Immunogenicity after Second ChAdOx1 nCoV-19 (AZD1222) Vaccination According to the Individual Reactogenicity, Health Status and Lifestyle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Measurement of Adverse Events
2.3. Measurements of Individual Health Status
2.4. Measurement of Cellular and Humoral Immune Responses
2.4.1. Humoral Immunity
2.4.2. Cellular Immunity
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. Humoral and Cellular Immunogenicity
3.3. Reactogenicity
3.4. Association between Immunogenicity, Reactogenicity, and Individual Health Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Anti-S1 Antibody (S/co) | sVNT (%) | T-Spot (/250,000 PMCs) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive Symptom | Negative Symptom | p | Positive Symptom | Negative Symptom | p | Positive Symptom | Negative Symptom | p | ||
| 1st dose | Fever (58.2%) | 5.2 (±1.5) | 5.6 (±1.8) | 0.26 | 74.9 (±17.7) | 75.1 (±21.4) | 0.97 | 10.4 (±8.1) | 9.7 (±7.0) | 0.69 |
| Chills (62.0%) | 5.2 (±1.5) | 5.67 (±1.81) | 0.17 | 74.0 (±18.3) | 76.6 (±30.8) | 0.60 | 10.8 (±1.6) | 9.1 (±7.1) | 0.34 | |
| Headache (54.4%) | 5.1 (±1.3) | 5.61 (±1.9) | 0.20 | 74.9 (±17.6) | 75.0 (±21.2) | 0.97 | 10.5 (±7.9) | 9.7 (±7.4) | 0.68 | |
| Muscle pain (72.2%) | 5.3 (±1.4) | 5.52 (±2.11) | 0.62 | 76.0 (±17.6) | 72.2 (±23.0) | 0.49 | 9.9 (±7.1) | 10.9 (±9.1) | 0.59 | |
| Fatigue (79.7%) | 5.3 (±1.4) | 5.6 (±2.32) | 0.61 | 75.6 (±17.7) | 72.6 (±24.8) | 0.66 | 10.4 (±7.9) | 8.9 (±6.2) | 0.51 | |
| Nausea & Vomiting (12.7%) | 5.9 (±1.4) | 5.27 (±1.64) | 0.27 | 80.8 (±14.8) | 74.1 (±19.7) | 0.31 | 14.4 (±11.1) | 9.5 (±6.8) | 0.21 | |
| Rash (5.1%) | 6.0 (±0.8) | 5.31 (±1.64) | 0.41 | 89.4 (±7.7) | 74.2 (±19.3) | 0.02 | 13.9 (±15.3) | 9.9 (±7.1) | 0.64 | |
| Anaphylactoid reaction (1.3%) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A) | N/A | |
| Tenderness (84.8%) | 5.5 (±1.6) | 4.72 (±1.91) | 0.15 | 76.5 (±17.9) | 66.4 (±24.4) | 0.09 | 10.4 (±8) | 9.0 (±5.4) | 0.58 | |
| Pain (53.2%) | 10.2 (±8.5) | 10.08 (±6.54) | 0.32 | 74.1 (±18.5) | 75.9 (±20.2) | 0.68 | 10.2 (±1.3) | 10.1 (±1.1) | 0.95 | |
| Redness (20.3%) | 5.1 (±1.1) | 5.4 (±1.74) | 0.42 | 72.4 (±17.3) | 75.6 (±19.7) | 0.55 | 7.8 (±5.0) | 10.7 (±8.1) | 0.19 | |
| Swelling (30.4%) | 5.3 (±1.2) | 5.38 (±1.77) | 0.73 | 73.6 (±17.0) | 75.5 (±20.2) | 0.66 | 8.5 (±7.0) | 10.9 (±7.8) | 0.20 | |
| 2nd dose | Fever (20.3%) | 5.4 (±1.9) | 5.32 (±1.55) | 0.81 | 76.1 (±20.3) | 74.7 (±19.1) | 0.79 | 10.2 (±10) | 10.1 (±7.0) | 0.96 |
| Chills (20.3%) | 6.2 (±1.7) | 5.16 (±1.55) | 0.03 | 85.1 (±14.7) | 72.8 (±19.4) | 0.03 | 13.0 (±9.6) | 9.6 (±7.1) | 0.15 | |
| Headache (36.7%) | 5.1 (±1.5) | 5.47 (±1.71) | 0.37 | 75.4 (±17.8) | 74.7 (±20.1) | 0.88 | 11.4 (±6.9) | 9.3 (±6.6) | 0.27 | |
| Muscle pain (43.0%) | 5.5 (±1.6) | 5.21 (±1.65) | 0.41 | 78.0 (±17.7) | 72.7 (±20.1) | 0.23 | 10.2 (±8.9) | 10.1 (±6.6) | 0.98 | |
| Fatigue (59.5%) | 5.4 (±1.5) | 5.26 (±1.85) | 0.71 | 77.6 (±16.5) | 71.0 (±22.2) | 0.16 | 10.8 (±8.4) | 9.1 (±6.2) | 0.37 | |
| Nausea & Vomiting (3.8%) | 6.1 (±1.3) | 5.31 (±1.63) | 0.40 | 83.7 (±14.1) | 74.6 (±19.4) | 0.43 | 16.3 (±16) | 9.9 (±7.2) | 0.56 | |
| Rash (1.3%) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Anaphylactoid reaction (0.0%) | N/A | N/A) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Tenderness (67.1%) | 5.4 (±1.5) | 5.18 (±1.88) | 0.52 | 77.8 (±16.4) | 69.3 (±23.3) | 0.10 | 9.5 (±7.3) | 11.5 (±8.3) | 0.29 | |
