SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search
2.5. Study Selection
2.6. Data Collection Process
2.7. Data Items
2.8. Endpoints
2.9. Summary Measures
2.10. Data Synthesis and Analysis
2.11. Quality of Studies, Risk Bias, and Evidence Profile
2.12. Software and Statistical Significance
3. Results
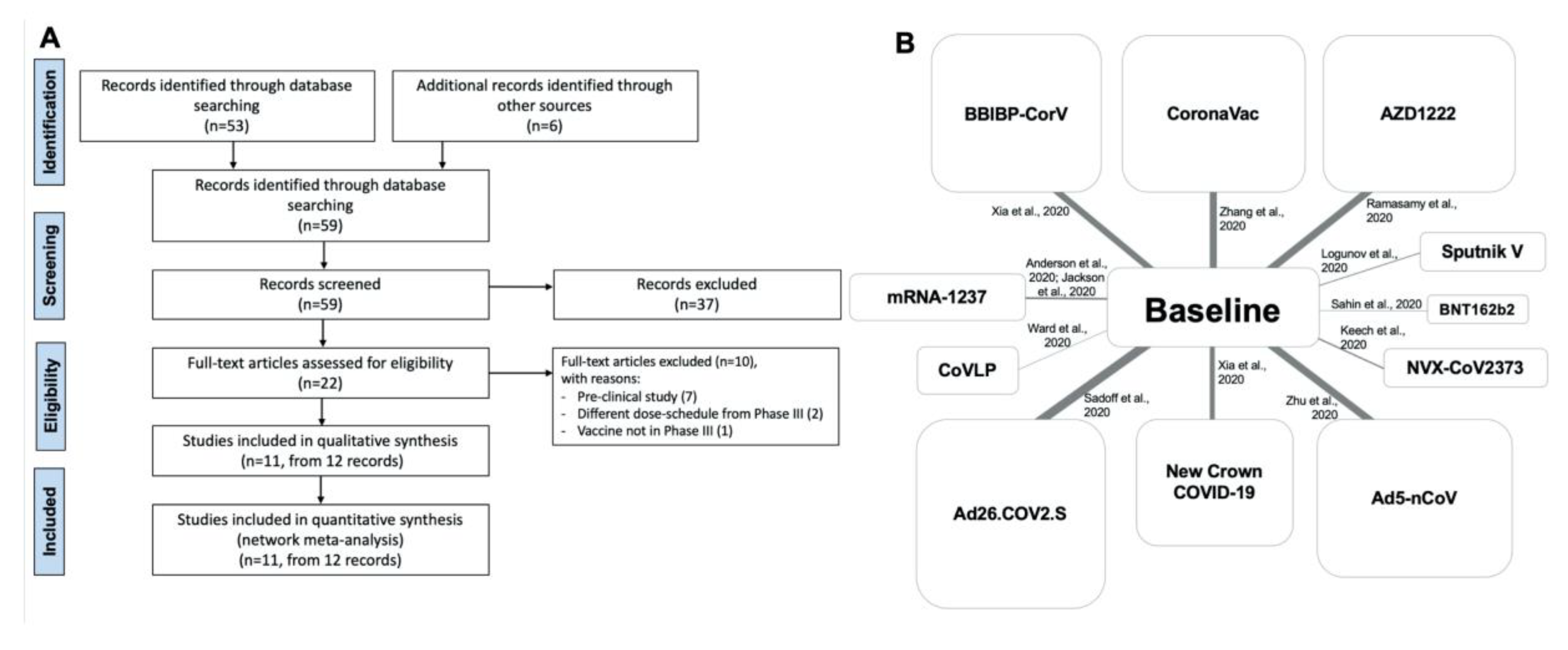
3.1. Study Selection and Characteristics
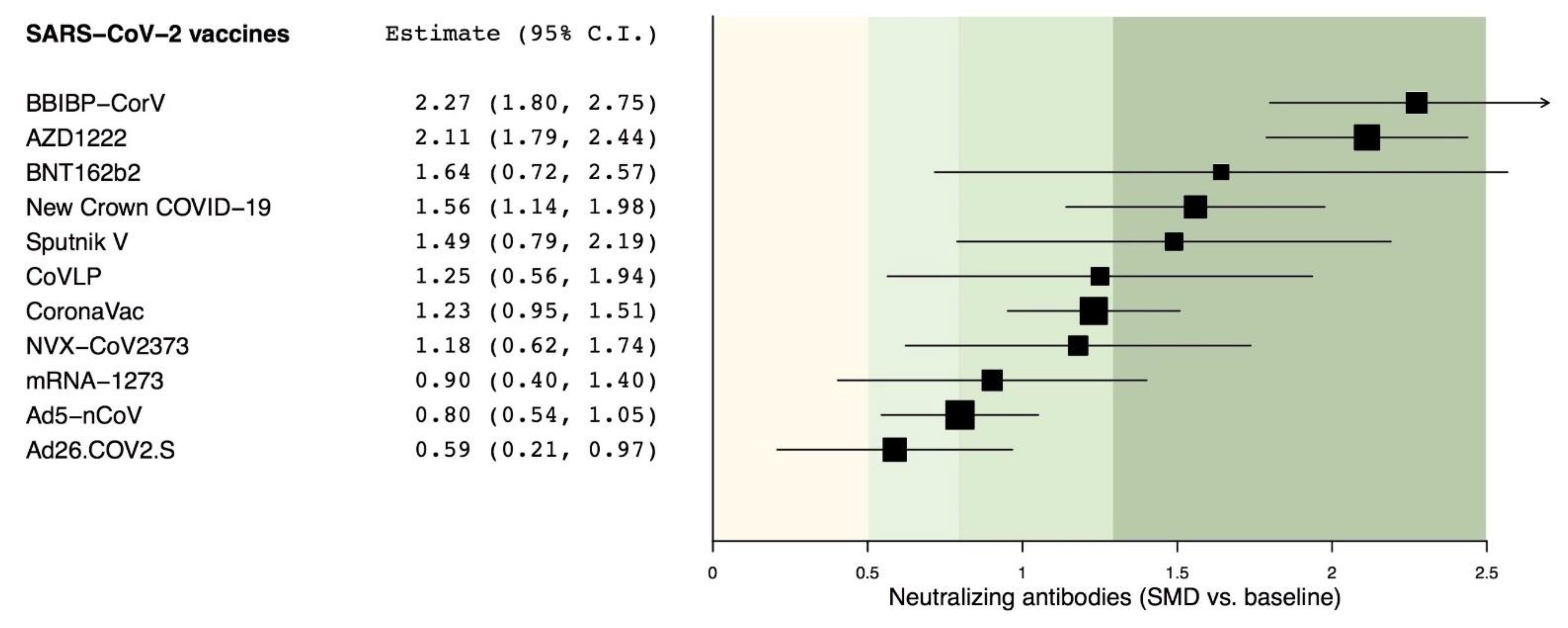
3.2. Primary Endpoint
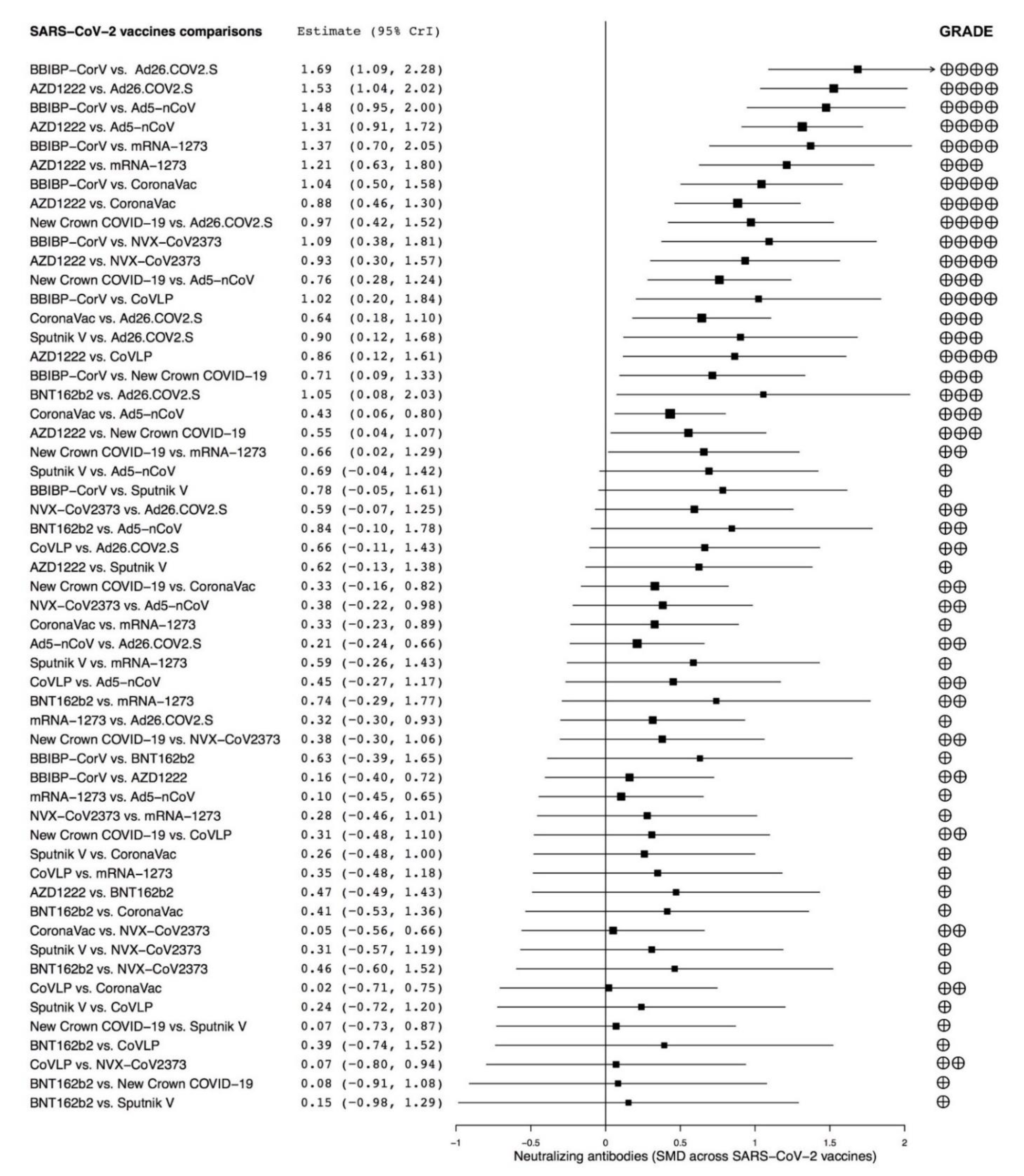
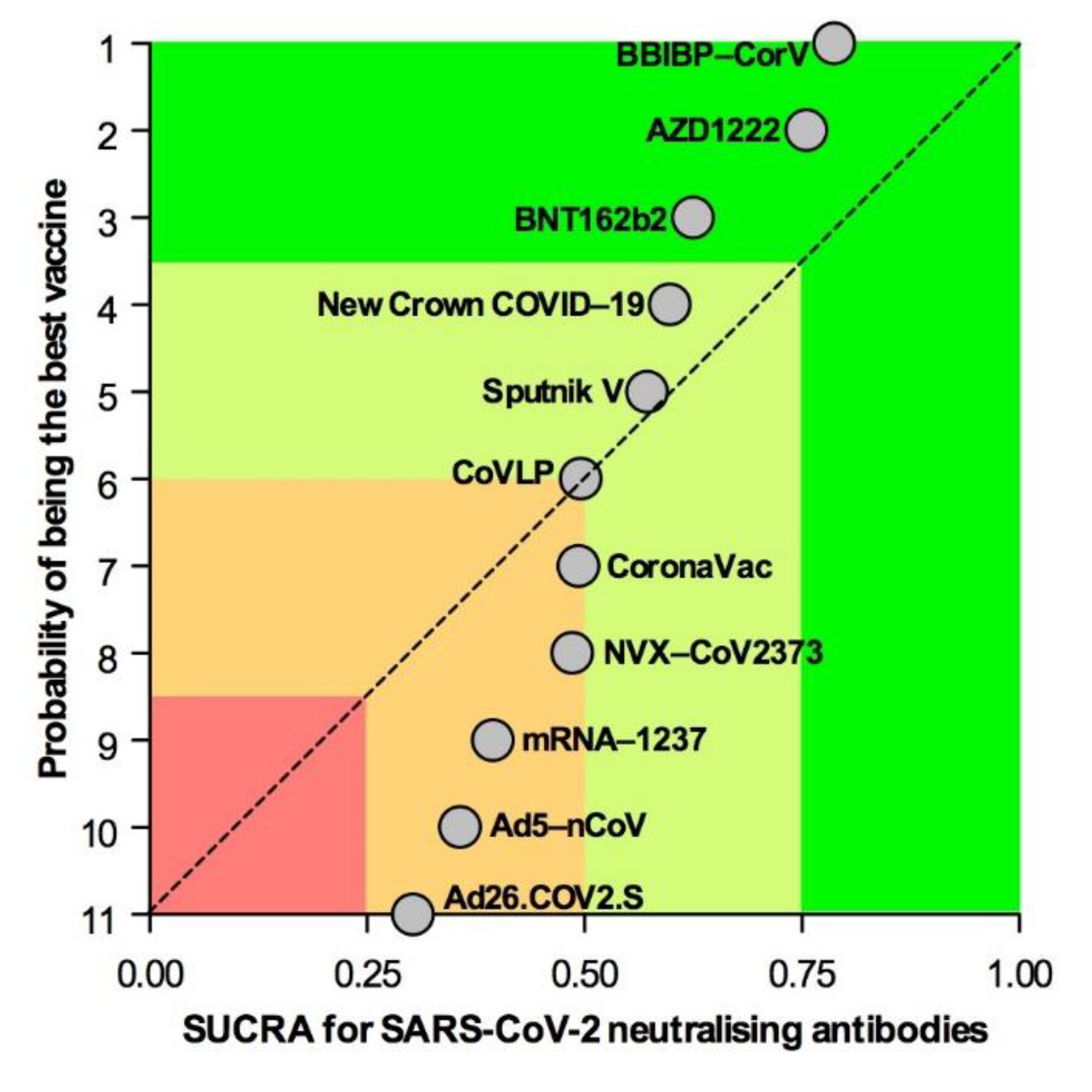
3.3. Subset Analyses
3.4. Secondary Endpoint
3.5. Risk of Bias and Quality of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control Prevention. Ten great public health achievements—United States, 2001–2010. MMWR Morb. Mortal. Wkly Rep. 2011, 60, 619. [Google Scholar]
- Gavi. The Gavi COVAX AMC: An Investment Opportunity. Available online: http://www.gavi.org/covax-facility (accessed on 30 December 2020).
