 2017, 137, 82–92. [Google Scholar] [CrossRef]
- Wilson, J.A.; Hart, M.K. Protection from Ebola Virus Mediated by Cytotoxic T Lymphocytes Specific for the Viral Nucleoprotein. J. Virol. 2001, 75, 2660–2664. [Google Scholar] [CrossRef]
- Olinger, G.G.; Bailey, M.A.; Dye, J.M.; Bakken, R.; Kuehne, A.; Kondig, J.; Wilson, J.; Hogan, R.J.; Hart, M.K. Protective Cytotoxic T-Cell Responses Induced by VenezuelanEquine Encephalitis Virus Replicons Expressing Ebola Virus Proteins. J. Virol. 2005, 79, 14189–14196. [Google Scholar] [CrossRef]
- Gupta, M.; Greer, P.; Mahanty, S.; Shieh, W.-J.; Zaki, S.R.; Ahmed, R.; Rollin, P.E. CD8-Mediated Protection against Ebola Virus Infection Is Perforin Dependent. J. Immunol. 2005, 174, 4198–4202. [Google Scholar] [CrossRef]
- Sullivan, N.J.; E Hensley, L.; Asiedu, C.; Geisbert, T.W.; Stanley, D.; Johnson, J.C.; Honko, A.N.; Olinger, G.G.; Bailey, M.Q.; Geisbert, J.B.; et al. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat. Med. 2011, 17, 1128–1131. [Google Scholar] [CrossRef]
- Shedlock, D.J.; Aviles, J.; Talbott, K.T.; Wong, G.; Wu, S.J.; O Villarreal, D.; Myles, D.J.; A Croyle, M.; Yan, J.; Kobinger, G.P.; et al. Induction of Broad Cytotoxic T Cells by Protective DNA Vaccination Against Marburg and Ebola. Mol. Ther. 2013, 21, 1432–1444. [Google Scholar] [CrossRef]
- Sakabe, S.; Sullivan, B.M.; Hartnett, J.N.; Robles-Sikisaka, R.; Gangavarapu, K.; Cubitt, B.; Ware, B.C.; Kotliar, D.; Branco, L.M.; Goba, A.; et al. Analysis of CD8+ T cell response during the 2013–2016 Ebola epidemic in West Africa. Proc. Natl. Acad. Sci. USA 2018, 115, E7578–E7586. [Google Scholar] [CrossRef]
- Schotsaert, M.; Ibañez, L.I.; Fiers, W.; Saelens, X. Controlling Influenza by Cytotoxic T-Cells: Calling for Help from Destroyers. J. Biomed. Biotechnol. 2010, 2010, 1–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cargnelutti, D.E.; Sánchez, M.V.; Mattion, N.M.; Scodeller, E.A. Development of a universal CTL-based vaccine for influenza. Bioengineered 2013, 4, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Morikawa, K.; Suda, T.; Ohno, N.; Matsushita, S.; Akatsuka, T.; Handa, H.; Matsui, M. Chimeric SV40 virus-like particles induce specific cytotoxicity and protective immunity against influenza A virus without the need of adjuvants. Virology 2014, 448, 159–167. [Google Scholar] [CrossRef]
- Wu, T.; Hu, Y.; Lee, Y.-T.; Bouchard, K.R.; Benechet, A.; Khanna, K.; Cauley, L.S. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 2013, 95, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Gasper, D.J.; Neldner, B.; Plisch, E.H.; Rustom, H.; Carrow, E.; Imai, H.; Kawaoka, Y.; Suresh, M. Effective Respiratory CD8 T-Cell Immunity to Influenza Virus Induced by Intranasal Carbomer-Lecithin-Adjuvanted Non-replicating Vaccines. PLoS Pathog. 2016, 12, e1006064. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, S.A.; Josephs, T.M.; Clemens, E.B.; Grant, E.J.; Nguyen, T.H.O.; Wang, G.C.; Price, D.A.; Miller, A.; Tong, S.Y.C.; Thomas, P.G.; et al. Molecular basis for universal HLA-A*0201–restricted CD8+ T-cell immunity against influenza viruses. Proc. Natl. Acad. Sci. USA 2016, 113, 4440–4445. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Kang, J.-O.; Chang, J. Nucleoprotein vaccine induces cross-protective cytotoxic T lymphocytes against both lineages of influenza B virus. Clin. Exp. Vaccine Res. 2019, 8, 54–63. [Google Scholar] [CrossRef]
- Jang, Y.H.; Seong, B.L. The Quest for a Truly Universal Influenza Vaccine. Front. Cell. Infect. Microbiol. 2019, 9, 344. [Google Scholar] [CrossRef]