| Pain (36.7%) | 5.3 (±1.5) | 5.39 (±1.7) | 0.72 | 75.1 (±17.9) | 74.9 (±20.1) | 0.97 | 8.7 (±7.4) | 11.0 (±7.7) | 0.22 | |
| Redness (17.7%) | 5.1 (±1.3) | 5.4 (±1.68) | 0.48 | 71.2 (±18.4) | 75.8 (±19.4) | 0.42 | 7.8 (±5.4) | 10.6 (±8.0) | 0.23 | |
| Swelling (16.5%) | 5.1 (±1.1) | 5.38 (±1.71) | 0.63 | 71.2 (±16.3) | 75.7 (±19.7) | 0.44 | 7.0 (±5.2) | 10.7 (±7.9) | 0.12 | |
| Anti-S1 Antibody | sVNT | T-Spot | ||||||
|---|---|---|---|---|---|---|---|---|
| Variables (N) | Mean (SD) | p | Mean (SD) | p | Mean (SD) | p | ||
| Sex | Male (21) | 5.2 (±1.9) | 0.73 | 73.7 (±20.9) | 0.73 | 12.9 (±7.8) | 0.08 | |
| Female (58) | 5.4 (±1.5) | 75.4 (±18.7) | 9.3 (±7.4) | |||||
| Age | <40 (55) | 5.4 (±1.6) | 0.47 | 76.6 (±17.7) | 0.31 | 9.8 (±7.6) | 0.58 | |
| ≥40 (24) | 5.1 (±1.8) | 71.2 (±22.2) | 10.9 (±7.7) | |||||
| Smoking | No (70) | 5.3 (±1.7) | 0.26 | 74.0 (±19.5) | 0.23 | 10.1 (±7.9) | 0.83 | |
| Yes (9) | 5.9 (±1.3) | 82.2 (±16.1) | 10.7 (±4.8) | |||||
| Alcohol consumption | Mild to moderate (45) | 5.3 (±1.5) | 0.98 | 75.5 (±18.6) | 0.78 | 8.9 (±5.9) | 0.13 | |
| Heavy or binge (34) | 5.3 (±1.7) | 74.2 (±20.2) | 11.9 (±9.3) | |||||
| BMI | <25 (57) | 5.2 (±1.5) | 0.44 | 74.2 (±18.3) | 0.51 | 10.1 (±7.9) | 0.08 | |
| 25–30 (18) | 5.7 (±0.4) | 78.7 (±19.8) | 11.8 (±6.7) | |||||
| >30 (4) | 6.1 (±0.8) | 68.8 (±31.4) | 4.0 (±5.0) | |||||
| Pulmonary tuberculosis | No (75) | 5.3 (±1.6) | 0.43 | 74.4 (±19.3) | 0.27 | 10.2 (±7.7) | 0.92 | |
| Yes(4) | 6.0 (±0.8) | 85.4 (±15.7) | 7.8 (±6.0) | |||||
| HTN | No (76) | 5.3 (±1.6) | 0.90 | 75.2 (±19.0) | 0.50 | 10.3 (±7.7) | 0.47 | |
| Yes (3) | 5.5 (±2.8) | 67.7 (±26.4) | 7.0 (±3.5) | |||||
| Dyslipidemia | No (76) | 5.3 (±1.6) | 0.94 | 74.7 (±19.0) | 0.62 | 10.1 (±7.7) | 0.78 | |
| Yes (3) | 5.4 (±1.8) | 80.4 (±28.5) | 11.3 (±5.8) | |||||
| DM | No (78) | 5.4 (±1.6) | 0.24 | 75.3 (±19.1) | 0.15 | 10.2 (±7.6) | 0.50 | |
| Yes (1) | 3.5 (±NA) | 47.5 (±NA) | 5.0 (±NA) | |||||
| Abnormal LFT | No (71) | 5.4 (±1.7) | 0.90 | 75.1 (±18.7) | 0.84 | 10.3 (±7.8) | 0.67 | |
| Yes (8) | 5.3 (±1.4) | 73.7 (±24.5) | 9.1 (±5.9) | |||||
| Abnormal RFT | No (72) | 5.3 (±1.6) | 0.25 | 74.7 (±19.5) | 0.75 | 9.6 (±7.0) | 0.19 | |
| Yes (7) | 6.0 (±1.4) | 77.1 (±16.8) | 15.7 (±11.0) | |||||
| Physical activity | Lack (42) | 5.2 (±1.6) | 0.52 | 73.1 (±21.2) | 0.36 | 10.6 (±7.4) | 0.60 | |
| Adequate (37) | 5.5 (±1.6) | 77.0 (±16.7) | 9.6 (±7.9) | |||||
| Muscle exercise | Lack (52) | 5.3 (±1.4) | 0.80 | 74.0 (±18.7) | 0.55 | 9.1 (±7.0) | 0.09 | |
| Adequate (27) | 5.4 (±1.9) | 76.8 (±20.4) | 12.4 (±8.6) | |||||
| 1st dose | Antipyretics | No (8) | 5.4 (±1.5) | 0.68 | 76.2 (±17.9) | 0.14 | 10.1 (±7.7) | 0.97 |
| Yes (69) | 5.0 (±2.5) | 66.5 (±26.1) | 10.3 (±7.3) | |||||
| Antipyretics intake | Prophylactic (30) | 5.4 (±1.5) | 0.58 | 75.1 (±17.5) | 0.62 | 10.5 (±7.4) | 0.76 | |
| After symptom onset (39) | 5.4 (±1.5) | 77.4 (±18.4) | 9.9 (±8.1) | |||||
| 2nd dose | Antipyretics | No (28) | 5.4 (±1.5) | 0.83 | 76.1 (±17.6) | 0.46 | 10.6 (±8.4) | 0.51 |
| Yes (51) | 5.3 (±1.8) | 72.8 (±21.9) | 9.3 (±5.7) | |||||
| Antipyretics intake | Prophylactic (24) | 5.5 (±1.8) | 0.51 | 76.3 (±17.2) | 0.85 | 11.9 (±8.9) | 0.33 | |