- World Health Organization. Draft Landscape of COVID-19 Candidate Vaccines. Available online: http://www.dropbox.com/s/jiqqdl96g7qf3f1/20210106-Novel Coronavirus_Landscape_COVID.xlsx?dl=0 (accessed on 7 January 2021).
- Le, T.T.; Cramer, J.P.; Chen, R.; Mayhew, S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 667–668. [Google Scholar] [CrossRef] [PubMed]
- NCT04683224. A Study to Evaluate the Safety, Immunogenicity, and Efficacy of UB-612 COVID-19 Vaccine. Available online: https://clinicaltrials.gov/ct2/show/NCT04683224 (accessed on 6 January 2021).
- NCT04642638. Safety, Immunogenicity, and Efficacy of INO-4800 for COVID-19 in Healthy Seronegative Adults at High Risk of SARS-CoV-2 Exposure. Available online: https://clinicaltrials.gov/ct2/show/NCT04642638 (accessed on 7 January 2021).
- INOVIO. Pharmaceuticals INOVIO and Advaccine Announce First Dosing of Subject in Phase 2 Clinical Trial for COVID-19 DNA Vaccine Candidate INO-4800 in China. Available online: http://ir.inovio.com/news-releases/news-releases-details/2020/INOVIO-and-Advaccine-Announce-First-Dosing-of-Subject-in-Phase-2-Clinical-Trial-for-COVID-19-DNA-Vaccine-Candidate-INO-4800-in-China/default.aspx (accessed on 7 January 2021).
- Reuters. Japan’s AnGes Begins Phase 2/3 Clinical Trial of DNA-Based COVID-19 Vaccine. Available online: http://www.reuters.com/article/us-anges-covid-vaccine/japans-anges-begins-phase-2-3-clinical-trial-of-dna-based-covid-19-vaccine-idINKBN28I0EA (accessed on 7 January 2021).
- NCT04672395. A Controlled Phase 2/3 Study of Adjuvanted Recombinant SARS-CoV-2 Trimeric S-protein Vaccine (SCB-2019) for the Prevention of COVID-19 (SCB-2019). Available online: https://clinicaltrials.gov/ct2/show/NCT04672395 (accessed on 7 January 2021).
- AstraZeneca. AstraZeneca’s COVID-19 Vaccine Authorised for Emergency Supply in the UK. Available online: http://www.astrazeneca.com/media-centre/press-releases/2020/astrazenecas-covid-19-vaccine-authorised-in-uk.html (accessed on 30 December 2020).
- Aislinn Laing Argentine Regulator Approves AstraZeneca/Oxford COVID-19 Vaccine—AstraZeneca. Available online: http://www.reuters.com/article/us-health-coronavirus-argentina-astrazen/argentine-regulator-approves-astrazeneca-oxford-covid-19-vaccine-astrazeneca-idUSKBN29421P (accessed on 6 January 2021).
- Bryan Pietsch Mexico Approved the Oxford-AstraZeneca Vaccine for Emergency Use. Available online: http://www.nytimes.com/2021/01/04/world/mexico-oxford-astrazeneca-vaccine.html (accessed on 6 January 2021).
- Schmall, E.; Yasir, S. India Approves Oxford-AstraZeneca Covid-19 Vaccine and 1 Other. Available online: http://www.nytimes.com/2021/01/03/world/asia/india-covid-19-vaccine.html (accessed on 6 January 2021).
- Wu, H.; Moritsugu, K. China Gives Conditional Approval to the Sinopharm Vaccine. Available online: https://time.com/5925600/china-approval-sinopharm-vaccine/ (accessed on 30 December 2020).
- Cyranoski, D. Arab Nations First to Approve Chinese COVID Vaccine—Despite Lack of Public Data. Nature 2020, 588, 548. [Google Scholar] [CrossRef]
- Muhamad Abu Zaid Egypt approves Chinese Sinopharm COVID-19 Vaccine. Available online: http://www.arabnews.com/node/1786531/middle-east (accessed on 6 January 2021).
