Are We Ready for the Arrival of the New COVID-19 Vaccinations? Great Promises and Unknown Challenges Still to Come
Abstract
1. Introduction
2. Type of Anti-COVID-19 Vaccines, Their Efficacy and Safety, and Vaccination Strategies
3. Acceptance towards Anti-COVID-19 Vaccination
4. Role of Social Media in the Information/Communication Campaign, with a Particular Focus on Vaccine Hesitancy
5. A Case Study for Italy: A Twitter Volumes Analysis
6. Main Considerations and Challenges Ahead
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pneumonia of Unknown Cause—China. Available online: https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ (accessed on 31 January 2021).
- Abbasi, J. COVID-19 and mRNA Vaccines-First Large Test for a New Approach. JAMA 2020, 324, 1125–1127. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. The race for coronavirus vaccines: A graphical guide. Nature 2020, 580, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Increased Risk of Hospitalization or Death. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html (accessed on 31 January 2021).
- Fantini, M.P.; Reno, C.; Biserni, G.B.; Savoia, E.; Lanari, M. COVID-19 and the re-opening of schools: A policy maker’s dilemma. Ital. J. Pediatr. 2020, 46, 79. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, M.C.; Galvani, A.P. Optimizing age-specific vaccination. Science 2021, eabg2334. [Google Scholar] [CrossRef]
- Baldo, V.; Reno, C.; Cocchio, S.; Fantini, M.P. SARS-CoV-2/COVID-19 Vaccines: The Promises and the Challenges Ahead. Vaccines 2021, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Piedrahita-Valdés, H.; Piedrahita-Castillo, D.; Bermejo-Higuera, J.; Guillem-Saiz, P.; Bermejo-Higuera, J.R.; Guillem-Saiz, J.; Sicilia-Montalvo, J.A.; Machío-Regidor, F. Vaccine Hesitancy on Social Media: Sentiment Analysis from June 2011 to April 2019. Vaccines 2021, 9, 28. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Peacock, R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: Audit of primary sources. BMJ 2005, 331, 1064–1065. [Google Scholar] [CrossRef]
- COVID-19 Vaccines: Key Facts. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-vaccines-key-facts (accessed on 31 January 2021).
- The COVID-19 Pandemic Has Prompted Numerous Research Institutes and Companies to Develop Vaccine Candidates Targeting This Novel Disease. Available online: https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/ (accessed on 31 January 2021).
- Coronavirus Vaccines Strategy. Available online: https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/public-health/coronavirus-vaccines-strategy_en (accessed on 31 January 2021).
- The Story of mRNA: How a Once-Dismissed Idea Became a Leading Technology in the Covid Vaccine Race. Available online: https://www.statnews.com/2020/11/10/the-story-of-mrna-how-a-once-dismissed-idea-became-a-leading-technology-in-the-covid-vaccine-race/ (accessed on 31 January 2021).
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef]
- EMA Recommends COVID-19 Vaccine AstraZeneca for Authorisation in the EU. Available online: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-astrazeneca-authorisation-eu (accessed on 31 January 2021).
- AIFA: Autorizzato Vaccino AstraZeneca. Available online: https://www.aifa.gov.it/en/-/aifa-autorizzato-vaccino-astrazeneca (accessed on 31 January 2021).
- The Oxford/AstraZeneca COVID-19 Vaccine: What You Need to Know. Available online: https://www.who.int/news-room/feature-stories/detail/the-oxford-astrazeneca-covid-19-vaccine-what-you-need-to-know (accessed on 31 January 2021).
- Suspicions Grow That Nanoparticles in Pfizer’s COVID-19 Vaccine Trigger Rare Allergic Reactions. Available online: https://www.sciencemag.org/news/2020/12/suspicions-grow-nanoparticles-pfizer-s-covid-19-vaccine-trigger-rare-allergic-reactions (accessed on 31 January 2021).
- Risk Assessment: Risk Related to the Spread of New SARS-CoV-2 Variants of Concern in the EU/EEA—First Update. Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-risk-assessment-spread-new-variants-concern-eueea-first-update (accessed on 31 January 2021).