| After symptom onset (27) | 5.2 (±1.3) | 75.4 (±18.3) | 9.5 (±8.3) | |||||
| SRI | <13 (37) | 5.3 (±1.8) | 0.81 | 72.9 (±21.0) | 0.38 | 10.8 (±7.8) | 0.49 | |
| ≥13 (42) | 5.4 (±1.5) | 76.8 (±17.5) | 9.6 (±7.5) | |||||
| SRID | <29 (40) | 5.4 (±1.8) | 0.75 | 74.6 (±20.8) | 0.88 | 11.3 (±7.7) | 0.21 | |
| ≥29 (39) | 5.3 (±1.5) | 75.3 (±17.6) | 9.1 (±7.5) | |||||
References
- McDonald, I.; Murray, S.M.; Reynolds, C.J.; Altmann, D.M.; Boyton, R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines 2021, 6, 74. [Google Scholar] [CrossRef]
- Ni, L.; Ye, F.; Cheng, M.-L.; Feng, Y.; Deng, Y.-Q.; Zhao, H.; Wei, P.; Ge, J.; Gou, M.; Li, X.; et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 2020, 52, 971–977.e3. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hillyer, C.; Du, L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol. 2020, 41, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.-B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168.e14. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Jeon, M.; Kim, J.; Oh, C.E.; Lee, J.-Y. Adverse Events Following Immunization Associated with Coronavirus Disease 2019 Vaccination Reported in the Mobile Vaccine Adverse Events Reporting System. J. Korean Med. Sci. 2021, 36, e114. [Google Scholar] [CrossRef] [PubMed]
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines 2019, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Luk, T.T.; Zhao, S.; Wu, Y.; Wong, J.Y.-H.; Wang, M.P.; Lam, T.H. Prevalence and determinants of SARS-CoV-2 vaccine hesitancy in Hong Kong: A population-based survey. Vaccine 2021, 39, 3602–3607. [Google Scholar] [CrossRef]
- Fisher, K.A.; Bloomstone, S.J.; Walder, J.; Crawford, S.; Fouayzi, H.; Mazor, K.M. Attitudes toward a potential SARS-CoV-2 vaccine: A survey of U.S. adults. Ann. Intern. Med. 2020, 173, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.H.; Song, K.-H.; Choi, Y.; Go, S.; Choi, S.-J.; Jung, J.; Kang, C.K.; Choe, P.G.; Kim, N.-J.; Park, W.B.; et al. Can reac-togenicity predict immunogenicity after COVID-19 vaccination? Korean J. Intern. Med. 2021, 36, 1486–1491. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, N.; Lee, S.K.; Cho, E.-J.; Hyun, J.; Park, M.-J.; Song, W.; Jung, E.J.; Woo, H.; Seo, Y.B.; et al. Comparing Five SARS-CoV-2 Antibody Assay Results before and after the First and Second ChAdOx1 nCoV-19 Vaccination among Health Care Workers: A Prospective Multicenter Study. J. Clin. Microbiol. 2021, 59, e0178821. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Choi, S.-H.; Chung, J.-W.; Hwang, M.-H.; Kim, M.-C. Systemic Adverse Events and Use of Antipyretics Predict the Neutralizing Antibody Positivity Early after the First Dose of ChAdOx1 Coronavirus Disease Vaccine. J. Clin. Med. 2021, 10, 2844. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Lee, N.; Lee, S.K.; Cho, E.-J.; Hyun, J.; Park, M.-J.; Song, W.; Jung, E.J.; Woo, H.; Seo, Y.B.; et al. Comparing Results of Five SARS-CoV-2 Antibody Assays before and after the First Dose of ChAdOx1 nCoV-19 Vaccine among Health Care Workers. J. Clin. Microbiol. 2021, 59, e0110521. [Google Scholar] [CrossRef]
- Guidance for Industry. Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. Available online: https://www.fda.gov/media/73679/download (accessed on 25 November 2021).
