Immunogenetic Predictors of Severe COVID-19
Abstract
1. Introduction
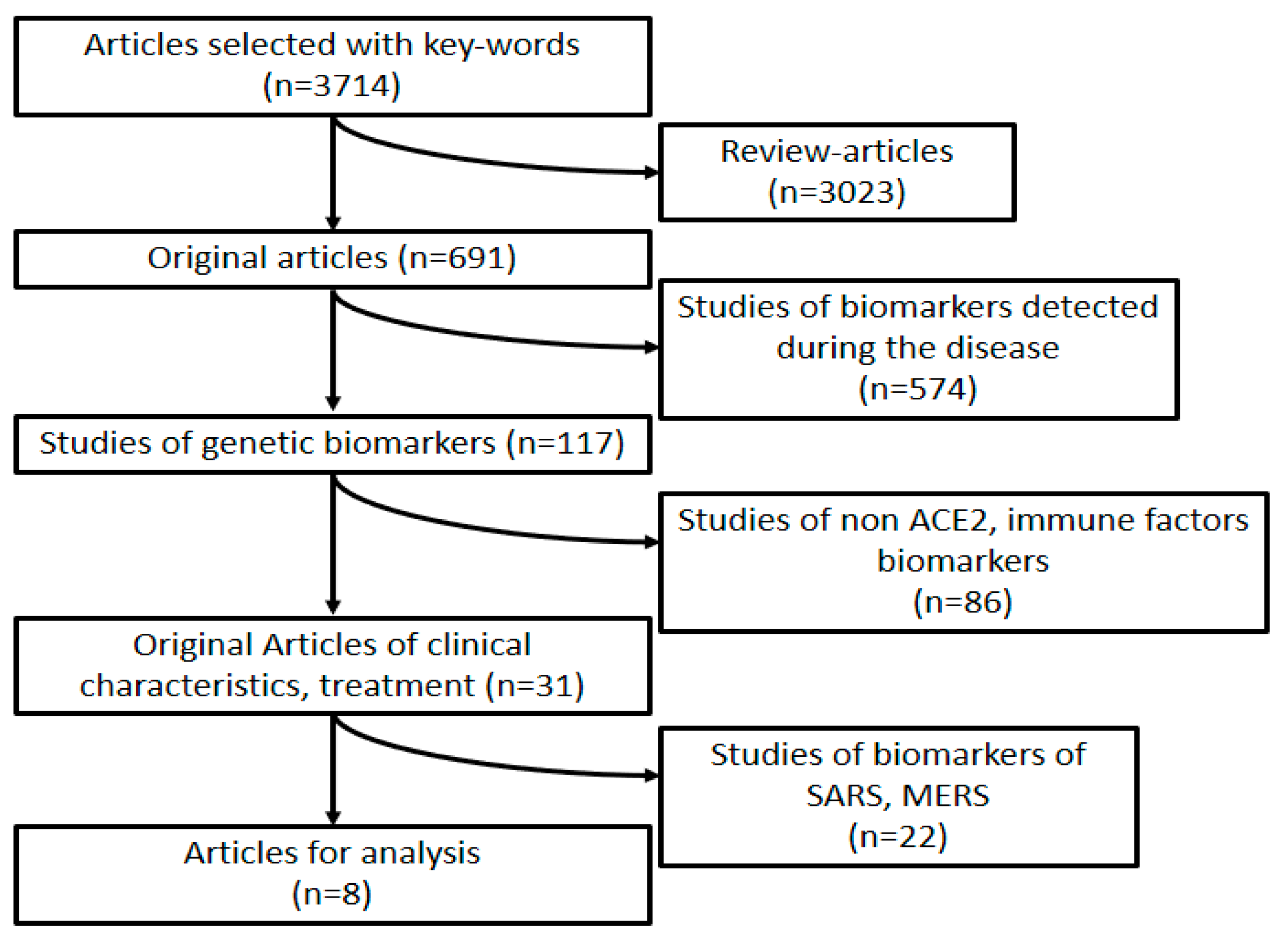
2. Design of the Study
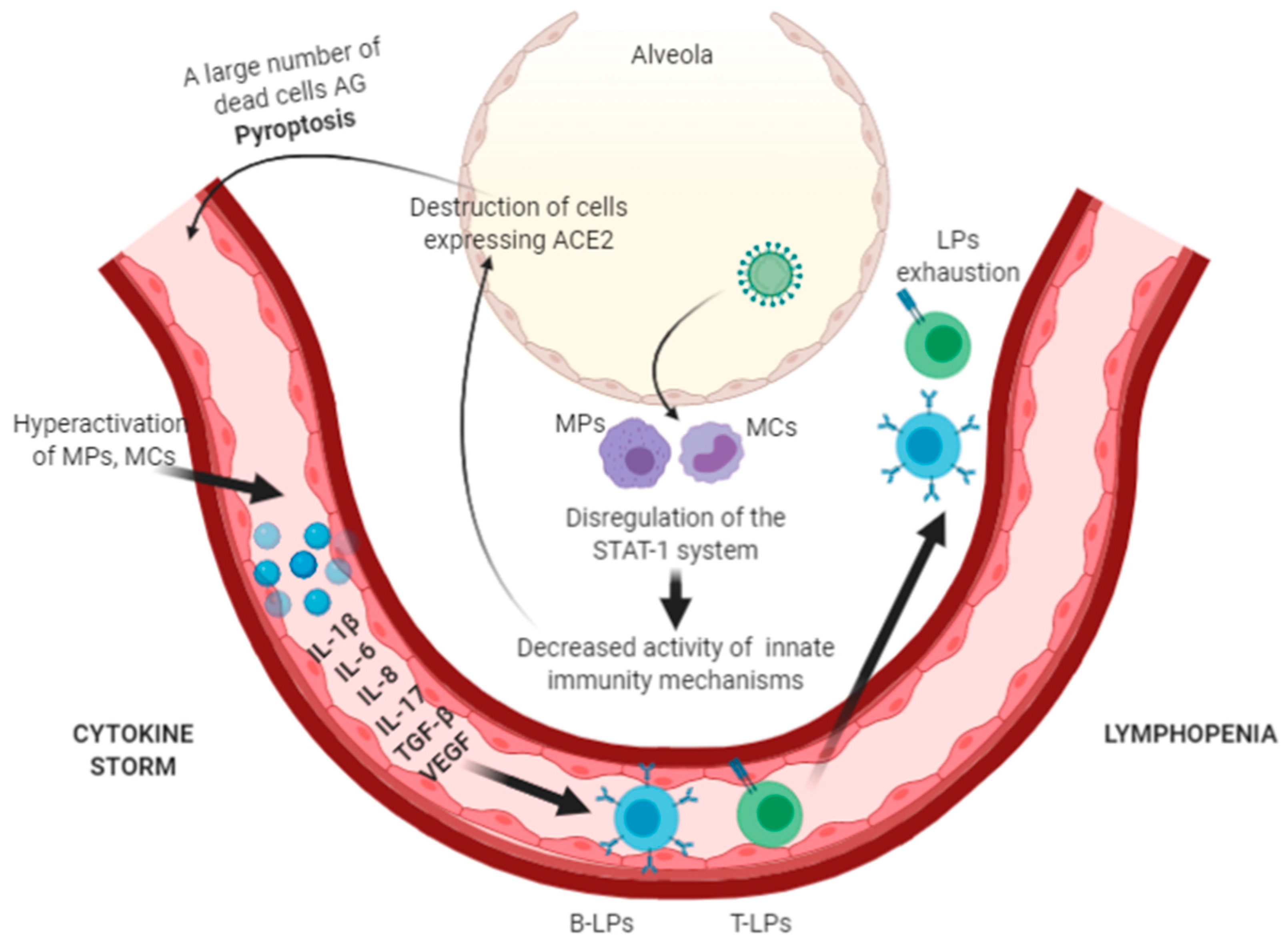
3. Pathogenesis of the New Coronavirus Infection
4. Possible Genetic Predictors of Severe COVID-19
4.1. Genetic Characteristics of the Immune Response in Patients with Severe COVID-19
4.2. Specificity of ACE-2 Protein Expression in Patients with Severe COVID-19
4.3. Gender-Related Specifics of COVID-19
4.4. Specifics of the COVID-19 Course in Various Populations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE-2 | Angiotension-Converting Enzyme 2 |
| AG | Antigens |
| COVID-19 | Corona virus disease 2019 |
| HLA | Human leukocyte antigens |
| GWAS | Genome-wide association study |
| IL | Interleukins |
| LP | lymphocytes |
| MHC | Major histocompatibility complex |
| MPs | Macrophages |
| MCs | Monocytes |
| RBD | Receptor-binding domain |
| SARS-COV-2 | Severe acute respiratory syndrome related coronavirus 2 |
| SpO2 | Oxygen saturation |
| TGF-β | Transforming Growth Factor Beta |
| VEGF | Vascular Endothelial Growth Factor |
References
- Starshinova, A.A.; Kushnareva, E.A.; Malkova, A.M.; Dovgalyuk, I.F.; Kudlay, D.A. New coronaviral infection: Features of clinical course, capabilities of diagnostics, treatment and prevention in adults and children. Vopr. Sovrem. Pediatr. Curr. Pediatr. 2020, 19, 123–131. [Google Scholar] [CrossRef]
- Azer, S.A. COVID-19: Pathophysiology, diagnosis, complications and investigational therapeutics. New Microbes New Infect. 2020, 37, 100738. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Beitler, J.R.; Brochard, L.; Calfee, C.S.; Ferguson, N.D.; Slutsky, A.S.; Brodie, D. COVID-19-associated acute respiratory distress syndrome: Is a different approach to management warranted? Lancet Respir. Med. 2020, 8, 816–821. [Google Scholar] [CrossRef]
- Tan, C.W.; Low, J.G.H.; Wong, W.H.; Chua, Y.Y.; Goh, S.L.; Ng, H.J. Critically ill COVID-19 infected patients exhibit increased clot waveform analysis parameters consistent with hypercoagulability. Am. J. Hematol. 2020, 95, E156–E158. [Google Scholar] [CrossRef]
