Characterization of a Novel Cysteine Protease Inhibitor from Poultry Red Mites: Potential Vaccine for Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Consent of Participants
2.2. PRM Samples
2.3. RNA Isolation and cDNA Synthesis
2.4. Identification of the Full-Length Nucleotide Sequence of Dg-Cys in PRMs
2.5. Laser-Capture Microdissection
2.6. Gene Expression Analysis
2.7. Real-Time Quantitative RT-PCR
2.8. Preparation of Recombinant Dg-Cys
2.9. Enzymatic Activity of Dg-Cys-his
2.10. Immunization of Chickens with Dg-Cys-his
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. Western Blotting
2.13. Evaluation of the Potential of Dg-Cys as a Vaccine Antigen
- (1)
- The SR:
- (2)
- The RC:
- (3)
- The molting rate (MR):
2.14. Evaluation of the Efficacy of the “Cocktail Vaccine”
2.15. Statistics
3. Results
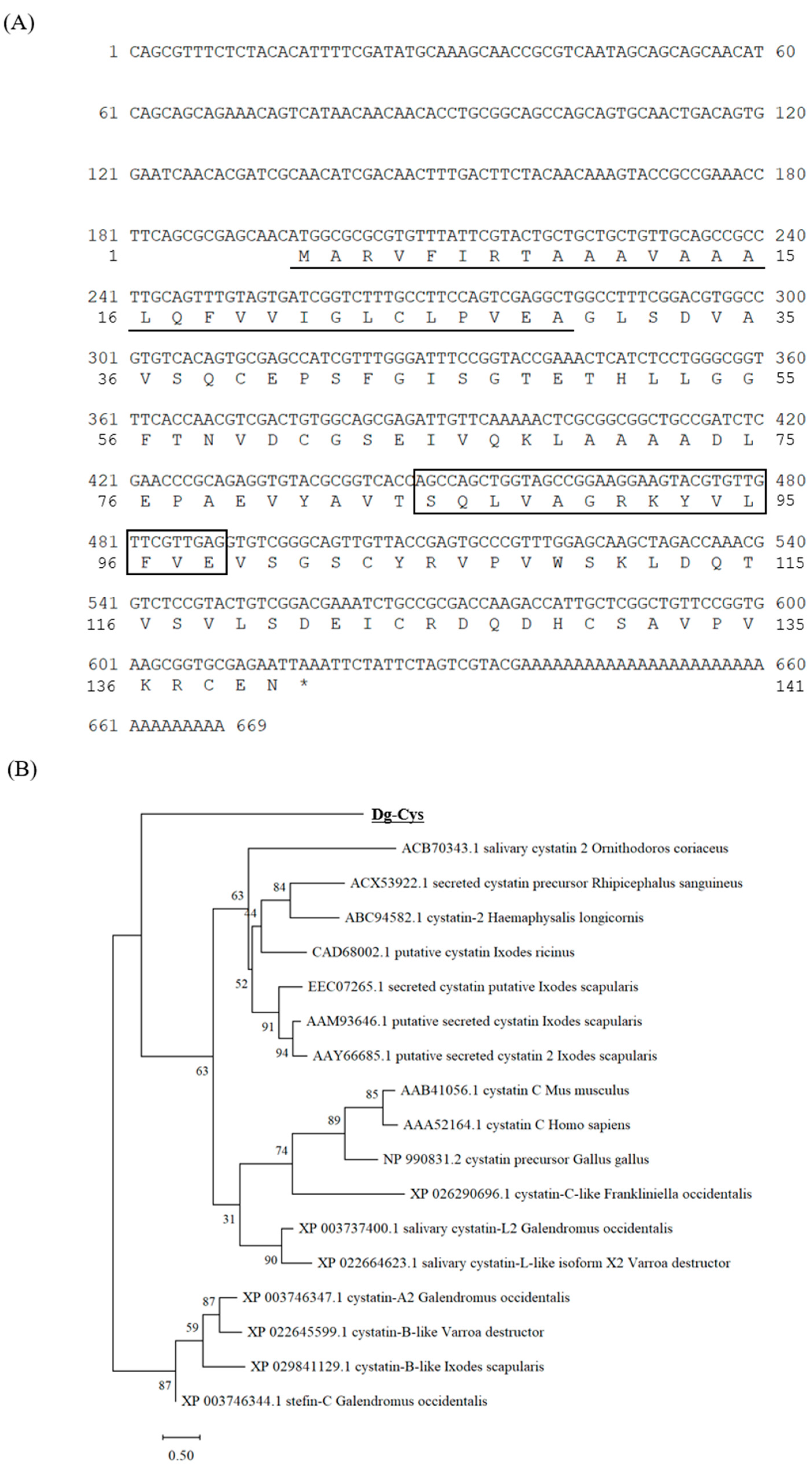
3.1. Cloning and Sequence Analysis of Dg-Cys from PRMs
3.2. Dg-Cys Expression Profile
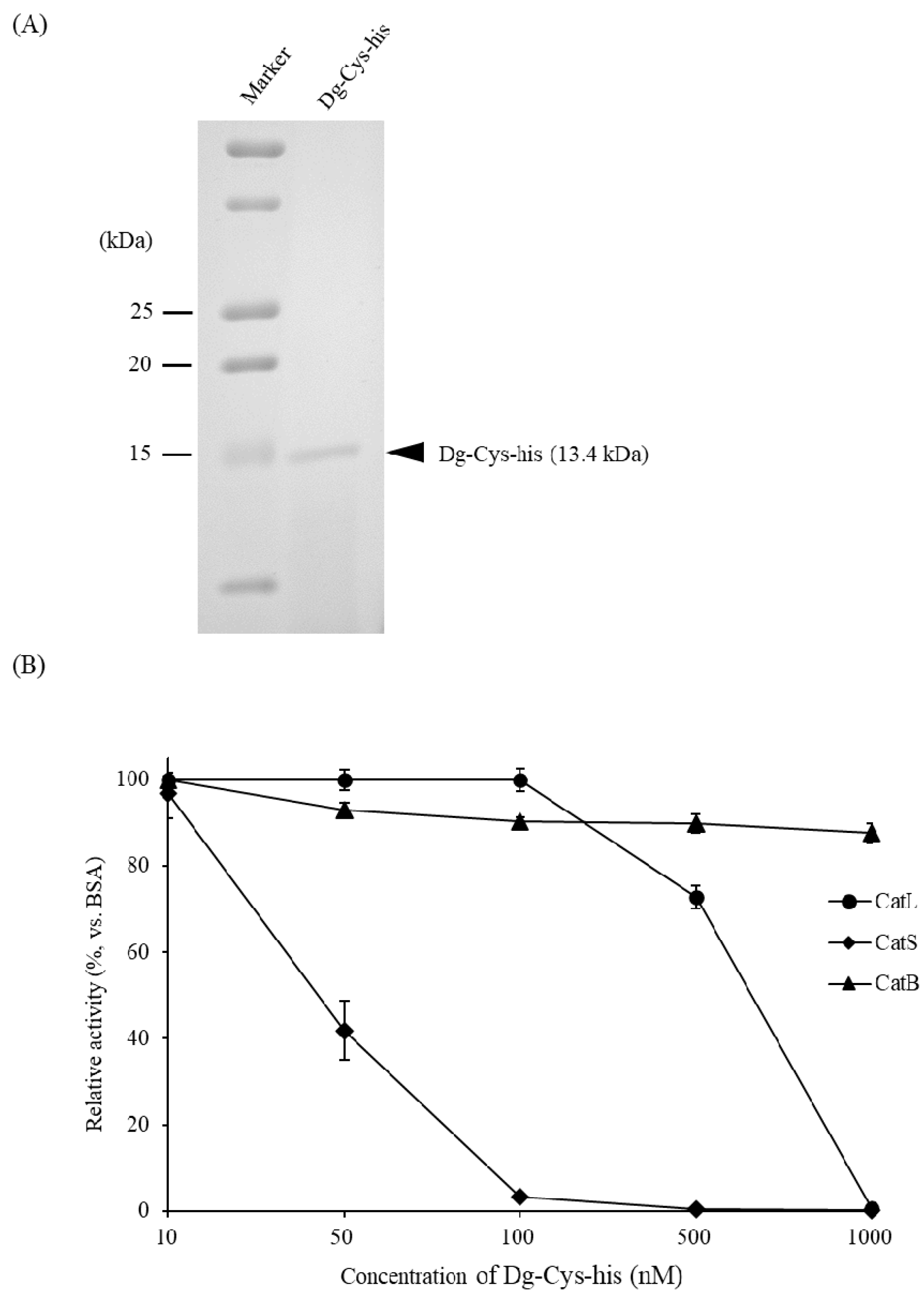
3.3. Dg-Cys Enzymatic Activity
3.4. Acaricidal Potential of the Plasma from Chickens Immunized with Dg-Cys-his
3.5. Enhanced Acaricidal Effect of Dg-Cys-his-Immunized Plasma in Combination with Dg-Ctr1- or Dg-APMAP-Immunized Plasma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartley, K.; Wright, H.W.; Huntley, J.F.; Manson, E.D.T.; Inglis, N.F.; McLean, K.; Nath, M.; Bartley, Y.; Nisbet, A.J. Identification and evaluation of vaccine candidate antigens from the poultry red mite (Dermanyssus gallinae). Int. J. Parasitol. 2015, 45, 819–830. [Google Scholar] [CrossRef] [Green Version]
- Wright, H.W.; Bartley, K.; Huntley, J.F.; Nisbet, A.J. Characterization of tropomyosin and paramyosin as vaccine candidate molecules for the poultry red mite, Dermanyssus gallinae. Parasites Vectors 2016, 9, 544. [Google Scholar] [CrossRef] [Green Version]
- Lima-Barbero, J.F.; Contreras, M.; Mateos-Hernández, L.; Mata-Lorenzo, F.M.; Triguero-Ocaña, R.; Sparagano, O.; Finn, R.D.; Strube, C.; Price, D.R.G.; Nunn, F.; et al. A vaccinology approach to the identification and characterization of Dermanyssus gallinae candidate protective antigens for the control of poultry red mite infestations. Vaccines 2019, 7, 190. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, S.; Murata, S.; Isezaki, M.; Ariizumi, T.; Sato, T.; Oishi, E.; Taneno, A.; Maekawa, N.; Okagawa, T.; Ichii, O.; et al. Characterization of a copper transporter 1 from Dermanyssus gallinae as a vaccine antigen. Parasitology 2021, 1–11. [Google Scholar] [CrossRef]
- Fujisawa, S.; Murata, S.; Takehara, M.; Aoyama, J.; Morita, A.; Isezaki, M.; Win, S.Y.; Ariizumi, T.; Sato, T.; Oishi, E.; et al. In vitro characterization of adipocyte plasma membrane-associated protein from poultry red mites, Dermanyssus gallinae, as a vaccine antigen for chickens. Vaccine 2021, 39, 6057–6066. [Google Scholar] [CrossRef]
- Bartley, K.; Turnbull, F.; Wright, H.W.; Huntley, J.F.; Palarea-Albaladejo, J.; Nath, M.; Nisbet, A.J. Field evaluation of poultry red mite (Dermanyssus gallinae) native and recombinant prototype vaccines. Vet. Parasitol. 2017, 244, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.C.; Francischetti, I.M.B. Role of arthropod saliva in blood feeding: Sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003, 48, 73–88. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, J.; Kuster, T.; Sparagano, O.; Tomley, F. Understanding the biology and control of the poultry red mite Dermanyssus gallinae: A review. Avian Pathol. 2015, 44, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Abrahamson, M.; Alvarez-Fernandez, M.; Nathanson, C.M. Cystatins. Biochem. Soc. Symp. 2003, 70, 179–199. [Google Scholar] [CrossRef]
- Honey, K.; Rudensky, A.Y. Lysosomal cysteine proteases regulate antigen presentation. Nat. Rev. Immunol. 2003, 3, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Wille, A.; Gerber, A.; Heimburg, A.; Reisenauer, A.; Peters, C.; Saftig, P.; Reinheckel, T.; Welte, T.; Bühling, F. Cathepsin L is involved in cathepsin D processing and regulation of apoptosis in A549 human lung epithelial cells. Biol. Chem. 2004, 385, 665–670. [Google Scholar] [CrossRef]