- Schoenborn, C.A.; Adams, P.F.; Peregoy, J.A. Health behaviors of adults: United States, 2008–2010. Vital Health Stat. Ser. 10 Data Natl. Health Surv. 2013, 257, 1–184. [Google Scholar]
- Jung, J.-G.; Kim, J.-S.; Yoon, S.-J.; Lee, S.; Ahn, S.-K. Korean Alcohol Guidelines for Primary Care Physician. Korean J. Fam. Pract. 2021, 11, 14–21. [Google Scholar] [CrossRef]
- Bull, F.C.; Maslin, T.S.; Armstrong, T. Global physical activity questionnaire (GPAQ): Nine country reliability and validity study. J. Phys. Act. Health 2009, 6, 790–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, K.-Y. Physical activity level in Korean adults: The Korea National Health and Nutrition Examination Survey 2017. Epidemiol. Health 2019, 41, e2019047. [Google Scholar] [CrossRef]
- Prendecki, M.; Clarke, C.; Edwards, H.; McIntyre, S.; Mortimer, P.; Gleeson, S.; Martin, P.; Thomson, T.; Randell, P.; Shah, A.; et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann. Rheum. Dis. 2021, 80, 1322. [Google Scholar] [CrossRef] [PubMed]
- Chia, W.N.; Zhu, F.; Ong, S.W.X.; Young, B.E.; Fong, S.-W.; Le Bert, N.; Tan, C.W.; Tiu, C.; Zhang, J.; Tan, S.Y.; et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: A longitudinal study. Lancet Microbe 2021, 2, e240–e249. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Tan, A.T.; Linster, M.; Tan, C.W.; Le Bert, N.; Chia, W.N.; Kunasegaran, K.; Zhuang, Y.; Tham, C.Y.L.; Chia, A.; Smith, G.J.D.; et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021, 34, 108728. [Google Scholar] [CrossRef] [PubMed]
- Bertoletti, A.; Le Bert, N.; Qui, M.; Tan, A.T. SARS-CoV-2-specific T cells in infection and vaccination. Cell. Mol. Immunol. 2021, 18, 2307–2312. [Google Scholar] [CrossRef]
- Stankov, M.V.; Cossmann, A.; Bonifacius, A.; Dopfer-Jablonka, A.; Ramos, G.M.; Gödecke, N.; Scharff, A.Z.; Happle, C.; Boeck, A.-L.; Tran, A.T.; et al. Humoral and cellular immune responses against SARS-CoV-2 variants and human coronaviruses after single BNT162b2 vaccination. medRxiv 2021, 73, 2000–2008. [Google Scholar] [CrossRef]
- Patel, S.S.; Molnar, M.Z.; Tayek, J.A.; Ix, J.H.; Noori, N.; Benner, D.; Heymsfield, S.; Kopple, J.D.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum creatinine as a marker of muscle mass in chronic kidney disease: Results of a cross-sectional study and review of literature. J. Cachexia Sarcopenia Muscle 2013, 4, 19–29. [Google Scholar] [CrossRef]
- Li, H.; Manwani, B.; Leng, S.X. Frailty, inflammation, and immunity. Aging Dis. 2011, 2, 466–473. [Google Scholar]