- Lei, J.; Li, J.; Li, X.; Qi, X. CT Imaging of the 2019 Novel Coronavirus (2019-NCoV) Pneumonia. Radiology 2020, 295, 18. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Zhang, J.; He, J.; Zhu, F. Distribution of HLA allele frequencies in 82 Chinese individuals with coronavirus disease-2019 (COVID-19). HLA 2020, 96, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Severe Covid-19 GWAS Group; Ellinghaus, D.; Degenhardt, F.; Bujanda, L.; Buti, M.; Albillos, A.; Invernizzi, P.; Fernández, J.; Prati, D.; Baselli, G.; et al. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar]
- Lu, C.; Gam, R.; Pandurangan, A.P.; Gough, J. Genetic risk factors for death with SARS-CoV-2 from the UK Biobank. medRxiv 2020. [Google Scholar] [CrossRef]
- Van Der Made, C.I.; Simons, A.; Schuurs-Hoeijmakers, J.; Van Den Heuvel, G.; Mantere, T.; Kersten, S.; Van Deuren, R.C.; Steehouwer, M.; Van Reijmersdal, S.V.; Jaeger, M.; et al. Presence of Genetic Variants among Young Men with Severe COVID-19. JAMA J. Am. Med. Assoc. 2020, 324, 663–673. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, J.; Martin, W.; Kallianpur, A.; Chung, M.K.; Jehi, L.; Cheng, F. New insights into genetic susceptibility of COVID-19: An ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020, 18, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, Z.; Wang, Y.; Zhou, Y.; Ma, Y.; Zuo, W. Single-Cell RNA Expression Profiling of ACE2, the Receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020, 202, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, L.; Feng, Z.; Wan, S.; Huang, P.; Sun, X.; Wen, F.; Huang, X.; Ning, G.; Wang, W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, L.; Zhao, Y.; Zhang, P.; Xu, B.; Li, K.; Liang, L.; Zhang, C.; Dai, Y.; Feng, Y.; et al. Interferon-induced transmembrane protein-3 genetic variant rs12252-C is associated with disease severity in COVID-19. J. Infect. Dis. 2020, 222, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Shi, Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.B.; Lyu, J.R.; Lei, X.M.; Li, W.; Wu, G.; Lyu, J.; Dai, Z.M. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int. J. Infect. Dis. 2020, 96, 19–24. [Google Scholar] [CrossRef]
- Gu, J.; Gong, E.; Zhang, B.; Zheng, J.; Gao, Z.; Zhong, Y.; Zou, W.; Zhan, J.; Wang, S.; Xie, Z.; et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005, 202, 415–424. [Google Scholar] [CrossRef]