- Lombardi, G.; Burzyn, D.; Mundiñano, J.; Berguer, P.; Bekinschtein, P.; Costa, H.; Castillo, L.F.; Goldman, A.; Meiss, R.; Piazzon, I.; et al. Cathepsin-L influences the expression of extracellular matrix in lymphoid organs and plays a role in the regulation of thymic output and of peripheral T cell number. J. Immunol. 2005, 174, 7022–7032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, C.A.; Sasaki, S.D.; Tanaka, A.S. Bmcystatin, a cysteine proteinase inhibitor characterized from the tick Boophilus microplus. Biochem. Biophys. Res. Commun. 2006, 347, 44–50. [Google Scholar] [CrossRef]
- Grunclová, L.; Horn, M.; Vancová, M.; Sojka, D.; Franta, Z.; Mares, M.; Kopáček, P. Two secreted cystatins of the soft tick Ornithodoros moubata: Differential expression pattern and inhibitory specificity. Biol. Chem. 2006, 387, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liao, M.; Ueda, M.; Gong, H.; Xuan, X.; Fujisaki, K. Characterization of an intracellular cystatin homolog from the tick Haemaphysalis longicornis. Vet. Parasitol. 2009, 160, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Tsuji, N.; Miyoshi, T.; Hatta, T.; Alim, M.A.; Anisuzzaman, K.S.; Kushibiki, S.; Fujisaki, K. Hlcyst-1 and Hlcyst-2 are potential inhibitors of HlCPL-A in the midgut of the ixodid tick Haemaphysalis longicornis. J. Vet. Med. Sci. 2010, 72, 599–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karim, S.; Miller, N.J.; Valenzuela, J.; Sauer, J.R.; Mather, T.N. RNAi-mediated gene silencing to assess the role of synaptobrevin and cystatin in tick blood feeding. Biochem. Biophys. Res. Commun. 2005, 334, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Salát, J.; Paesen, G.C.; Rezácová, P.; Kotsyfakis, M.; Kovárová, Z.; Sanda, M.; Majtán, J.; Grunclová, L.; Horká, H.; Andersen, J.F.; et al. Crystal structure and functional characterization of an immunomodulatory salivary cystatin from the soft tick Ornithodoros moubata. Biochem. J. 2010, 429, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujisawa, S.; Murata, S.; Isezaki, M.; Oishi, E.; Taneno, A.; Maekawa, N.; Okagawa, T.; Konnai, S.; Ohashi, K. Transcriptome dynamics of blood-fed and starved poultry red mites, Dermanyssus gallinae. Parasitol. Int. 2020, 78, 102156. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef]