- Montgomery, R.R.; Shaw, A.C. Paradoxical changes in innate immunity in aging: Recent progress and new directions. J. Leukoc. Biol. 2015, 98, 937–943. [Google Scholar] [CrossRef]
- Fischinger, S.; Boudreau, C.M.; Butler, A.L.; Streeck, H.; Alter, G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 2019, 41, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Ruggieri, A.; Anticoli, S.; D’Ambrosio, A.; Giordani, L.; Viora, M. The influence of sex and gender on immunity, infection and vaccination. Ann. Dell’istituto Super. Sanita 2016, 52, 198–204. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Young, K.M.; Gray, C.M.; Bekker, L.-G. Is obesity a risk factor for vaccine non-responsiveness? PLoS ONE 2013, 8, e82779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohut, M.L.; Cooper, M.M.; Nickolaus, M.S.; Russell, D.R.; Cunnick, J.E. Exercise and Psychosocial Factors Modulate Immunity to Influenza Vaccine in Elderly Individuals. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M557–M562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, J.A.; Vieira, V.J.; Keylock, K.T. Exercise, Inflammation, and Innate Immunity. Immunol. Allergy Clin. N. Am. 2009, 29, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, A.R.; Singh, M.A.F.; Edwards, K.M. The effects of exercise on vaccination responses: A review of chronic and acute exercise interventions in humans. Brain Behav. Immun. 2014, 39, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Gross, P.A.; Levandowski, R.A.; Russo, C.; Weksler, M.; Bonelli, J.; Dran, S.; Munk, G.; Deichmiller, S.; Hilsen, R.; Panush, R.F. Vaccine immune response and side effects with the use of acetaminophen with influenza vaccine. Clin. Diagn. Lab. Immunol. 1994, 1, 134–138. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Pasalic, L.; Lippi, G. Review and evolution of guidelines for diagnosis of COVID-19 vaccine induced thrombotic thrombocytopenia (VITT). Clin. Chem. Lab. Med. 2021, 60, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Von Hundelshausen, P.; Lorenz, R.; Siess, W.; Weber, C. Vaccine-induced immune thrombotic thrombocytopenia (VITT): Targeting pathomechanisms with Bruton tyrosine kinase inhibitors. Thromb. Haemost. 2021, 121, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J. Laboratory testing for suspected COVID-19 vaccine-induced (immune) thrombotic thrombocytopenia. Int. J. Lab. Hematol. 2021, 43, 559–570. [Google Scholar] [CrossRef] [PubMed]
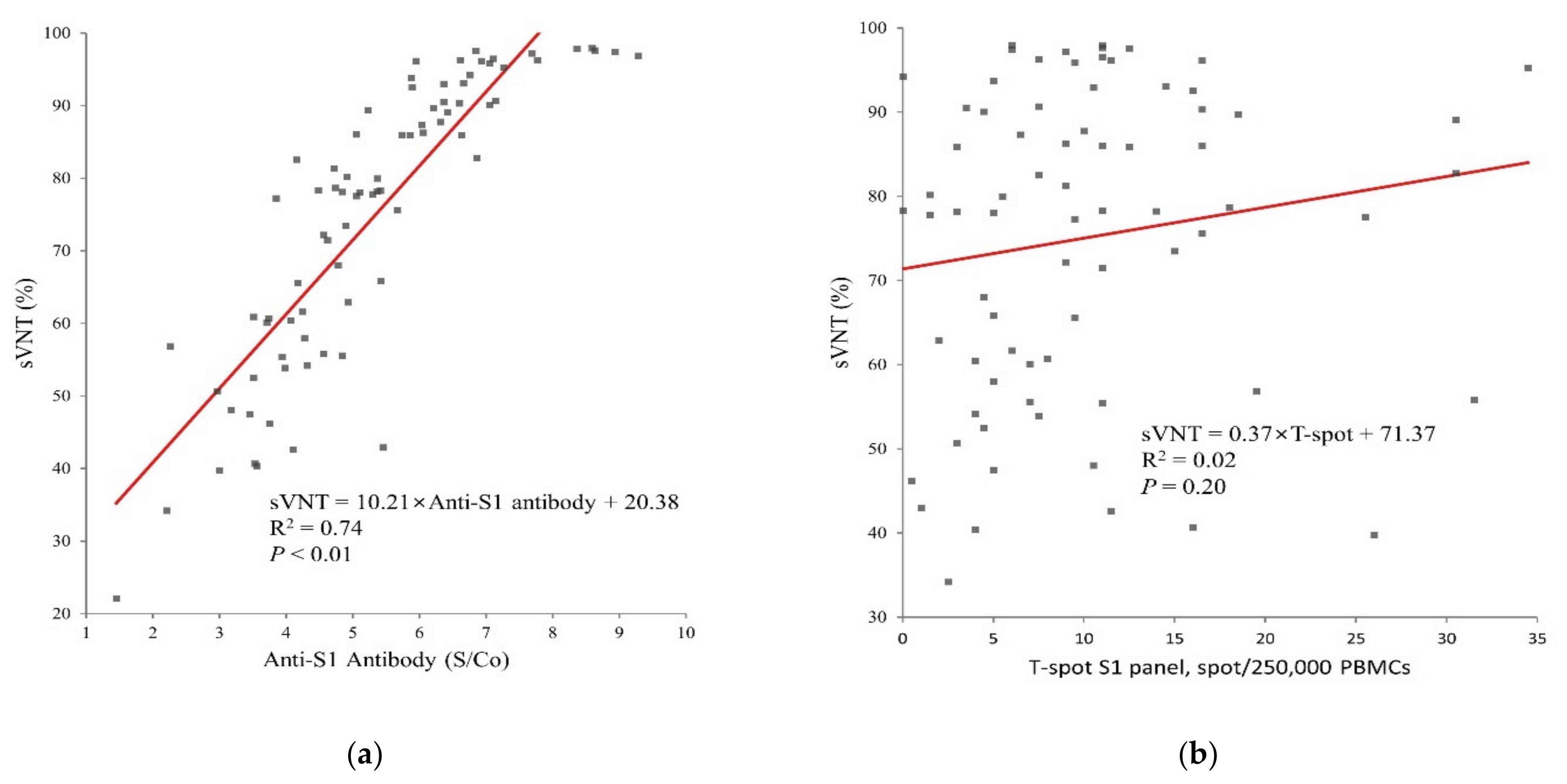
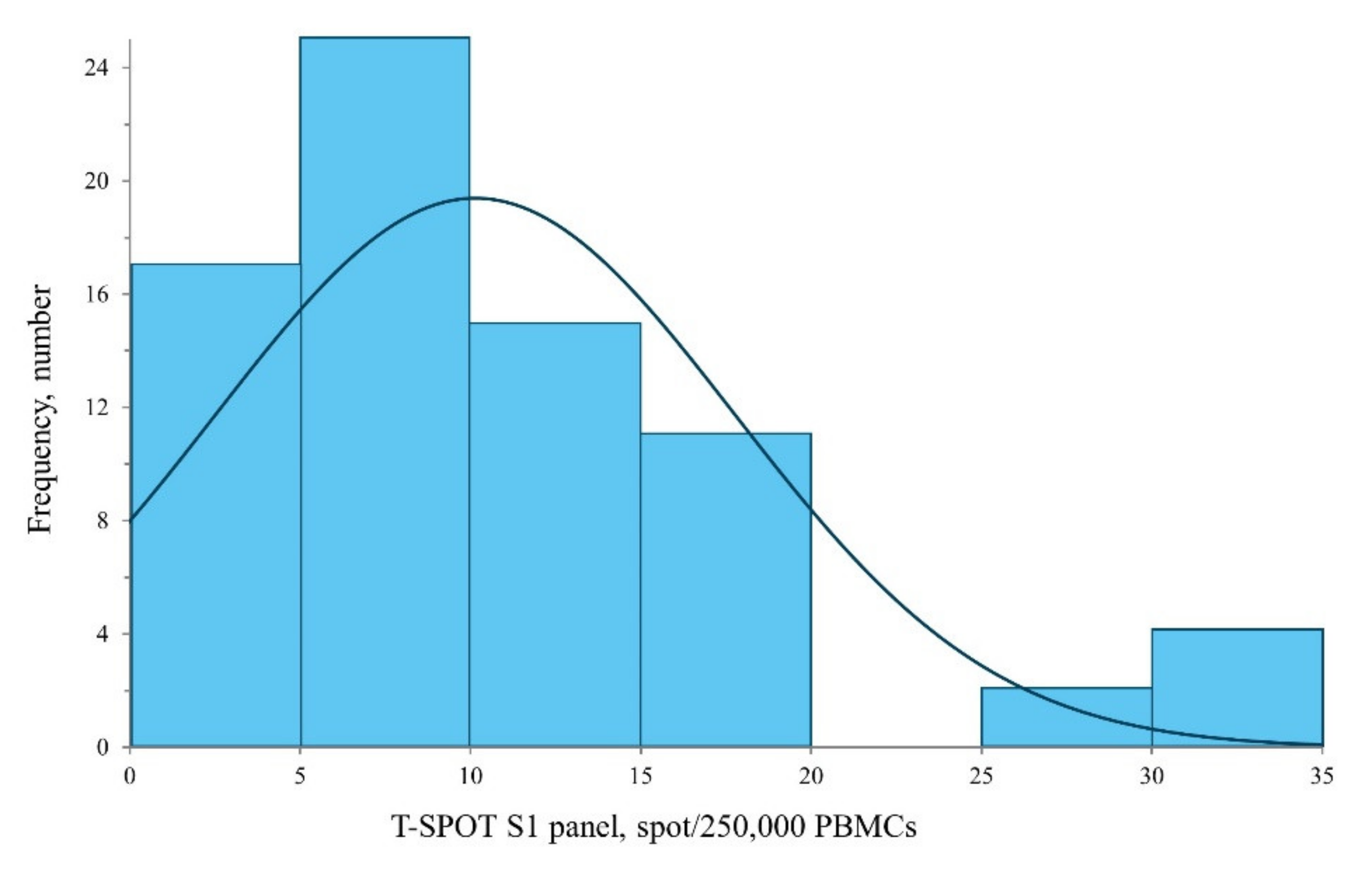