- Gu, J.; Korteweg, C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007, 170, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.-L.; Yeung, M.L.; Kok, K.-H.; Yuen, K.-S.; Kew, C.; Lui, P.-Y.; Chan, C.-P.; Tse, H.; Woo, P.C.Y.; Yuen, K.-Y.; et al. Middle East Respiratory Syndrome Coronavirus 4a Protein Is a Double-Stranded RNA-Binding Protein That Suppresses PACT-Induced Activation of RIG-I and MDA5 in the Innate Antiviral Response. J. Virol. 2014, 88, 4866–4876. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xing, Y.; Chen, X.; Zheng, Y.; Yang, Y.; Nichols, D.B.; Clementz, M.A.; Banach, B.S.; Li, K.; Baker, S.C.; et al. Coronavirus Papain-like Proteases Negatively Regulate Antiviral Innate Immune Response through Disruption of STING-Mediated Signaling. PLoS ONE 2012, 7, e30802. [Google Scholar] [CrossRef] [PubMed]
- Frieman, M.; Ratia, K.; Johnston, R.E.; Mesecar, A.D.; Baric, R.S. Severe Acute Respiratory Syndrome Coronavirus Papain-Like Protease Ubiquitin-Like Domain and Catalytic Domain Regulate Antagonism of IRF3 and NF-κB Signaling. J. Virol. 2009, 83, 6689–6705. [Google Scholar] [CrossRef]
- Narayanan, K.; Huang, C.; Lokugamage, K.; Kamitani, W.; Ikegami, T.; Tseng, C.-T.K.; Makino, S. Severe Acute Respiratory Syndrome Coronavirus nsp1 Suppresses Host Gene Expression, Including That of Type I Interferon, in Infected Cells. J. Virol. 2008, 82, 4471–4479. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, P.; Wei, Y.; Yue, H.; Wang, Y.; Hu, M.; Zhang, S.; Cao, T.; Yang, C.; Li, M.; et al. Histopathologic changes and SARS-COV-2 immunostaining in the lung of a patient with coviD-19. Ann. Intern. Med. 2020, 172, 629–632. [Google Scholar] [CrossRef]
- Yang, M. Cell Pyroptosis, a Potential Pathogenic Mechanism of 2019-nCoV Infection. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Ciceri, F.; Beretta, L.; Scandroglio, A.M.; Colombo, S.; Landoni, G.; Ruggeri, A.; Peccatori, J.; D’Angelo, A.; De Cobelli, F.; Rovere-Querini, P.; et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): An atypical acute respiratory distress syndrome working hypothesis. Crit. Care Resusc. 2020, 22, 95–97. [Google Scholar] [PubMed]
- Wan, S.; Yi, Q.; Fan, S.; Lv, J.; Zhang, X.; Guo, L.; Lang, C.; Xiao, Q.; Xiao, K.; Yi, Z.; et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv 2020. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 55, 105954. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 2016, 8, 959–970. [Google Scholar] [CrossRef]
- Arango, M.T.; Perricone, C.; Kivity, S.; Cipriano, E.; Ceccarelli, F.; Valesini, G.; Shoenfeld, Y. HLA-DRB1 the notorious gene in the mosaic of autoimmunity. Immunol. Res. 2017, 65, 82–98. [Google Scholar] [CrossRef]