- Ichii, O.; Ohta, H.; Horino, T.; Nakamura, T.; Hosotani, M.; Mizoguchi, T.; Morishita, K.; Nakamura, K.; Hoshino, Y.; Takagi, S.; et al. Urinary exosome-derived microRNAs reflecting the changes of renal function and histopathology in dogs. Sci. Rep. 2017, 7, 40340. [Google Scholar] [CrossRef] [Green Version]
- Ariizumi, T.; Murata, S.; Fujisawa, S.; Isezaki, M.; Maekawa, N.; Okagawa, T.; Sato, T.; Oishi, E.; Taneno, A.; Konnai, S.; et al. Selection of reference genes for quantitative PCR analysis in Poultry red mite (Dermanyssus gallinae). J. Vet. Med. Sci. 2021, 83, 558–565. [Google Scholar] [CrossRef]
- Almazán, C.; Moreno-Cantú, O.; Moreno-Cid, J.A.; Galindo, R.C.; Canales, M.; Villar, M.; de la Fuente, J. Control of tick infestations in cattle vaccinated with bacterial membranes containing surface-exposed tick protective antigens. Vaccine 2012, 30, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, R.; Manzano-Román, R.; Obolo-Mvoulouga, P.; Oleaga, A. In silico selection of functionally important proteins from the mialome of Ornithodoros erraticus ticks and assessment of their protective efficacy as vaccine targets. Parasites Vectors 2019, 12, 508. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, A.; Valdés, J.J.; Kotsyfakis, M. The role of cystatins in tick physiology and blood feeding. Ticks Tick-Borne Dis. 2012, 3, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Kobpornchai, P.; Flynn, R.J.; Reamtong, O.; Rittisoonthorn, N.; Kosoltanapiwat, N.; Boonnak, K.; Boonyuen, U.; Ampawong, S.; Jiratanh, M.; Tattiyapong, M.; et al. A novel cystatin derived from Trichinella spiralis suppresses macrophage-mediated inflammatory responses. PLoS Negl. Trop. Dis. 2020, 14, e0008192. [Google Scholar] [CrossRef] [PubMed]
- Lieskovská, J.; Páleníková, J.; Širmarová, J.; Elsterová, J.; Kotsyfakis, M. Tick salivary cystatin sialostatin L2 suppresses IFN responses in mouse dendritic cells. Parasite Immunol. 2015, 37, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Chmelař, J.; Kotál, J.; Langhansová, H.; Kotsyfakis, M. Protease inhibitors in tick saliva: The role of serpins and cystatins in tick-host–pathogen interaction. Front. Cell. Infect. Microbiol. 2017, 7, 216. [Google Scholar] [CrossRef]