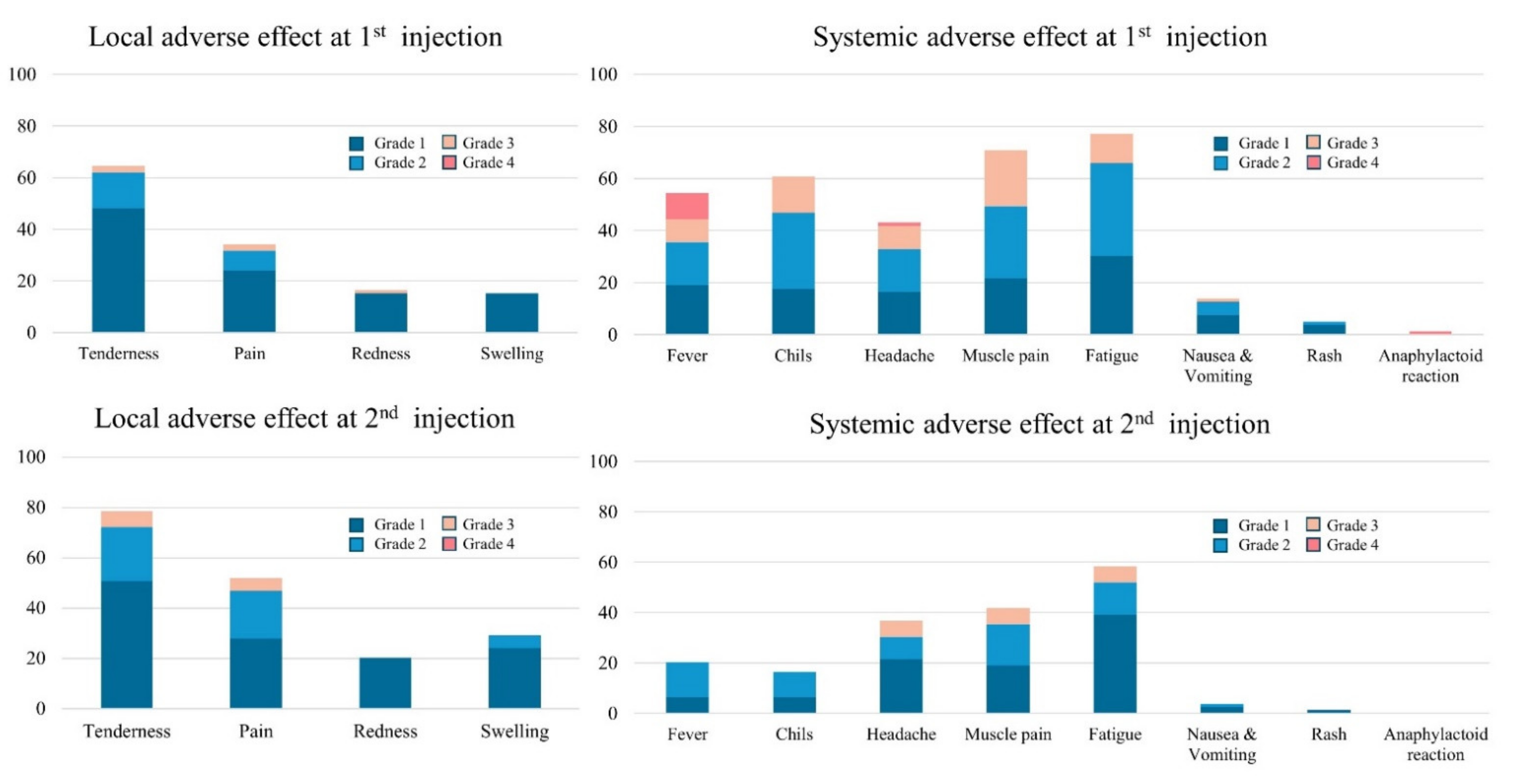
| Male (n = 21) | Female (n = 58) | Total | p | ||
|---|---|---|---|---|---|
| Age | Age (years) | 40.4 ± 7.5 | 35.5 ± 6.3 | 36.8 ± 7 | 0.01 |
| Days between 1st and 2nd shot | 77.8 ± 13.4 | 78.9 ± 8.6 | 78.6 ± 10.0 | 0.65 | |
| Days after 2nd shot | 16.8 ± 4.8 | 15.3 ± 2.4 | 15.7 ± 3.2 | 0.20 | |
| Immunogenicity | S1 antibody (S/Co value) | 5.2 ± 1.9 | 5.4 ± 1.5 | 5.3 ± 1.6 | 0.73 |
| S1 antibody positive | 21/21 (100%) | 58/58 (100%) | 79/79 (100%) | N/A | |
| T-spot (spots) | 12.9 ± 7.8 | 9.3 ± 7.4 | 10.1 ± 7.6 | 0.08 | |
| T-spot positive | 10/17 (52.9%) | 22/46 (19.3%) | 32/74 (43.2%) | 0.14 | |
| sVNT (%) | 73.7 ± 20.9 | 75.4 ± 18.7 | 75.0 ± 19.2 | 0.73 | |
| sVNT positive | 20/21 (95.2%) | 58/58 (100%) | 78/79 (98.7%) | 0.09 | |
| Blood pressure | SBP (mmHg) | 123.7 ± 13.0 | 112.8 ± 10.1 | 115.7 ± 11.9 | <0.01 |
| DBP (mmHg) | 80.5 ± 8.5 | 72 ± 8.4 | 74.2 ± 9.2 | <0.01 | |
| Anthropometrics | BMI (kg/m²) | 26.8 ± 7.1 | 22.5 ± 3.3 | 23.7 ± 5 | <0.01 |
| WC (cm) | 77.5 ± 8.5 | 73.6 ± 13.1 | 74.7 ± 12.1 | <0.01 | |
| Physical activities | Lack | 9 (42.9%) | 33 (56.9%) | 42 (53.2%) | 0.28 |
| Adequate | 12 (57.1%) | 25 (43.1%) | 37 (46.8%) | ||
| Weight training | Lack | 8 (38.1%) | 44 (75.9%) | 52 (65.8%) | <0.01 |
| Adequate | 13 (61.9%) | 14 (24.1%) | 27 (34.2%) | ||
| Smoking | No | 9/21 (42.9%) | 58/58 (100%) | 70/79 (88.6%) | <0.01 |
| Yes | 12/21 (57.1%) | 0/58 (0%) | 9/79 (11.4%) | ||
| Alcohol consumption | Mild to moderate | 6/21 (28.6%) | 39/58 (67.2%) | 45/79 (57.0%) | 0.02 |
| Heavy or binge | 15/21 (71.4%) | 19/58 (32.8%) | 34/79 (43.0%) | ||
| Comorbidity | Pulmonary tuberculosis | 2/21 (9.5%) | 2/58 (3.4%) | 4/79 (5.1%) | 0.28 |
| Hypertension | 2/21 (9.5%) | 1/58 (1.7%) | 3/79 (3.8%) | 0.11 | |
| Dyslipidemia | 2/21 (9.5%) | 1/58 (1.7%) | 3/79 (3.8%) | 0.11 | |
| Diabetes mellitus | 1/21 (4.8%) | 0/58 (0.0%) | 1/79 (1.3%) | 0.09 | |
| Laboratory analysis | Hb (g/dL) | 15.0 ± 0.8 | 13.1 ± 1.05 | 13.6 ± 1.3 | <0.01 |
| FBG (mg/dL) | 102.2 ± 14.4 | 91.5 ± 13.1 | 94.4 ± 13.1 | <0.01 | |
| Cr (mg/dL) | 0.8 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | <0.01 | |
| eGFR (mL/min/1.73 m2) | 85.0 ± 15.7 | 92.6 ± 20.4 | 90.6 ± 19.4 | 0.13 | |
| AST (IU/L) | 23.7 ± 5.8 | 21.8 ± 13.7 | 22.3 ± 12.1 | 0.55 | |
| ALT (IU/L) | 32.4 ± 18.6 | 19.4 ± 31.9 | 22.8 ± 29.4 | 0.08 | |
| GGT (IU/L) | 32.5 ± 24.0 | 16.6 ± 11.1 | 20.8 ± 16.9 | <0.01 | |
| TC (mg/dL) | 225.6 ± 41.9 | 198.1 ± 35.2 | 205.4 ± 38.8 | <0.01 | |