- Choo, S.Y. The HLA system: Genetics, immunology, clinical testing, and clinical implications. Yonsei Med. J. 2007, 48, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Tseng, H.K.; Trejaut, J.A.; Lee, H.L.; Loo, J.H.; Chu, C.C.; Chen, P.J.; Su, Y.W.; Lim, K.H.; Tsai, Z.U.; et al. Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med. Genet. 2003, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; David, J.K.; Maden, S.K.; Wood, M.A.; Weeder, B.R.; Nellore, A.; Thompson, R.F. Human leukocyte antigen susceptibility map for SARS-CoV-2. J. Virol. 2020, 94, e00510-20. [Google Scholar] [CrossRef] [PubMed]
- Wein, A.N.; McMaster, S.R.; Takamura, S.; Dunbar, P.R.; Cartwright, E.K.; Hayward, S.L.; McManus, D.T.; Shimaoka, T.; Ueha, S.; Tsukui, T.; et al. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J. Exp. Med. 2019, 216, 2748–2762. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J.M. Trilogy of ACE2: A peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010, 128, 119–128. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Züst, R.; Weber, F.; Spiegel, M.; Lang, K.S.; Akira, S.; Thiel, V.; Ludewig, B. Control of coronavirus infection through plasmacytoid dendritic-cell- derived type I interferon. Blood 2007, 109, 1131–1137. [Google Scholar] [CrossRef]
- Moreno-Eutimio, M.A.; López-Macías, C.; Pastelin-Palacios, R. Bioinformatic Analysis and Identification of Single-Stranded RNA Sequences Recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV Genomes. Microbes Infect. 2020, 22, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chakraborti, S.; Dimitrov, A.S.; Gramatikoff, K.; Dimitrov, D.S. The SARS-CoV S Glycoprotein: Expression and Functional Characterization. Biochem. Biophys. Res. Commun. 2003, 312, 1159–1164. [Google Scholar] [CrossRef]
- Brass, A.L.; Huang, I.C.; Benita, Y.; John, S.P.; Krishnan, M.N.; Feeley, E.M.; Ryan, B.J.; Weyer, J.L.; van der Weyden, L.; Fikrig, E.; et al. The IFITM Proteins Mediate Cellular Resistance to Influenza A H1N1 Virus, West Nile Virus, and Dengue Virus. Cell 2009, 139, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Wakim, L.M.; Gupta, N.; Mintern, J.D.; Villadangos, J.A. Enhanced survival of lung tissue-resident memory CD8 + T cells during infection with influenza virus due to selective expression of IFITM3. Nat. Immunol. 2013, 14, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.J.; Zhang, W.N.; Wang, W.D.; He, S.Y.; Liang, C.C.; Wang, D.W. Two Potential Novel SARS-CoV-2 Entries, TMPRSS2 and IFITM3, in Healthy Individuals and Cancer Patients. SSRN Electron. J. 2020, 16, 3028–3036. [Google Scholar] [CrossRef]