- Moore, J.P.; Offit, P.A. SARS-CoV-2 Vaccines and the Growing Threat of Viral Variants. JAMA 2021. [Google Scholar] [CrossRef]
- Callaway, E.; Ledford, H. How to redesign covid vaccines so they protect against variants. Nature 2021. [Google Scholar] [CrossRef]
- Graffigna, G.; Palamenghi, L.; Boccia, S.; Barello, S. Relationship between Citizens’ Health Engagement and Intention to Take the COVID-19 Vaccine in Italy: A Mediation Analysis. Vaccines 2020, 8, 576. [Google Scholar] [CrossRef] [PubMed]
- AP-NORC Poll: Only Half in US Want Shots as Vaccine Nears. Available online: https://apnews.com/article/ap-norc-poll-us-half-want-vaccine-shots-4d98dbfc0a64d60d52ac84c3065dac55 (accessed on 31 January 2021).
- Szilagyi, P.G.; Thomas, K.; Shah, M.D.; Vizueta, N.; Cui, Y.; Vangala, S.; Kapteyn, A. National Trends in the US Public’s Likelihood of Getting a COVID-19 Vaccine—April 1 to December 8, 2020. JAMA 2021, 325, 396–398. [Google Scholar] [CrossRef]
- COVID-19 Vaccination Intent is Decreasing Globally. Available online: https://www.ipsos.com/en/global-attitudes-covid-19-vaccine-october-2020 (accessed on 31 January 2021).
- World Health Organization. Ten Threats to Global Health in 2019. 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 31 January 2021).
- MacDonald, N.E. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, R.; Villani, L.; Mariani, M.; Ricciardi, W.; Graffigna, G.; Boccia, S. Impact of COVID-19 Pandemic on Flu and COVID-19 Vaccination Intentions among University Students. Vaccines 2021, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.; Madden, K. HPV vaccine information in the blogosphere: How positive and negative blogs influence vaccine-related risk perceptions, attitudes, and behavioral intentions. Health Commun. 2012, 27, 829–836. [Google Scholar] [CrossRef]
- Buller, D.B.; Walkosz, B.J.; Berteletti, J.; Pagoto, S.L.; Bibeau, J.; Baker, K.; Hillhouse, J.; Henry, K.L. Insights on HPV vaccination in the United States from mothers’ comments on Facebook posts in a randomized trial. Hum. Vaccines Immunother. 2019, 15, 1479–1487. [Google Scholar] [CrossRef]
- Johnson, N.F.; Velásquez, N.; Restrepo, N.J.; Leahy, R.; Gabriel, N.; El Oud, S.; Zheng, M.; Manrique, P.; Wuchty, S.; Lupu, Y. The online competition between pro- and anti-vaccination views. Nature 2020, 582, 230–233. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Systematic Scoping Review on Social Media Monitoring Methods and Interventions Relating to Vaccine Hesitancy; ECDC: Stockholm, Sweden, 2020. [Google Scholar]