| HDL-C (mg/dL) | 54.7 ± 8.8 | 62.1 ± 9.9 | 60.2 ± 10.1 | <0.01 | |
| LDL-C (mg/dL) | 134.9 ± 37.5 | 116.3 ± 36.1 | 121.3 ± 35 | 0.04 | |
| TG (mg/dL) | 172.7 ± 82.5 | 98.9 ± 53.9 | 118.6 ± 70.3 | <0.01 | |
| Reactogenicity | SIR | 9.3 ± 8.5 | 14.9 ± 9.3 | 13.4 ± 9.3 | 0.02 |
| SIRD | 19.1 ± 22.9 | 39.9 ± 32.8 | 34.4 ± 31.7 | 0.01 |
| T-Spot Negative | T-Spot Positive | Total | p | ||
|---|---|---|---|---|---|
| Sex | Female | 35 (61.4%) | 22 (38.6%) | 57 | 0.14 |
| Male | 7 (41.2%) | 10 (58.8%) | 17 | ||
| Age | (years) | 35.5 ± 6.7 | 37.4 ± 5.8 | 36.8 ± 7.0 | 0.18 |
| Occupation | Doctor | 3 (50.0%) | 3 (50.0%) | 6 | 0.45 |
| Nurse | 25 (58.1%) | 18 (41.9%) | 43 | ||
| Researchers | 1 (50%) | 1 (50%) | 2 | ||
| Medical technologist | 7 (43.8%) | 9 (56.3%) | 16 | ||
| Hospital administrative assistant | 6 (85.7%) | 1 (14.3%) | 7 | ||
| Physical activities | Lack | 22 (53.7%) | 19 (46.3%) | 41 | 0.55 |
| Adequate | 20 (60.6%) | 13 (39.4%) | 33 | ||
| Weight training | Lack | 31 (60.8%) | 20 (39.2%) | 51 | 0.30 |
| Adequate | 11 (47.8%) | 12 (52.2%) | 23 | ||
| Smoking | No | 38 (57.6%) | 28 (42.4%) | 66 | 0.68 |
| Yes | 4 (50%) | 4 (50%) | 8 | ||
| Alcohol consumption | Mild to moderate | 26 (60.5%) | 17 (39.5%) | 43 | 0.45 |
| Heavy | 16 (51.6%) | 15 (48.4%) | 31 | ||
| Anthropometrics | BMI | 23.4 ± 3.5 | 23.2 ± 3.2 | 23.7 ± 5.0 | 0.72 |
| WC | 74.7 ± 9.3 | 73.8 ± 15.6 | 74.7 ± 12.1 | 0.76 | |
| SBP | 114.26 ± 10.6 | 117.6 ± 13.7 | 115.7 ± 11.9 | 0.25 | |
| DBP | 73.1 ± 8.7 | 75.6 ± 9.2 | 74.2 ± 9.2 | 0.24 | |
| Pulmonary tuberculosis | No | 40 (57.1%) | 30 (42.9%) | 70 | 0.78 |
| Yes | 2 (50.0%) | 2 (50.0%) | 4 | ||
| Hypertension | No | 40 (56.3%) | 31 (43.7%) | 71 | 0.74 |
| Yes | 2 (66.7%) | 1 (33.3%) | 3 | ||
| Dyslipidemia | No | 41 (57.7%) | 30 (42.3%) | 71 | 0.40 |
| Yes | 1 (33.3%) | 2 (66.7%) | 3 | ||
| Diabetes mellitus | No | 41 (56.2%) | 32 (43.8%) | 73 | 0.38 |
| Yes | 1 (100.0) | 0 (0.0%) | 1 | ||
| Laboratory analysis | Hb (g/dL) | 13.4 ± 1.3 | 13.8 ± 1.3 | 13.6 ± 1.3 | 0.29 |
| FBG (mg/dL) | 94.9 ± 11.8 | 93.2 ± 15.3 | 94.4 ± 13.1 | 0.60 | |
| Cr (mg/dL) | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.01 | |
| eGFR (mL/min/1.73 m2) | 95.4 ± 19.8 | 83.8 ± 16.7 | 90.6 ± 19.5 | <0.01 | |
| AST (IU/L) | 20.5 ± 5.7 | 24.8 ± 17.7 | 22.3 ± 12.1 | 0.14 | |
| ALT (IU/L) | 19.1 ± 14.8 | 28.1 ± 42.7 | 22.8 ± 29.5 | 0.21 | |
| GGT (IU/L) | 19.4 ± 13.5 | 22.7 ± 21.6 | 20.8 ± 17.0 | 0.42 | |
| TC (mg/dL) | 205.7 ± 36.6 | 204.1 ± 43.6 | 205.4 ± 38.8 | 0.87 | |