- Babcock, G.J.; Esshaki, D.J.; Thomas, W.D.; Ambrosino, D.M. Amino Acids 270 to 510 of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein Are Required for Interaction with Receptor. J. Virol. 2004, 78, 4552–4560. [Google Scholar] [CrossRef]
- Bosch, B.J.; Martina, B.E.E.; Van Der Zee, R.; Lepault, J.; Haijema, B.J.; Versluis, C.; Heck, A.J.R.; De Groot, R.; Osterhaus, A.D.M.E.; Rottier, P.J.M. Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection Inhibition Using Spike Protein Heptad Repeat-Derived Peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 8455–8460. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, G.; Chen, Y.; He, Y.; Niu, J.; Escalante, C.R.; Xiong, H.; Farmar, J.; Debnath, A.K.; Tien, P.; et al. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: Implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet 2004, 363, 938–947. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Y.; Pan, Y.; Zhao, Z.J. Structure analysis of the receptor binding of 2019-nCoV. Biochem. Biophys. Res. Commun. 2020, 525, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, W.; Farzan, M.; Harrison, S.C. Structural biology: Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 2005, 309, 1864–1868. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Coutard, B.; Valle, C.; de Lamballerie, X.; Canard, B.; Seidah, N.G.; Decroly, E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020, 176, 104742. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Penninger, J.M. Angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Cell. Mol. Life Sci. 2007, 64, 2006–2012. [Google Scholar] [CrossRef]
- Yang, X.H.; Deng, W.; Tong, Z.; Liu, Y.X.; Zhang, L.F.; Zhu, H.; Qin, C. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp. Med. 2007, 57, 450–459. [Google Scholar]
- Zhang, H.; Kang, Z.; Gong, H.; Xu, D.; Wang, J.; Li, Z.; Li, Z.; Cui, X.; Xiao, J.; Zhan, J.; et al. Digestive system is a potential route of COVID-19: An analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 2020, 69, 1010–1018. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team China CDC The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronaviurs Diseases. Vital Surveill. 2020, 41, 145–151.