- Bharat Biotech. Bharat Biotech’s ‘COVAXINTM’ Emergency Use Authorization Approval by DCGI-CDSCO, MoH&FW, a Significant Landmark in India’s Scientific Discovery, and Scientists Capability. Available online: http://www.bharatbiotech.com/images/press/bharat-biotech-covaxin-emergency-use-authorization-approval-by-dcgi-cdsco-moh-and-fw.pdf (accessed on 7 January 2021).
- Tanne, J.H. Covid-19: Pfizer-BioNTech vaccine is rolled out in US. BMJ 2020, 371, m483. [Google Scholar] [CrossRef] [PubMed]
- The Rio Times. Argentina Grants Emergency Approval to Pfizer/BioNTech Vaccine. Available online: https://riotimesonline.com/brazil-news/mercosur/argentine-extends-emergency-approval-to-the-pfizer-vaccine/ (accessed on 30 December 2020).
- Juan Martinez Chile and Ecuador Authorize Pfizer Vaccine in South America. Available online: https://riotimesonline.com/brazil-news/mercosur/chile-and-ecuador-authorize-pfizer-vaccine-in-south-america/ (accessed on 30 December 2020).
- The Q Media. Costa Rica Authorizes Use of Pfizer and BioNTech Vaccine. Available online: https://qcostarica.com/costa-rica-authorizes-use-of-pfizer-and-biontech-vaccine/ (accessed on 30 December 2020).
- Kuwait News Agency (KUNA). Kuwait Authorities Emergency Use of Pfizer-BiNTech Covid-19 Vaccine. Available online: https://www.kuna.net.kw/ArticleDetails.aspx?id=2945421&language=en (accessed on 30 December 2020).
- ALJAZZERA. Mexico Approves Emergency Use of Pfizer-BioNTech COVID Vaccine. Available online: http://www.aljazeera.com/news/2020/12/12/mexico-regulator-approves-pfizer-biontech-coronavirus-vaccine (accessed on 30 December 2020).
- The Japan Times. In Asia First, Singapore Approves Pfizer’s COVID-19 Vaccine. Available online: http://www.japantimes.co.jp/news/2020/12/15/asia-pacific/singapore-pfizer-coronavirus-vaccine/ (accessed on 30 December 2020).
- European Medicines Agency. EMA Recommends First COVID-19 Vaccine for Authorisation in the EU. Available online: http://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu (accessed on 30 December 2020).
- Government of Canada. Regulatory Decision Summary—Pfizer-BioNTech COVID-19 Vaccine—Health Canada. Available online: https://covid-vaccine.canada.ca/info/regulatory-decision-summary-detailTwo.html?linkID=RDS00730 (accessed on 30 December 2020).
- ALJAZZERA. Bahrain Becomes Second Country to Approve Pfizer COVID-19 Vaccine. Available online: http://www.aljazeera.com/news/2020/12/4/bahrain-becomes-second-country-to-approve-pfizer-covid-19-vaccine (accessed on 30 December 2020).
- Swiss Agency for Therapeutic Products. Swissmedic Grants Authorisation for the First COVID-19 Vaccine in Switzerland. Available online: http://www.bag.admin.ch/bag/en/home/das-bag/aktuell/medienmitteilungen.msg-id-81761.html (accessed on 30 December 2020).
- Thomas, K.; LaFraniere, S.; Weiland, N.; Goodnough, A.; Maggie Haberman, F.D.A. Clears Pfizer Vaccine, and Millions of Doses Will Be Shipped Right Away. Available online: http://www.nytimes.com/2020/12/11/health/pfizer-vaccine-authorized.html (accessed on 30 December 2020).
- Government of Canada. Moderna COVID-19 Vaccine (mRNA-1273 SARS-CoV-2). Available online: https://covid-vaccine.canada.ca/moderna-covid-19-vaccine/product-details (accessed on 30 December 2020).
- Moderna—Press Release, Moderna Announces FDA Authorization of Moderna COVID-19 Vaccine in U.S. Available online: https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-fda-authorization-moderna-covid-19-vaccine-us (accessed on 30 December 2020).
- European Medicines Agency. EMA Recommends COVID-19 Vaccine Moderna for Authorisation in the EU. Available online: http://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-moderna-authorisation-eu (accessed on 7 January 2021).
- The Times of Israel. Moderna Says Israel Approved Its Vaccine, 1st Shipment Set to Arrive This Month. Available online: http://www.timesofisrael.com/health-ministry-approves-moderna-vaccine-1st-shipment-set-to-arrive-this-month/ (accessed on 7 January 2021).
- Gamaleya National Center of Epidemiology and Microbiology. BELARUS STARTS VACCINATION WITH SPUTNIK V. Available online: https://sputnikvaccine.com/newsroom/pressreleases/belarus-starts-vaccination-with-sputnik-v-/ (accessed on 30 December 2020).