- Sajiki, Y.; Konnai, S.; Ochi, A.; Okagawa, T.; Githaka, N.; Isezaki, M.; Yamada, S.; Ito, T.; Ando, S.; Kawabata, H.; et al. Immunosuppressive effects of sialostatin L1 and L2 isolated from the taiga tick Ixodes persulcatus Schulze. Ticks Tick-Borne Dis. 2020, 11, 101332. [Google Scholar] [CrossRef]
- Alim, M.A.; Tsuji, N.; Miyoshi, T.; Islam, M.K.; Huang, X.; Hatta, T.; Fujisaki, K. HlLgm2, a member of asparaginyl endopeptidases/legumains in the midgut of the ixodid tick Haemaphysalis longicornis, is involved in blood-meal digestion. J. Insect Physiol. 2008, 54, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Alim, M.A.; Tsuji, N.; Miyoshi, T.; Islam, M.K.; Hatta, T.; Fujisaki, K. Legumains from the hard tick Haemaphysalis longicornis play modulatory roles in blood feeding and gut cellular remodelling and impact on embryogenesis. Int. J. Parasitol. 2009, 39, 97–107. [Google Scholar] [CrossRef]
- Yamaji, K.; Tsuji, N.; Miyoshi, T.; Islam, M.K.; Hatta, T.; Alim, M.A.; Anisuzzaman, T.A.; Takenaka, A.; Fujisaki, K. Hemoglobinase activity of a cysteine protease from the ixodid tick Haemaphysalis longicornis. Parasitol. Int. 2009, 58, 232–237. [Google Scholar] [CrossRef]
- Dai, L.S.; Chu, S.H.; Yu, X.M.; Li, Y.Y. A role of cathepsin L gene in innate immune response of crayfish (Procambarus clarkii). Fish Shellfish. Immunol. 2017, 71, 246–254. [Google Scholar] [CrossRef]
- Yan, X.; Wu, Z.; Wang, B.; Yu, T.; Hu, Y.; Wang, S.; Deng, C.; Zhao, B.; Nakanishi, H.; Zhang, X. Involvement of cathepsins in innate and adaptive immune responses in periodontitis. Evid.-Based Complement. Altern. Med. 2020, 2020, 4517587. [Google Scholar] [CrossRef] [PubMed]
- Turk, V.; Stoka, V.; Turk, D. Cystatins: Biochemical and structural properties, and medical relevance. Front. Biosci. 2008, 13, 5406–5420. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhou, Y.; Gong, H.; Cao, J.; Zhang, H.; Li, X.; Zhou, J. Functional characterization of a cystatin from the tick Rhipicephalus haemaphysaloides. Parasites Vectors 2015, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.K.; Islam, M.S.; You, M. Impact of subolesin and cystatin knockdown by RNA