- Zeng, F.; Dai, C.; Cai, P.; Wang, J.; Xu, L.; Li, J.; Hu, G.; Wang, L. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between gender. J. Med. Virol. 2020, 92, 2050–2054. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Hejblum, B.P.; Simon, N.; Jojic, V.; Dekker, C.L.; Thiébaut, R.; Davis, M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Taneja, V. Sex hormones determine immune response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Hughes, G.C.; Choubey, D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat. Rev. Rheumatol. 2014, 10, 740–751. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fett, C.; Mack, M.; Ten Eyck, P.P.; Meyerholz, D.K.; Perlman, S. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. J. Immunol. 2017, 198, 4046–4053. [Google Scholar] [CrossRef]
- Straub, R.H.; Bijlsma, J.W.; Masi, A.; Cutolo, M. Role of neuroendocrine and neuroimmune mechanisms in chronic inflammatory rheumatic diseases-The 10-year update. Semin. Arthritis Rheum. 2013, 43, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Mandel, M.; Sheonefeld, Y. Coronavirus 2019 outbreak pathogenesis: Why China and Italy? Eur. J. Rheumatol. 2020, 7, S99–S101. [Google Scholar] [CrossRef]
- Pathak, G.A.; Singh, K.; Miller-Fleming, T.W.; Wendt, F.; Ehsan, N.; Hou, K.; Johnson, R.; Lu, Z.; Gopalan, S.; Dimbou, L.Y.; et al. Integrative analyses identify susceptibility genes underlying COVID-19 hospitalization. medRxiv 2020. [Google Scholar] [CrossRef]
| Author | Country | Number of Patients | Study Variables | Method |
|---|---|---|---|---|
| Wang et al., 2020 [7] | China | 82 | HLA | Next-generation sequencing (NGS) |
| The Severe Covid-19 genome-wide association study (GWAS) Group, 2020 [8] | Spain Italy | 1980 | Single-nucleotide polymorphisms | Global Screening Array (GSA) |
| Lu et al., 2020 [9] | UK | 193 deaths from 1412 confirmed infections in the 5871 group | Distribution of genetic variants | GWAS |
| Van Der Made et al., 2020 [10] | The Netherlands | 4 | Distribution of genetic variants | Rapid clinical whole-exome sequencing |
| Hou et al., 2020 [11] | China | 81,000 human genomes | Distribution of genetic variants | Work with databases |
| Zhao et al., 2020 [12] | China | 8 | Single-cell RNA-sequencing | Unsupervised graph-based clustering |
| Cao et al., 2020 [13] | China | 1700 variants | Functional coding variants in ACE-2 | Work with databases |
| Zhang et al., 2020 [14] | China | 80 | 300 bp locus spanning rs12252 | Next-generation sequencing (NGS) |
| Marker Type | Marker | Pathogenetic Effect |
|---|---|---|
| Associated with the immune response | HLA-B*46:01/A25:01/C01:02 | Suitable MHC molecules have a low affinity for the virus proteins, which can make the representatives of this genotype especially vulnerable |
| Hypoexpression of the CXCR6 gene | Encodes chemokine receptor 6 with the CXC motif (CXCR6), which regulates the specific location of lung tissue-resident-memory CD8+ T cells specific against respiratory pathogens | |