- Wen, J.; Ngono, A.E.; Regla-Nava, J.A.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Dengue virus-reactive CD8+ T cells mediate cross-protection against subsequent Zika virus challenge. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Hensley, L.E.; Mulangu, S.; Asiedu, C.; Johnson, J.; Honko, A.N.; Stanley, D.; Fabozzi, G.; Nichol, S.T.; Ksiazek, T.G.; Rollin, P.E.; et al. Demonstration of Cross-Protective Vaccine Immunity against an Emerging Pathogenic Ebolavirus Species. PLoS Pathog. 2010, 6, e1000904. [Google Scholar] [CrossRef]
- Netland, J.; Bevan, M.J. CD8 and CD4 T Cells in West Nile Virus Immunity and Pathogenesis. Viruses 2013, 5, 2573–2584. [Google Scholar] [CrossRef]
- Dandekar, A.A.; Perlman, S. Immunopathogenesis of coronavirus infections: Implications for SARS. Nat. Rev. Immunol. 2005, 5, 917–927. [Google Scholar] [CrossRef]
- Yip, M.S.; Leung, N.H.L.; Cheung, C.Y.; Li, P.H.; Lee, H.H.Y.; Daëron, M.; Peiris, J.S.M.; Bruzzone, R.; Jaume, M. Antibody-Dependent Infection of Human Macrophages bySevere Acute Respiratory Syndrome Coronavirus. Virol. J. 2014, 11, 82. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Q.; Lin, Q.; Fang, J.; Wang, H.; Kwok, H.; Tang, H.; Nishiura, K.; Peng, J.; Tan, Z.; et al. Anti–spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Gattinger, P.; Borochova, K.; Dorofeeva, Y.; Henning, R.; Kiss, R.; Kratzer, B.; Mühl, B.; Perkmann, T.; Trapin, D.; Trella, M.; et al. Antibodies in serum of convalescent patients following mild COVID-19 do not always prevent virus-receptor binding. Allergy 2020. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Fett, C.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Virus-Specific Memory CD8 T Cells Provide Substantial Protection from Lethal Severe Acute Respira-tory Syndrome Coronavirus Infection. J. Virol. 2014, 88, 11034–11044. [Google Scholar] [CrossRef]
- Arenaccio, C.; Chiozzini, C.; Ferrantelli, F.; Leone, P.; Olivetta, E.; Federico, M. Exosomes in Therapy: Engineering, Pharmacokinetics and Future Applications. Curr. Drug Targets 2018, 20, 87–95. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrantelli, F.; Chiozzini, C.; Manfredi, F.; Giovannelli, A.; Leone, P.; Federico, M. Simultaneous CD8+ T-Cell Immune Response against SARS-Cov-2 S, M, and N Induced by Endogenously Engineered Extracellular Vesicles in Both Spleen and Lungs. Vaccines 2021, 9, 240. https://doi.org/10.3390/vaccines9030240
Ferrantelli F, Chiozzini C, Manfredi F, Giovannelli A, Leone P, Federico M. Simultaneous CD8+ T-Cell Immune Response against SARS-Cov-2 S, M, and N Induced by Endogenously Engineered Extracellular Vesicles in Both Spleen and Lungs. Vaccines. 2021; 9(3):240. https://doi.org/10.3390/vaccines9030240
Chicago/Turabian StyleFerrantelli, Flavia, Chiara Chiozzini, Francesco Manfredi, Andrea Giovannelli, Patrizia Leone, and Maurizio Federico. 2021. "Simultaneous CD8+ T-Cell Immune Response against SARS-Cov-2 S, M, and N Induced by Endogenously Engineered Extracellular Vesicles in Both Spleen and Lungs" Vaccines 9, no. 3: 240. https://doi.org/10.3390/vaccines9030240
APA StyleFerrantelli, F., Chiozzini, C., Manfredi, F., Giovannelli, A., Leone, P., & Federico, M. (2021). Simultaneous CD8+ T-Cell Immune Response against SARS-Cov-2 S, M, and N Induced by Endogenously Engineered Extracellular Vesicles in Both Spleen and Lungs. Vaccines, 9(3), 240. https://doi.org/10.3390/vaccines9030240