interference in adult female Haemaphysalis longicornis (Acari: Ixodidae) on blood engorgement and reproduction. Insects 2018, 9, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndawula, C., Jr.; Tabor, A.E. Cocktail anti-tick vaccines: The unforeseen constraints and approaches toward enhanced efficacies. Vaccines 2020, 8, 457. [Google Scholar] [CrossRef]
- McKenna, R.V.; Riding, G.A.; Jarmey, J.M.; Pearson, R.D.; Willadsen, P. Vaccination of cattle against the Boophilus microplus using a mucin-like membrane glycoprotein. Parasite Immunol. 1998, 20, 325–336. [Google Scholar] [CrossRef]
- Hope, M.; Jiang, X.; Gough, J.; Josh, P.; Jonsson, N.; Willadsen, P. Experimental vaccination of sheep and cattle against tick infestation using recombinant 5’-nucleotidase. Parasite Immunol. 2010, 32, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]

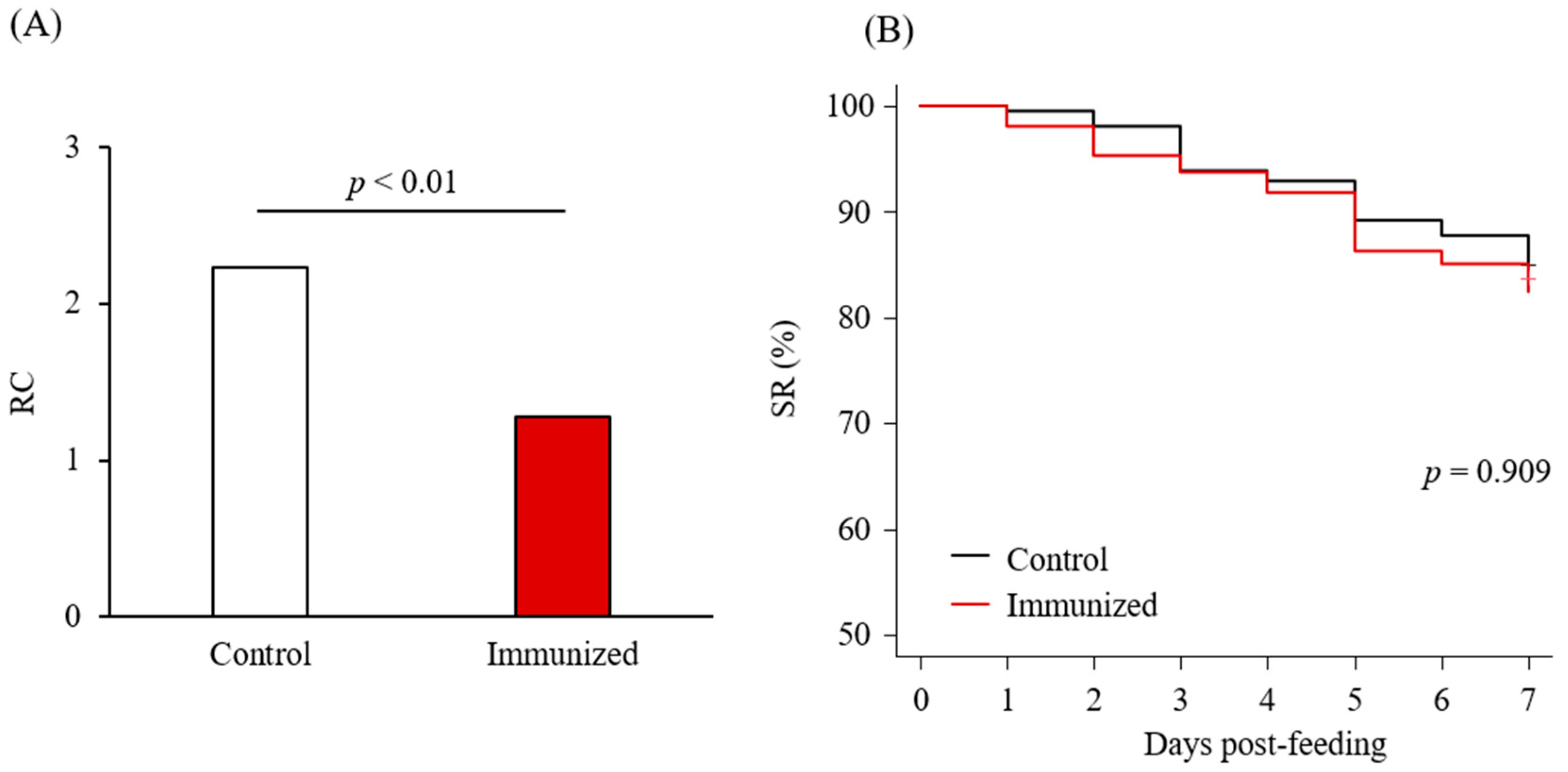


| Days Post-Feeding | RC | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Immunized (n = 255, nymphs: n = 38, adults: n = 219) | ||||||||
| No. of dead PRMs | 5 | 12 | 16 | 21 | 35 | 38 | 45 | 1.29 |
| SR (%) | 98.04 | 95.29 | 93.73 | 91.76 | 86.27 | 85.10 | 82.35 | |
| Control (n = 211, nymphs: n = 51, adults: n = 160) | ||||||||
| No. of dead PRMs | 1 | 4 | 13 | 15 | 23 | 26 | 37 | 2.24 |
| SR (%) | 99.53 | 98.10 | 93.84 | 92.89 | 89.10 | 87.68 | 82.46 | |
| p value (Fisher’s exact) | 0.228 | 0.126 | 1 | 0.729 | 0.399 | 0.499 | 1 | 2.38 × 10−5 * |
| Odds ratio | 4.19 | 2.55 | 1.01 | 1.17 | 1.30 | 1.25 | 1.01 | 0.57 |
| 95% CI (lower limit) | 0.46 | 0.76 | 0.45 | 0.56 | 0.72 | 0.71 | 0.61 | 0.44 |
| 95% CI (upper limit) | 199.39 | 11.02 | 2.36 | 2.52 | 2.39 | 2.22 | 1.68 | 0.75 |
| Days Post-Feeding | MR (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Immunized (n = 189, all nymphs) | 48.68 | |||||||
| No. of dead PRMs | 5 | 6 | 10 | 13 | 34 | 57 | 74 | |
| SR (%) | 97.35 | 96.83 | 94.71 | 93.12 | 82.01 | 69.84 | 60.85 | |
| Control (n = 150, all nymphs) | 49.33 | |||||||
| No. of dead PRMs | 1 | 3 | 4 | 4 | 14 | 28 | 38 | |
| SR (%) | 99.33 | 98.00 | 97.33 | 97.33 | 90.67 | 81.33 | 74.67 | |
| p value | 0.233 | 0.736 | 0.280 | 0.085 | 0.028 * | 0.017 * | 0.008 * | 1 |