| HDL-C (mg/dL) | 61.5 ± 10.4 | 59.0 ± 9.7 | 60.2 ± 10.1 | 0.30 | |
| LDL-C (mg/dL) | 120.5 ± 35.4 | 122.1 ± 35.3 | 121.4 ± 35.0 | 0.86 | |
| TG (mg/dL) | 118.3 ± 72.8 | 117.6 ± 72.0 | 118.6 ± 70.4 | 0.97 | |
| Reactogenicity | SIR | 14.5 ± 10.0 | 13.4 ± 8.4 | 13.4 ± 9.3 | 0.61 |
| SIRD | 38.1 ± 31.3 | 34.0 ± 32.9 | 34.4 ± 31.7 | 0.58 |
| Anti-S1 Antibody | sVNT | T-Spot | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Model | Multivariate Model | Univariate Model | Multivariate Model | Univariate Model | Multivariate Model | |||||||
| Coefficient (SE) | p | Coefficient (SE) | p | Coefficient (SE) | p | Coefficient (SE) | p | Coefficient (SE) | p | Coefficient (SE) | p | |
| Men | −0.20(0.51) | 0.7 | −1.78(6.28) | 0.77 | 3.63 (2.07) | 0.08 | 5.94 (2.24) | 0.01 | ||||
| Age | −0.07(0.03) | 0.02 | −0.06(0.03) | 0.02 | −0.60(0.34) | 0.09 | −0.61(0.31) | 0.05 | 0.16 (0.14) | 0.27 | ||
| BMI | 0.10(0.06) | 0.12 | 0.10(0.05) | 0.06 | 0.42(0.73) | 0.57 | −0.32 (0.27) | 0.24 | −0.69 (0.29) | 0.02 | ||
| Muscle exercise | 0.02(0.42) | 0.97 | 3.25(5.07) | 0.52 | 3.30 (1.88) | 0.09 | ||||||
| SRI | −0.04(0.03) | 0.22 | 0.19(0.41) | 0.65 | 0.08 (0.04) | 0.59 | −0.25 (0.15) | 0.11 | ||||
| SRID | 0.01(0.01) | 0.52 | −0.08(0.12) | 0.50 | −0.13 (0.06) | 0.89 | 0.07 (0.04) | 0.10 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Lee, S.-M.; Lim, S.; Shin, K.-H.; Kim, T.; Kim, W.-j.; Yun, M.; Oh, S.-H. Immunogenicity after Second ChAdOx1 nCoV-19 (AZD1222) Vaccination According to the Individual Reactogenicity, Health Status and Lifestyle. Vaccines 2021, 9, 1473. https://doi.org/10.3390/vaccines9121473
Choi H, Lee S-M, Lim S, Shin K-H, Kim T, Kim W-j, Yun M, Oh S-H. Immunogenicity after Second ChAdOx1 nCoV-19 (AZD1222) Vaccination According to the Individual Reactogenicity, Health Status and Lifestyle. Vaccines. 2021; 9(12):1473. https://doi.org/10.3390/vaccines9121473
Chicago/Turabian StyleChoi, Hyunji, Sun-Min Lee, Seungjin Lim, Kyung-Hwa Shin, Taeyun Kim, Won-joo Kim, Misook Yun, and Seung-Hwan Oh. 2021. "Immunogenicity after Second ChAdOx1 nCoV-19 (AZD1222) Vaccination According to the Individual Reactogenicity, Health Status and Lifestyle" Vaccines 9, no. 12: 1473. https://doi.org/10.3390/vaccines9121473
APA StyleChoi, H., Lee, S.-M., Lim, S., Shin, K.-H., Kim, T., Kim, W.-j., Yun, M., & Oh, S.-H. (2021). Immunogenicity after Second ChAdOx1 nCoV-19 (AZD1222) Vaccination According to the Individual Reactogenicity, Health Status and Lifestyle. Vaccines, 9(12), 1473. https://doi.org/10.3390/vaccines9121473