- Gamaleya National Center of Epidemiology and Microbiology. ARGENTINA HAS REGISTERED THE SPUTNIK V VACCINE BASED ON RUSSIAN CLINICAL TRIAL DATA. Available online: https://sputnikvaccine.com/newsroom/pressreleases/argentina-has-registered-the-sputnik-v-vaccine-based-on-russian-clinical-trial-data-/ (accessed on 30 December 2020).
- Hodgson, S.H.; Mansatta, K.; Mallett, G.; Harris, V.; Emary, K.R.W.; Pollard, A.J. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect. Dis. 2020, 21, e26–e35. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2020, 397, 99–111. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- World Health Organization. Draft Landscape of COVID-19 Candidate Vaccines. Available online: http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwixxvq8koruAhWBjaQKHUrQCi0QFjAAegQIAhAC&url=https%3A%2F%2Fwww.who.int%2Fdocs%2Fdefault-source%2Fa-future-for-children%2Fnovel-coronavirus_landscape_covid-19.pdf%3Fsfvrsn%3D4d8bd201_1%26download%3Dtrue&usg=AOvVaw2qsH-YawjFeXQVTvKbPdHu (accessed on 18 December 2020).
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduc. Targ. Ther. 2020, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing Risk of Bias in a Randomized Trial. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. pp. 205–228. Available online: http://www.training.cochrane.org/handbook (accessed on 20 December 2020).
- Pedder, H.; Sarri, G.; Keeney, E.; Nunes, V.; Dias, S. Data extraction for complex meta-analysis (DECiMAL) guide. Syst. Rev. 2016, 5, 212. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Gianinazzi, M.E.; Rueegg, C.S.; Zimmerman, K.; Kuehni, C.E.; Michel, G.; Swiss Paediatric Oncology, G. Intra-rater and inter-rater reliability of a medical record abstraction study on transition of care after childhood cancer. PLoS ONE 2015, 10, e0124290. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 9.2.3.2. The standardized mean difference. 2011. Available online: https://handbook-5-1.cochrane.org/chapter_9/9_2_3_2_the_standardized_mean_difference.htm (accessed on 20 December 2020).
- Cohen, J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Dobler, C.C.; Wilson, M.E.; Murad, M.H. A pulmonologist’s guide to understanding network meta-analysis. Eur. Respir. J. 2018, 52, 1800525. [Google Scholar] [CrossRef] [PubMed]
- Calzetta, L.; Rogliani, P.; Ora, J.; Puxeddu, E.; Cazzola, M.; Matera, M.G. LABA/LAMA combination in COPD: A meta-analysis on the duration of treatment. Eur. Respir. Rev. 2017, 26, 160043. [Google Scholar] [CrossRef]
- Lu, G.; Ades, A.E. Assessing evidence inconsistency in mixed treatment comparisons. J. Am. Stat. Assoc. 2006, 101, 447–459. [Google Scholar] [CrossRef]
- Spiegelhalter, D.J.; Abrams, K.R.; Myles, J.P. Bayesian Approaches to Clinical Trials and Health-Care Evaluation; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 13. [Google Scholar]
- Van Valkenhoef, G.; Lu, G.; de Brock, B.; Hillege, H.; Ades, A.E.; Welton, N.J. Automating network meta-analysis. Res. Synth. Methods 2012, 3, 285–299. [Google Scholar] [CrossRef]
- van Valkenhoef, G.; Dias, S.; Ades, A.E.; Welton, N.J. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Re. Synth. Methods 2016, 7, 80–93. [Google Scholar] [CrossRef]
- Dias, S.; Welton, N.J.; Sutton, A.J.; Caldwell, D.M.; Lu, G.; Ades, A.E. Evidence synthesis for decision making 4: Inconsistency in networks of evidence based on randomized controlled trials. Med. Dec. Mak. 2013, 33, 641–656. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the gap between methodologists and end-users: R as a computational back-end. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- McGuinness, L.A. robvis: An R package and web application for visualising risk-of-bias assessments. Available online: https://github.com/mcguinlu/robvis (accessed on 20 December 2020).