| Odds ratio | 4.04 | 1.60 | 2.04 | 2.69 | 2.13 | 1.88 | 1.89 | 1.01 |
| 95% CI (lower limit) | 0.44 | 0.34 | 0.57 | 0.81 | 1.06 | 1.09 | 1.16 | 0.68 |
| 95% CI (upper limit) | 192.59 | 10.08 | 9.07 | 11.57 | 4.48 | 3.28 | 3.13 | 1.50 |
| Days Post-Feeding | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Control (n = 70) | ||||||||||
| No. of dead PRMs | 0 | 1 | 2 | 2 | 3 | 5 | 8 | 19 | 25 | 34 |
| SR (%) | 100 | 98.57 | 97.14 | 97.14 | 95.71 | 92.86 | 88.57 | 72.86 | 64.29 | 51.43 |
| Dg-Cys (n = 78) | ||||||||||
| No. of dead PRMs | 0 | 5 | 7 | 11 | 16 | 18 | 24 | 32 | 38 | 46 |
| SR (%) | 100 | 93.59 | 91.03 | 85.90 | 79.49 | 76.92 | 69.23 | 58.97 | 51.28 | 41.03 |
| Dg-Ctr1 (n = 57) | ||||||||||
| No. of dead PRMs | 1 | 2 | 4 | 4 | 6 | 7 | 11 | 13 | 21 | 29 |
| SR (%) | 98.25 | 96.49 | 92.98 | 92.98 | 89.47 | 87.72 | 80.70 | 77.19 | 63.16 | 49.12 |
| Combination (n = 80) | ||||||||||
| No. of dead PRMs | 0 | 1 | 5 | 8 | 11 | 16 | 25 | 33 | 47 | 54 |
| SR (%) | 100.00 | 98.72 | 93.59 | 89.74 | 85.90 | 79.49 | 67.95 | 57.69 | 39.74 | 30.77 |
| p value | ||||||||||
| Control vs. Dg-Cys | 1 | 1 | 1 | 0.116 | 0.019 * | 0.068 | 0.025 * | 0.256 | 0.539 | 0.970 |
| Control vs. Dg-Ctr1 | 1 | 1 | 1 | 1 | 0.891 | 0.754 | 0.634 | 1 | 1 | 0.970 |
| Control vs. Combination | 1 | 1 | 1 | 0.513 | 0.254 | 0.16 | 0.018 * | 0.239 | 0.019 * | 0.074 |
| Dg-Cys vs. Dg-Ctr1 | 1 | 1 | 1 | 1 | 0.633 | 0.491 | 0.496 | 0.161 | 0.595 | 0.970 |
| Dg-Cys vs. Combination | 1 | 1 | 1 | 1 | 0.891 | 0.847 | 1 | 1 | 0.595 | 0.970 |
| Dg-Ctr1 vs. Combination | 1 | 1 | 1 | 1 | 0.891 | 0.754 | 0.468 | 0.161 | 0.045 * | 0.168 |
| Days Post-Feeding | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Control (n = 71) | ||||||||||
| No. of dead PRMs | 2 | 4 | 6 | 10 | 11 | 14 | 21 | 27 | 33 | 40 |
| SR (%) | 97.18 | 94.37 | 91.55 | 85.92 | 84.51 | 80.28 | 70.42 | 61.97 | 53.52 | 43.66 |
| Dg-Cys (n = 52) | ||||||||||
| No. of dead PRMs | 0 | 1 | 2 | 4 | 6 | 8 | 10 | 16 | 20 | 28 |
| SR (%) | 100 | 98.08 | 96.15 | 92.31 | 88.46 | 84.62 | 80.77 | 69.23 | 61.54 | 46.15 |
| Dg-APMAP (n = 74) | ||||||||||
| No. of dead PRMs | 0 | 3 | 4 | 9 | 9 | 18 | 23 | 30 | 38 | 47 |