| Polymorphism of the CCR9 gene | Encodes a homeostatic and inflammatory chemokine receptor, which is involved in the pathogenesis of pneumonia of various origins and its expression is induced in the early stages of airway inflammation. | |
| X-chromosomal TLR7 gene | Encodes toll-like receptor 7, is associated with the maintenance of the innate immune response against coronaviruses, with the IFN-γ production | |
| rs150892504 mutation of the ERAP2 gene | Encodes metalloaminopeptidase involved in the final modification of antigens for presentation by MHC-I molecules | |
| rs12252 allele of IFITM3 gene | Encodes interferon-induced transmembrane protein 3, which enhances the accumulation of CD8+ T cells in airways to promote mucosal immune cell persistence | |
| ACE-2 protein-associated | ACE-2 wild-type or naïve expression | Risk of severe disease |
| ACE-2 allele P.Arg514Gly | Affects the renin-angiotensin system (RAS) function—the risk of cardiovascular and pulmonary complications | |
| ACE-2 alleles p.Arg708Trp p.Arg710Cys p.Arg710His, p.Arg716Cys | Facilitates the penetration of SARS-S into host cells | |
| Overexpression of the SLC6A20 gene | Encodes sodium/imino-acid (proline) transporter 1 (SIT1), which functionally interacts with angiotension-converting enzyme 2 | |
| TMPRSS2 wild-type or naïve expression | Risk of severe disease | |
| TMPRSS2 p.Val160Met allele | Risk of severe disease among men |
| Population | HLA Genotypes with a Possible Protective Function | Predisposing HLA Genotypes | ACE Expression | Number of Cases | Number of Severe Cases | % of Severe Cases |
|---|---|---|---|---|---|---|
| Africans/ African American (AFR) | C*12:03 A*02:02 B*15:03 | polymorphisms of differential genetic susceptibility to COVID-19 | 2,199,524 | 2.611 | 0.12% | |
| Non-Finnish Europeans (EUR) | C*12:03 | A*25:01 | polymorphisms of differential genetic susceptibility to COVID-19 | 17,480,113 | 28.587 | 0.16% |
| Hispanic/Mixed-race American (AMR) | A*02:02 C*12:03 | C*01:02 | 11,216,585 | 17.025 | 0.15% | |
| East Asians (EAS) | C*12:03 | A*25:01 B*46:01 C*01:02 | 16,893,952 | 27.397 | 0.16% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malkova, A.; Kudlay, D.; Kudryavtsev, I.; Starshinova, A.; Yablonskiy, P.; Shoenfeld, Y. Immunogenetic Predictors of Severe COVID-19. Vaccines 2021, 9, 211. https://doi.org/10.3390/vaccines9030211
Malkova A, Kudlay D, Kudryavtsev I, Starshinova A, Yablonskiy P, Shoenfeld Y. Immunogenetic Predictors of Severe COVID-19. Vaccines. 2021; 9(3):211. https://doi.org/10.3390/vaccines9030211
Chicago/Turabian StyleMalkova, Anna, Dmitriy Kudlay, Igor Kudryavtsev, Anna Starshinova, Piotr Yablonskiy, and Yehuda Shoenfeld. 2021. "Immunogenetic Predictors of Severe COVID-19" Vaccines 9, no. 3: 211. https://doi.org/10.3390/vaccines9030211
APA StyleMalkova, A., Kudlay, D., Kudryavtsev, I., Starshinova, A., Yablonskiy, P., & Shoenfeld, Y. (2021). Immunogenetic Predictors of Severe COVID-19. Vaccines, 9(3), 211. https://doi.org/10.3390/vaccines9030211