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I. Safety and immunogenicity of the Ad26. COV2. S COVID-19 vaccine candidate: Interim results of a phase 1/2a, double-blind, randomized, placebo-controlled trial. MedRxiv 2020. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2020, 21, 39–51. [Google Scholar] [CrossRef]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA 2020, 324, 951–960. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2020, 21, 181–192. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. MedRxiv 2020. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Eng. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Eng. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Eng. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.J.; Gobeil, P.; Séguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.-Y.; Couture, M.; D’Aoust, M.-A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 trial of a Candidate Recombinant Virus-Like Particle Vaccine for Covid-19 Disease Produced in Plants. MedRxiv 2020. [Google Scholar] [CrossRef]
- Rogliani, P.; Ritondo, B.L.; Ora, J.; Cazzola, M.; Calzetta, L. SMART and as-needed therapies in mild to severe asthma: A network meta-analysis. Eur. Respir. J. 2020, 56, 2000625. [Google Scholar] [CrossRef]
- Andrade, C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis: As simple as it gets. J. Clin. Psychiatry 2020, 81, 20f1368. [Google Scholar] [CrossRef]
- Ji, Z.; Jian, M.; Chen, T.; Luo, L.; Li, L.; Dai, X.; Bai, R.; Ding, Z.; Bi, Y.; Wen, S. Immunogenicity and safety of the M72/AS01E candidate vaccine against tuberculosis: A meta-analysis. Fron. Immunol. 2019, 10, 2089. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. SARS-CoV-2 immunity: Review and applications to phase 3 vaccine candidates. Lancet 2020, 396, 1595–1606. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D. COVID-19 vaccine BNT162b1 elicits human antibody and TH 1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Weiss, P. Drugmakers Expect Tests to Confirm Vaccines Effective Against New Coronavirus Variant. Available online: http://www.nasdaq.com/articles/drugmakers-expect-tests-to-confirm-vaccines-effective-against-new-coronavirus-variant-2020 (accessed on 7 January 2021).
| Study and Year and Reference | Trial Number Identifier | Study Characteristics | Vaccine Developer | SARS-CoV-2 Vaccine (Dose and Route of Administration as in Phase III Development) | Type of Candidate Vaccine | Study Duration with Follow-Up (Weeks) | Number of Scheduled Doses (Timing of Inoculations) as in Phase III Development | Number of Vaccine Recipients | Characteristics of Vaccine Recipients | Age (Mean and Range) | Male (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Xia et al., 2020 [66] | ChiCTR2000032459 | Phase I/II, single center, randomized, double-blind, placebo-controlled, parallel-group | Beijing Institute of Biological Products/Sinopharm | BBIBP-CorV (4 µg IM) | Inactivated SARS-CoV-2 vaccine | 7 | Prime and boost inoculation (0, 21 days) | 112 | Healthy adults with negative IgG and IgM to SARS-CoV-2, with no history of travelling to Hubei Province (China), regions outside of China, or regions with reported COVID-19 cases from December 2019, and no history of SARS-CoV-2 infection | 41.7 (18.0–59.0) | 47.0 |
| Ramasamy et al., 2020 [62] | NCT04400838, ISRCTN15281137 | Phase II/III, multicenter, randomized, single-blind, negative-controlled, parallel-group | University of Oxford/AstraZeneca | AZD1222 or ChAdOx1 nCoV-19 or COVISHIELD (3.5–6.5 × 1010 viral particles IM) | Replication-defective chimpanzee adenovirus-vectored vaccine expressing full-length SARS-CoV-2 spike glycoprotein gene | 8 | Prime and boost inoculation (0, 28 days) | 128 | Healthy adults, seronegative to SARS-CoV-2 before enrolment, apart from those aged 18.0–55.0 years old | 57.8 (19.0–83.0) | 52.3 |
| Sahin et al., 2020 [69] | NCT04380701, EudraCT: 2020-001038-36 | Phase I/II, single center, non-randomized, open-label, dose-ranging, non-controlled, parallel-group | BioNTech/Fosun Pharma/Pfizer | BNT162b2 (30 µg IM) | 3 LNP-encapsulated nucleoside-modified mRNA vaccine encoding trimerized SARS-CoV-2 RBD antigen of spike glycoprotein | 12 | Prime and boost inoculation (1, 22 days) | 12 | Healthy adults, with negative IgG and/or IgM to SARS-CoV-2, negative SARS-CoV-2 nucleic acid amplification test nasal swab, with no previous clinical or microbiological diagnosis of COVID-19, no receipt of medications to prevent COVID-19, and no previous vaccination with any coronavirus vaccine | 46.7 (35.0–55.0) | 66.7 |
| Xia et al., 2020 [67] | ChiCTR2000031809 | Phase I/II, single center, randomized, double-blind, placebo-controlled, parallel-group | Wuhan Institute of Biological Products/Sinopharm | New Crown COVID-19 (5 μg IM) | Inactivated SARS-CoV-2 vaccine | 5 | Prime and boost inoculation (0, 21 days) | 84 | Healthy adults with no history of SARS-CoV (via on-site inquiry) or SARS-CoV-2 infection (via serological test and PCR) | 41.4 (18.0–59.0) | 38.1 |