| SR (%) | 100 | 95.95 | 94.59 | 87.84 | 87.84 | 75.68 | 68.92 | 59.46 | 48.65 | 36.49 |
| Combination (n = 48) | ||||||||||
| No. of dead PRMs | 0 | 2 | 2 | 5 | 8 | 16 | 20 | 31 | 34 | 41 |
| SR (%) | 100 | 95.83 | 95.83 | 89.58 | 83.33 | 66.67 | 58.33 | 35.42 | 29.17 | 14.58 |
| p value | ||||||||||
| Control vs. Dg-Cys | 1 | 1 | 1 | 1 | 1 | 1 | 0.855 | 1 | 0.923 | 1 |
| Control vs. Dg-APMAP | 1 | 1 | 1 | 1 | 1 | 1 | 0.859 | 1 | 0.923 | 1 |
| Control vs. Combination | 1 | 1 | 1 | 1 | 1 | 0.657 | 0.855 | 0.026 * | 0.070 | 0.006 ** |
| Dg-Cys vs. Dg-APMAP | 1 | 1 | 1 | 1 | 1 | 1 | 0.772 | 1 | 0.611 | 1 |
| Dg-Cys vs. Combination | 1 | 1 | 1 | 1 | 1 | 0.357 | 0.104 | 0.007 ** | 0.008 ** | 0.006 ** |
| Dg-APMAP vs. Combination | 1 | 1 | 1 | 1 | 1 | 1 | 0.855 | 0.062 | 0.165 | 0.050 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, S.; Murata, S.; Isezaki, M.; Ariizumi, T.; Sato, T.; Oishi, E.; Taneno, A.; Maekawa, N.; Okagawa, T.; Ichii, O.; et al. Characterization of a Novel Cysteine Protease Inhibitor from Poultry Red Mites: Potential Vaccine for Chickens. Vaccines 2021, 9, 1472. https://doi.org/10.3390/vaccines9121472
Fujisawa S, Murata S, Isezaki M, Ariizumi T, Sato T, Oishi E, Taneno A, Maekawa N, Okagawa T, Ichii O, et al. Characterization of a Novel Cysteine Protease Inhibitor from Poultry Red Mites: Potential Vaccine for Chickens. Vaccines. 2021; 9(12):1472. https://doi.org/10.3390/vaccines9121472
Chicago/Turabian StyleFujisawa, Sotaro, Shiro Murata, Masayoshi Isezaki, Takuma Ariizumi, Takumi Sato, Eiji Oishi, Akira Taneno, Naoya Maekawa, Tomohiro Okagawa, Osamu Ichii, and et al. 2021. "Characterization of a Novel Cysteine Protease Inhibitor from Poultry Red Mites: Potential Vaccine for Chickens" Vaccines 9, no. 12: 1472. https://doi.org/10.3390/vaccines9121472
APA StyleFujisawa, S., Murata, S., Isezaki, M., Ariizumi, T., Sato, T., Oishi, E., Taneno, A., Maekawa, N., Okagawa, T., Ichii, O., Konnai, S., & Ohashi, K. (2021). Characterization of a Novel Cysteine Protease Inhibitor from Poultry Red Mites: Potential Vaccine for Chickens. Vaccines, 9(12), 1472. https://doi.org/10.3390/vaccines9121472