- Durazzi, F.; Müller, M.; Salathé, M.; Remondini, D. International Expert Communities on Twitter Become More Isolated during the COVID-19 Pandemic. Available online: https://arxiv.org/abs/2011.06845 (accessed on 31 January 2021).
- Yadollahi, A.; Shahraki, A.G.; Zaiane, O.R. Current state of text sentiment analysis from opinion to emotion mining. ACM Comput. Surv. 2017, 50, 1–33. [Google Scholar] [CrossRef]
- Cambria, E. Affective Computing and Sentiment Analysis. IEEE Intell. Syst. 2016, 31, 102–107. [Google Scholar] [CrossRef]
- Deiner, M.S.; Fathy, C.; Kim, J.; Niemeyer, K.; Ramirez, D.; Ackley, S.F.; Liu, F.; Lietman, T.M.; Porco, T.C. Facebook and Twitter vaccine sentiment in response to measles outbreaks. Health Inform. J. 2019, 25, 1116–1132. [Google Scholar] [CrossRef] [PubMed]
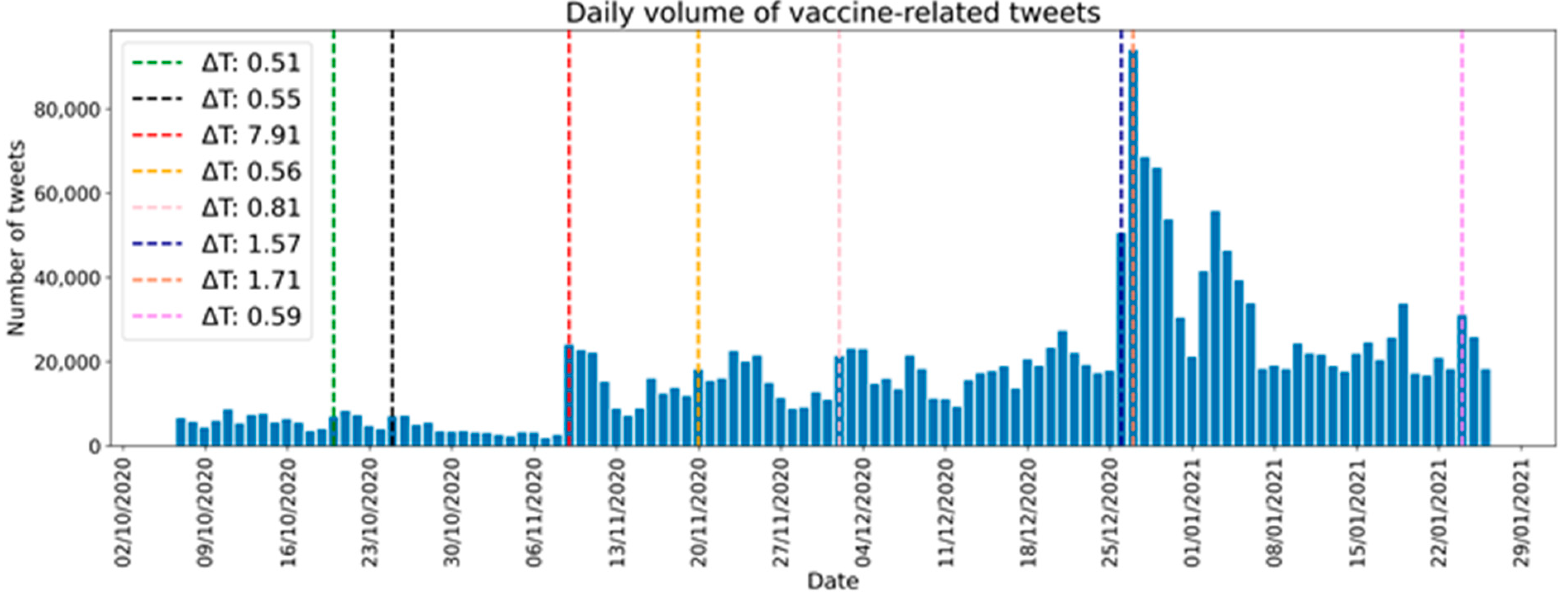
| Date | ΔT | Main Events |
|---|---|---|
| 20-10-2020 | 0.51 | Rumors about vaccine in December |
| 25-10-2020 | 0.55 | Press conference about vaccine in December |
| 9-11-2020 | 7.91 | Pfizer’s vaccine announced |
| 20-11-2020 | 0.56 | Skeptical statement on vaccine by a well-known physician |
| 2-12-2020 | 0.81 | Vaccination plan presented by Minister of Health |
| 26-12-2020 | 1.57 | First doses of vaccine arrive in Italy |
| 27-12-2020 | 1.71 | First day of vaccination in Italy |
| 24-01-2020 | 0.59 | Controversy over a call for a pavilion for vaccinations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gori, D.; Reno, C.; Remondini, D.; Durazzi, F.; Fantini, M.P. Are We Ready for the Arrival of the New COVID-19 Vaccinations? Great Promises and Unknown Challenges Still to Come. Vaccines 2021, 9, 173. https://doi.org/10.3390/vaccines9020173
Gori D, Reno C, Remondini D, Durazzi F, Fantini MP. Are We Ready for the Arrival of the New COVID-19 Vaccinations? Great Promises and Unknown Challenges Still to Come. Vaccines. 2021; 9(2):173. https://doi.org/10.3390/vaccines9020173
Chicago/Turabian StyleGori, Davide, Chiara Reno, Daniel Remondini, Francesco Durazzi, and Maria Pia Fantini. 2021. "Are We Ready for the Arrival of the New COVID-19 Vaccinations? Great Promises and Unknown Challenges Still to Come" Vaccines 9, no. 2: 173. https://doi.org/10.3390/vaccines9020173
APA StyleGori, D., Reno, C., Remondini, D., Durazzi, F., & Fantini, M. P. (2021). Are We Ready for the Arrival of the New COVID-19 Vaccinations? Great Promises and Unknown Challenges Still to Come. Vaccines, 9(2), 173. https://doi.org/10.3390/vaccines9020173