| Logunov et al., 2020 [63] | NCT04436471 | Phase I/II, single center, non-randomized, open-label, non-controlled, parallel-group | Gamaleya Research Institute | Sputnik V or Gam-COVID-Vac (1 × 1011 viral particles IM) | Recombinant adenovirus type 26 vector plus recombinant adenovirus type 5 vector carrying the gene for SARS-CoV-2 full-length spike glycoprotein | 6 | Prime and boost inoculation (0, 21 days) | 20 | Healthy adults with negative PCR and IgG and IgM to SARS-CoV-2, with no history of COVID-19 or contact with COVID-19 patients | 26.4 (18.0–60.0) | 70.0 |
| Ward et al., 2020 [73] | NCT04450004 | Phase I, single center, randomized, partially-blinded, dose-escalation, non-controlled, parallel-group | Medicago Inc./GlaxoSmithKline | CoVLP (3.75 μg IM) | Plant-derived virus-like particle vaccine adjuvanted with AS03 | 6 | Prime and boost inoculation (0, 21 days) | 20 | Healthy adults with negative IgG and IgM to SARS-CoV-2, with not known current or previous laboratory-confirmed SARS-CoV-1 or SARS-CoV-2/COVID-19 | 34.7 (19.0–49.0) | 25.0 |
| Zhang et al., 2020 [68] | NCT04352608 | Phase I/II, single center, randomized, double-blind, placebo-controlled, parallel-group | Sinovac | CoronaVac (3 μg IM) | Inactivated SARS-CoV-2 vaccine | 6 | Prime and boost inoculation (0, 14 days) | 120 | Healthy adults with negative PCR and negative IgG and IgM to SARS-CoV-2, with no history of contact with patients with SARS-CoV-2, or travelling or residence in Wuhan city and surrounding areas or other communities with case reports within 2 weeks before enrolment | 42.0 (18.0–59.0) | 45.0 |
| Keech et al., 2020 [72] | NCT04368988 | Phase I/II, single center, randomized, observer-blind, placebo-controlled, parallel-group | Novavax | NVX-CoV2373 (5 μg IM) | Full-length recombinant SARS-CoV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M1 | 5 | Prime and boost inoculation (0, 21 days) | 26 | Healthy adults with no history of SARS or COVID-19 or with negative PCR or ELISA, with no history of contact with SARS-COV-2 subjects or working in an occupation at high risk for exposure to SARS-CoV-2 | 29.5 (18.0–59.0) | 50.0 |
| Anderson et al., 2020 [71]; Jackson et al., 2020 [71] | NCT04283461 | Phase I, single center, non-randomized, open-label, dose-escalation, parallel-group | Moderna/National Institute of Allergy and Infectious Diseases’ Vaccine Research Center | mRNA-1273 (100 μg IM) | LNP-encapsulated nucleoside-modified mRNA vaccine encoding SARS-CoV-2 prefusion-stabilized full-length spike glycoprotein trimer | 6 | Prime and boost inoculation (0, 28 days) | 35 | Healthy adults, not screened for current or past SARS-CoV-2 infection by PCR or serology before enrolment | 55.9 (18.0–55.0; 56.0–70.0: ≥71.0) | 42.3 |
| Zhu et al., 2020 [64] | NCT04341389 | Phase II, single center, randomized, double-blind, placebo-controlled, parallel-group | CanSino Biological Inc./Beijing Institute of Biotechnology | Ad5-nCoV (5 × 1010 viral particles IM) | Recombinant adenovirus type 5 vector vaccine | 4 | Single inoculation | 129 | Healthy adults, HIV-negative, with no history of SARS-CoV-2 infection, confirmed by negative SARS-CoV-2 fingertip rapid blood test | 39.7 (18.0–44.0; 45.0 –54.0; ≥55.0) | 50.0 |
| Sadoff et al., 2020 [65] | NCT04436276 | Phase I/II, multicenter, randomized, double-blind (immunogenicity data were unblinded), placebo-controlled, parallel-group | Janssen Pharmaceutical Companies | Ad26.COV2.S or JNJ-78436735 or Ad26COVS1 (5 × 1010 viral particles IM) | Replication-defective recombinant adenovirus type 26 vector vaccine expressing SARS-CoV-2 stabilized prefusion spike glycoprotein | 4 | Single inoculation | 150 | Healthy adults with negative PCR to SARS-CoV-2 | 18.0–55.0; ≥65.0 (cohort 1a and 3)a | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogliani, P.; Chetta, A.; Cazzola, M.; Calzetta, L. SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines. Vaccines 2021, 9, 227. https://doi.org/10.3390/vaccines9030227
Rogliani P, Chetta A, Cazzola M, Calzetta L. SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines. Vaccines. 2021; 9(3):227. https://doi.org/10.3390/vaccines9030227
Chicago/Turabian StyleRogliani, Paola, Alfredo Chetta, Mario Cazzola, and Luigino Calzetta. 2021. "SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines" Vaccines 9, no. 3: 227. https://doi.org/10.3390/vaccines9030227
APA StyleRogliani, P., Chetta, A., Cazzola, M., & Calzetta, L. (2021). SARS-CoV-2 Neutralizing Antibodies: A Network Meta-Analysis across Vaccines. Vaccines, 9(3), 227. https://doi.org/10.3390/vaccines9030227






